Abstract
Studies show that a decrease in protein kinase CK2 (CK2) activity is associated with cellular senescence. However, the role of CK2 in organism aging is still poorly understood. Here, we investigated whether protein kinase CK2 (CK2) modulated longevity in Caenorhabditis elegans. CK2 activity decreased with advancing age in the worms. Knockdown of kin-10 (the ortholog of CK2β) led to a short lifespan phenotype and induced age-related biomarkers, including retardation of locomotion, decreased pharyngeal pumping rate, increased lipofuscin accumulation, and reduced resistance to heat and oxidative stress. The long lifespan of age-1 and akt-1 mutants was significantly suppressed by kin-10 RNAi, suggesting that CK2 acts downstream of AGE-1 and AKT-1. Kin-10 knockdown did not further shorten the short lifespan of daf-16 mutant worms but either decreased or increased the transcriptional activity of DAF-16 depending on the promoters of the target genes, indicating that CK2 is an upstream regulator of DAF-16 in C. elegans. Kin-10 knockdown increased production of reactive oxygen species (ROS) in the worms. Finally, the ROS scavenger N-acetyl-L-cysteine significantly counteracts the lifespan shortening and lipofuscin accumulation induced by kin-10 knockdown. Therefore, the present results suggest that age-dependent CK2 downregulation reduces longevity by associating with both ROS generation and the AGE-1-AKT-1-DAF-16 pathway in C. elegans.
Keywords: C. elegans, protein kinase CK2, longevity, daf-16, Gerotarget
INTRODUCTION
The nematode Caenorhabditis elegans has been widely used as a model for exploring the mechanisms underlying aging. A crucial pathway influencing C. elegans aging is the insulin/insulin-like growth factor (IGF)-1 signaling (IIS) pathway. Key components of the IIS pathway are daf-2/IGF receptor (IGFR), age-1/phosphoinositide 3-kinase (PI3K), and akt-1/AKT-1/2 [1–4]. The transcription factor DAF-16/FoxO lies downstream of the IIS pathway [5, 6]. DAF-2/IGFR activation leads to the activation of AGE-1/PI3K, which in turn activates AKT, resulting in phosphorylation and cytoplasmic sequestration of DAF-16/FoxO [7]. Inhibition of DAF-2/IGFR or AGE-1/PI3K allows translocation of DAF-16/FoxO into the nucleus, where DAF-16/FoxO activates the transcription of pro-longevity genes, including antioxidant and thermotolerant genes [8, 9].
Cellular senescence, which is defined as an irreversible arrest at the G1 phase of the cell cycle, is an important tumor suppression process in vivo. Because the number of senescent cells increases with organism aging, it is widely believed that cellular senescence plays an important role in aging. We have previously shown that protein kinase CK2 (CK2), which is a ubiquitous serine/threonine kinase, is downregulated during replicative senescence in human lung fibroblast IMR-90 cells and in aged rat tissues [10]. CK2 inhibition induces premature senescence in IMR-90 cells, human colon cancer HCT116 cells, and breast cancer MCF-7 cells. The p53-p21Cip1/WAF1 pathway is required for senescence induced by CK2 inhibition [11]. Reactive oxygen species (ROS) production and p53 acetylation by downregulation of NAD+-dependent protein deacetylase SIRT1 serve as upstream activators of p53 stabilization in senescent cells due to CK2 inhibition [12, 13]. Inhibition of PI3K, AKT, and mammalian target of rapamycin (mTOR), as well as ectopic expression of FoxO3a, attenuated ROS production and senescence in CK2-downregulated cells, indicating that the PI3K-AKT-mTOR pathway as well as FoxO3a are involved in CK2 inhibition-mediated ROS generation [14, 15].
The CK2 holoenzyme is a heterotetramer composed of two catalytic (α and/or α′) subunits and two regulatory β subunits. The β subunit stimulates the catalytic activity of the α or α′ subunit, thereby mediating tetramer formation and substrate recognition [16]. The expression level of CK2 is greatly enhanced in a variety of tumor or leukemic cells [17–19]. The biological role of CK2 has been extensively studied in diverse organisms. KIN-3 and KIN-10, the C. elegans orthologs of CK2α and CK2β, respectively, are known to be expressed ubiquitously in multiple tissues [20, 21]. In this study, we characterized the role of CK2 as a positive regulator of longevity in worms. We found that CK2 activity decreases with advancing age and that downregulation of CK2 activity by kin-10 RNAi causes expression of age-related biomarkers and lifespan reduction. This study provides the first evidence that age-dependent downregulation of CK2 activity may be a possible regulator of lifespan in C. elegans.
RESULTS
In C. elegans, CK2 activity is downregulated with advancing age, and kin-10 knockdown reduces lifespan and pumping rate
We first examined whether CK2 catalytic activity in C. elegans was modulated with advancing age. The phosphotransferase activity of CK2 decreased by 70% in worms at day 8 compared to worms at day 1 (Figure 1A). To investigate whether CK2 regulates the longevity of C. elegans, we compared the lifespan of worms treated with kin-10 RNAi with that of worms treated with empty vector control (L4440) RNAi. The phenotype of kin-3 RNAi was lethal under our experimental conditions. The growth rate of kin-10 RNAi worms was almost the same as that of wild-type worms (data not shown). CK2 phosphotransferase activity in kin-10 RNAi worms was reduced by approximately 50% compared to that of control RNAi worms (Figure 1B). After kin-10 RNAi treatment, the maximum lifespan of the worms was shortened from 24 to 17 days, and the median lifespan decreased from 16 to 10 days (Figure 1C; see also Supplemental Figure 1). We also carried out lifespan assays using worms with kin-10 RNAi only during adulthood and observed similarly shortened lifespans (Supplemental Figure 2A). This result eliminates the possibility that downregulation of kin-10 during larval development harmfully affected the health of worms and consequently decreased their longevity during adulthood. We next investigated the effect of kin-10 knockdown on the worms’ pharyngeal pumping. Compared with that of worms at day 1 of adulthood, the pumping rate was much lower in worms at day 8 of adulthood. However, pumping rates further decreased in worms fed with kin-10 RNAi (Figure 1D). Therefore, the results suggest that CK2 may function as a positive regulator of aging in C. elegans.
Figure 1. In C. elegans, CK2 activity was downregulated with advancing age, and kin-10 knockdown reduced lifespan and pumping rate.
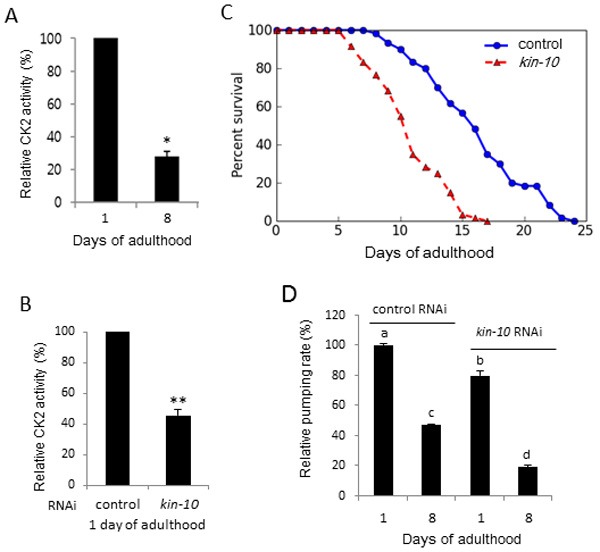
Age-synchronized L4 larvae were fed on empty vector control (L4440) or kin-10 RNAi plates under standard conditions. A. Age-dependent decrease in CK2 activity. Lysates from worms at days 1 and 8 of adulthood were utilized in kinase assays using a specific CK2 substrate peptide. 32P incorporation into the substrate peptide was measured by scintillation counting. B. Kin-10 knockdown reduced CK2 activity in worms. Lysates from worms at day 1 of adulthood fed with kin-10 RNAi or control RNAi were utilized in kinase assays using a specific CK2 substrate. 32P incorporation into the substrate peptide was measured by scintillation counting. C. Kin-10 RNAi reduced the lifespan of worms. Viability was scored as movement away from pick touch at the indicated days. Representative data from three independent RNAi experiments are shown (n = 60 per condition). D. Kin-10 knockdown reduced pharyngeal pumping rate. Each data point represents the mean of relative pumping rate per minute (n = 20 per condition). Values indicate mean ± SEM. *P < 0.05; **P < 0.01. Bars that do not share a common letter (a, b, c, d) are significantly different among the groups at P < 0.05. All experiments in this figure were carried out at 21 °C.
Kin-10 knockdown increases lipofuscin and fat accumulation in C. elegans
Increased lipofuscin and fat accumulation are also associated with accelerated aging [1]. To investigate the effect of kin-10 on lipofuscin accumulation in worms, lipofuscin accumulation was determined by autofluorescence [22]. Autofluorescence significantly increased with age but also increased in kin-10 RNAi worms relative to control RNAi worms (Figure 2A). To exclude the effect of kin-10 on lipofuscin accumulation during development, we examined worms fed with kin-10 RNAi only during adulthood and observed similar lipofuscin accumulation (Supplemental Figure 2B). Nile red staining indicated that kin-10 RNAi stimulated fat accumulation in the worms (Figure 2B). Therefore, these results suggest that kin-10 downregulation induced biomarkers of aging in C. elegans.
Figure 2. Kin-10 knockdown increased lipofuscin accumulation and Nile red staining in C. elegans.
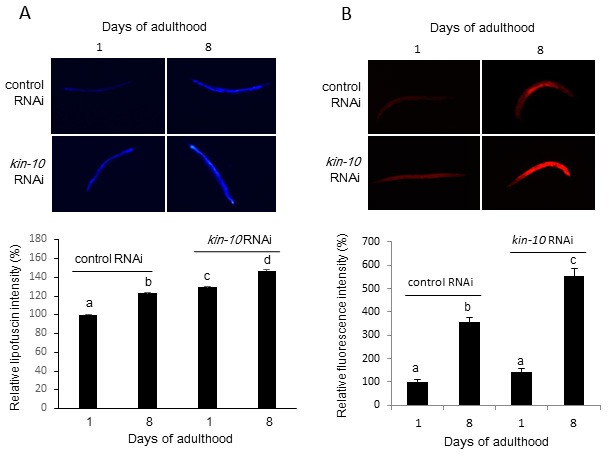
A. Knockdown of kin-10 increased lipofuscin accumulation. Representative autofluorescence images of worms at day 1 or 8 of adulthood (upper panel). The fluorescence intensity (bottom panel) was quantified using the ImageJ software by determining the average pixel intensity (n > 80 per condition). B. Representative Nile red staining images (upper panel) of worms at day 1 or 8 of adulthood and relative fluorescence intensity (bottom panel) are shown. The fluorescence intensity was quantified using ImageJ software by determining the average pixel intensity (n = 20 per condition). Values indicate mean ± SEM. Bars that do not share a common letter (a, b, c) are significantly different among the groups at P < 0.05. All experiments in this figure were carried out at 21 °C.
Kin-10 knockdown decreases stress resistance and motility in C. elegans
Aged worms display a general decrease in resistance to diverse stresses, including heat stress and oxidative stress [1]. We performed a thermotolerance assay and found that not only aging but also kin-10 RNAi decreased resistance to heat stress at 35 °C compared to control treatment with the empty vector (Figure 3A). Resistance to oxidative stress (3 mM H2O2) was also decreased by kin-10 RNAi (Figure 3B). Because all animals display age-related decline in physical ability, we measured the worms’ body movement. As shown in Figure 3C, kin-10 RNAi decreased the average worm velocity by approximately 35% and 50% compared to control RNAi at day 1 and 8 of adulthood, respectively. In addition, at day 8 of adulthood, kin-10 knockdown decreased the worms’ maximum velocity by 47% compared to control RNAi (Figure 3D). Taken together, these results also suggest that kin-10 downregulation induced biomarkers of aging in C. elegans.
Figure 3. Kin-10 knockdown decreased resistance against heat and oxidative stress and motility in C. elegans.
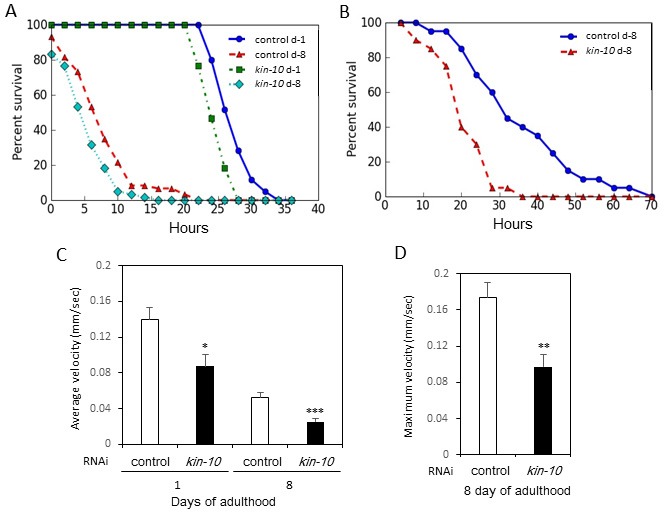
A. Kin-10 knockdown decreased resistance against heat stress at 35 °C. Worms at day 1 (d-1) or 8 (d-8) of adulthood were scored every 2 h for viability (n = 60 per condition). B. Kin-10 knockdown decreased resistance against oxidative stress. Survival curves of worms fed with control or kin-10 RNAi after exposure to 3 mM H2O2 for 5 h. Worms at day 8 (d-8) of adulthood were scored every 4 h for viability (n = 40 per condition). C and D. Kin-10 RNAi decreased average velocity C. and maximum velocity D. of worms. The average velocity and maximum velocity of individual worms were measured at day 1 or 8 of adulthood (n > 13 per condition). Data are shown as the means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
CK2 regulates lifespan downstream of AGE-1 and AKT-1 in C. elegans
The IIS pathway is a critical modulator of longevity, along with DAF-16/FoxO [1, 9]. To define the role of CK2 in the aging pathway, we examined the genetic interactions between CK2 and the IIS pathway. Our previous studies demonstrated that the AGE-1/PI3K-AKT pathway is involved in CK2 inhibition-mediated cellular senescence in human cells [14]. Therefore, we used the age-1(hx546) and akt-1(mg144) single mutants. There was a significant increase in the lifespans of both age-1(hx546) and akt-1(mg144) single mutants. The maximum and median lifespans of these mutant worms were extended to 56 and 37 days, respectively. However, kin-10 RNAi completely suppressed the longevity extension effect of the age-1(hx546) and akt-1(mg144) mutations. Upon kin-10 RNAi treatment, the maximum and median lifespans of the mutant worms were shortened to levels similar to those of wild-type worms fed with kin-10 RNAi (Figure 4A and 4B). Thus, these results suggest that CK2 is associated with the IIS pathway as a downstream regulator of AGE-1 and AKT-1 in C. elegans.
Figure 4. Effect of kin-10 knockdown on the lifespan of age-1, akt-1, and daf-16 single mutant worms.
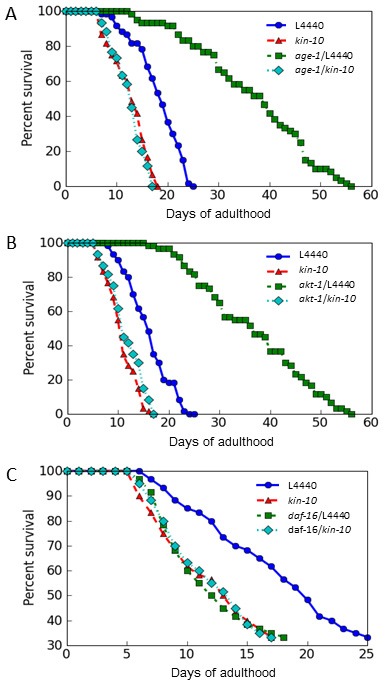
Age-1(hx546) A., akt-1(mg144) B., and daf-16(mu86) C. single mutant worms were fed on the control (L4440) or kin-10 RNAi plates under standard conditions. n = 60 per condition (A and B); n = 40 per condition (C). Viability was scored as movement away from pick touch at the indicated days. Kin-10 knockdown successfully suppressed the longevity extension by age-1(hx546) and akt-1(mg144) mutations, but did not synergistically shorten the lifespan of daf-16(mu86) mutant. All experiments in this figure were carried out at 21 °C.
Kin-10 knockdown in C. elegans either decreases or increases DAF-16 activity depending on the promoter of the target genes
To examine the role of DAF-16 in the cellular responses subsequent to kin-10 RNAi, daf-16 mutant worms were used. The lifespan of daf-16(mu86) mutant worms was not synergistically shortened by RNAi inhibition of kin-10 expression, suggesting the possibility that CK2 and DAF-16 are present in the same signaling pathway regulating longevity (Figure 4C). To examine whether CK2 activity regulates DAF-16 transcriptional activity in C. elegans, we measured the fluorescence of several reporter worms that are specific for certain stress pathways, including sod-3, which encodes manganese superoxide dismutase (MnSOD), dod-11, which encodes sorbitol dehydrogenase, mtl-1, which encodes metallothionein, and dod-8, which encodes 17 β-hydroxysteroid dehydrogenase [23]. kin-10 RNAi decreased the fluorescence of sod-3::gfp, dod-11::rfp, and mtl-1:rfp reporter worms compared to the control RNAi. By contrast, kin-10 RNAi enhanced the fluorescence of the dod-8::gfp reporter relative to the empty vector RNAi (Figure 5, upper panel). Further densitometric analysis of fluorescence images confirmed that kin-10 knockdown downregulates the expression of sod-3, dod-11, and mtl-1 but upregulates the expression of dod-8 (Figure 5, bottom panel). These results therefore suggest that in C. elegans, CK2 regulates the transcriptional activity of DAF-16 either negatively or positively, depending on the target genes upstream of DAF-16.
Figure 5. Kin-10 knockdown either decreases or increases the transcriptional activity of DAF-16 depending on the target genes in C. elegans.
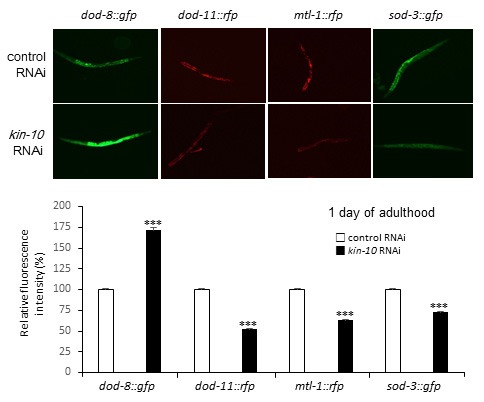
Worms with integrated sod-3::gfp, mtl-1::rfp, dod-11::rfp, and dod-8::gfp reporter constructs were fed with kin-10 RNAi or control (L4440) RNAi at L4 stage for 1 day. One day after RNAi treatment, GFP or RFP fluorescence was imaged with a 10× objective (upper panel). GFP or RFP fluorescence intensity (bottom panel) was quantified using the ImageJ software by determining the average pixel intensity (n > 70 per condition). Data are shown as the means ± SEM. ***P < 0.001. All experiments in this figure were carried out at 21 °C.
Kin-10 knockdown stimulates ROS production in C. elegans
Excess intracellular ROS cause DNA damage, which can induce cellular senescence. We have also previously shown that ROS play a key role in CK2 inhibition-mediated senescence in human cells [12]. To investigate the role of ROS in aging of kin-10 RNAi worms, the amount of ROS was quantified using DCFDA and DHE. Kin-10 RNAi significantly increased the production of ROS in the worms (Figure 6, upper panels). Quantification of ROS levels showed that they increased approximately 1.5- to 2.3-fold depending on age and fluorescent probe in kin-10 RNAi worms compared with control RNAi worms (Figure 6, bottom panels). Consistently, wild-type N2 worms at day 8 of adulthood exhibited a markedly higher level of ROS than worms at day 1 of adulthood (Figure 6).
Figure 6. Kin-10 knockdown stimulates ROS production in C. elegans.
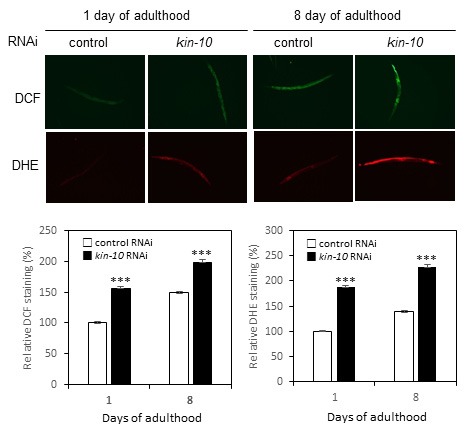
Worms fed with kin-10 RNAi or control RNAi were incubated with DCFDA or DHE. Representative images at day 1 or 8 of adulthood were obtained at 10× magnification (upper panels). The fluorescence intensity (bottom panels) was quantified using the ImageJ software by determining the average pixel intensity (n > 30 per condition). Data are shown as the means ± SEM. ***P < 0.001. All experiments in this figure were carried out at 21 °C.
The ROS scavenger NAC counteracts the lifespan shortening and lipofuscin accumulation mediated by kin-10 knockdown
To determine the role of ROS in aging subsequent to kin-10 knockdown, worms were incubated with N-acetyl-L-cysteine (NAC), which is a commonly used ROS scavenger. Treatment with NAC (3 or 6 μM) significantly counteracted the lifespan shortening (Figure 7A; see also Supplemental Figure 3) and lipofuscin accumulation (Figure 7B) mediated by kin-10 knockdown. To exclude effects of NAC and kin-10 RNAi treatment on lifespan and lipofuscin accumulation during development, worms were treated with kin-10 RNAi and NAC only during adulthood. NAC treatment during adulthood only also showed similar counteracting effects on the lifespan shortening and lipofuscin accumulation (Supplemental Figure 4). These findings indicate that in worms, ROS production is one of the major upstream causes of aging induced by CK2 downregulation.
Figure 7. The effect of NAC on lifespan shortening and lipofuscin accumulation in kin-10 RNAi worms.
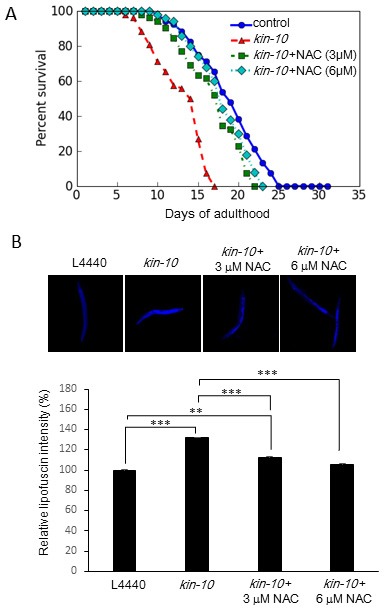
Age-synchronized L4 larvae were fed on empty vector control (L4440) plates or kin-10 RNAi plates containing NAC (0, 3, or 6 µM) under standard conditions. A. Viability was scored as movement away from pick touch at the indicated days. Representative data from three independent RNAi experiments are shown (n = 50 per condition). B. Representative autofluorescence images of worms at day 1 of adulthood (upper panel). The fluorescence intensity (bottom panel) was quantified using the ImageJ software by determining the average pixel intensity (n > 40 per condition). Data are shown as the means ± SEM. **P < 0.01; ***P < 0.001. All experiments in this figure were carried out at 21 °C.
DISCUSSION
Previous studies demonstrated that the activity of CK2 decreases during replicative senescence in human fibroblast cells and with advancing age in rat tissues [10, 24]. In addition, CK2 inhibition induces premature senescence through the ROS-p53-p21Cip1/WAF1 pathway in human cells such as lung fibroblast IMR-90, colon cancer HCT116, and breast cancer MCF-7cells [11–15]. In this study, we examined whether CK2 modulated longevity in C. elegans. The present results indicate that CK2 activity decreased with advancing age in C. elegans (Figure 1A). Moreover, we showed here that downregulation of CK2 activity by knockdown of kin-10 (the ortholog of CK2β) shortened the lifespan of C. elegans (Figure 1C; see also Supplemental Figures 1 and 2A), induced several age-related biomarkers, increased lipofuscin accumulation (Figure 2A; see also Supplemental Figure 2B) and fat content (Figure 2B), reduced the pharyngeal pumping rate (Figure 1D) and the velocity of body movement (Figure 3C and 3D), and reduced resistance to heat and oxidative stress (Figure 3A and 3B). These events may be consistent with previous findings showing that the capacity for exercise is fundamental to longevity and more notably healthy aging [25], as well as that the accumulation of fat in aged worms may be due to an age-associated decline in tissue function [26]. In addition, CK2 downregulation in C. elegans promoted tri-methylation of lysine 9 in histone H3 (H3K9me3), which is a hallmark of senescence associated heterochromatin foci (SAHF) (J.W. Park and Y.S. Bae, manuscript in preparation). It has been reported that SAHF formation represses expression of cell cycle progression associated genes, such as cyclin A2 and transcription factor E2F target genes [27]. How does CK2 activity decrease with advancing age in C. elegans? Because our previous studies demonstrated that DNA methylation and microRNAs (miRNAs) are primarily involved in CK2α gene silencing during replicative senescence of human cells [28–30], we speculate that the underlying cause of age-dependent CK2 downregulation in worms may be related to DNA methylation or miRNAs.
Components of IIS, such as PI3K and AKT, have been shown to modulate longevity in yeast, worms, and flies [1–4]. The present study indicated that the lifespan of age-1 or akt-1 single mutants was significantly suppressed by kin-10 RNAi, suggesting that CK2 functioned downstream of AKT in C. elegans (Figure 4A and 4B). DAF-16/FoxO is a well-known transducer of IIS [5–7]. The present data showing that the reduced longevity conferred by knockdown of kin-10 was not additive to the shortened lifespan caused by the daf-16 mutation suggested that both the daf-16 mutation and CK2 deficiency acted in the same pathway in C. elegans (Figure 4C). We also demonstrated here that CK2 downregulation either decreased or increased the transcriptional activity of DAF-16 depending on the target genes, suggesting that CK2 acts upstream of DAF-16 in C. elegans (Figure 5). Thus, we conclude that CK2 functions between AKT-1 and DAF-16 in worms. This is not consistent with our previous report that CK2 acts upstream of the AKT in human colon cancer cells [14, 15]. Although we currently cannot explain this discrepancy, we hypothesize that CK2 may function at different stages of the IIS pathway depending on the organism type (e.g., invertebrate or vertebrate).
A number of studies have shown that increased oxidative damage in intracellular molecules is an important mechanistic explanation for the aging process [31, 32]. A recent paper reported that the effect of antioxidants reveals an inverted U-shaped dose-response relationship between ROS amounts and lifespan in C. elegans [33], suggesting that sustaining ROS homeostasis is crucial for maintaining a healthy lifespan. Therefore, intracellular ROS levels should be maintained for preventing aging within narrow ranges by both ROS generating and scavenging systems [32]. We propose here that redox balance can be disrupted by decreased activity of CK2 in C. elegans, because kin-10 knockdown increased ROS levels in worms (Figure 6). Antioxidant NAC successfully counteracted the lifespan shortening and lipofuscin accumulation induced by kin-10 RNAi, indicating that in worms, ROS generation is a major upstream cause of aging induced by CK2 downregulation (Figure 7; see also Supplemental Figures 3 and 4). The transcriptional activity of DAF-16 on the promoters of sod-3 and dod-11 was decreased by kin-10 RNAi (Figure 5). Since SOD-3/MnSOD detoxifies ROS [34] and DOD-11/sorbitol dehydrogenase reduces the nicotinamide adenine dinucleotide (NAD+) level in the conversion of sorbitol to fructose [35], our observations suggest that CK2 inhibition-mediated downregulation of SOD-3 and DOD-11 may cause an imbalance between ROS production and removal in C. elegans. These results are consistent with our previous report indicating that CK2 inhibition stimulates ROS production through inhibition of FoxO3a activity in human cells [15]. The present study found that kin-10 knockdown decreased the transcriptional activity of DAF-16 on the mtl-1 promoters, but it enhanced that of DAF-16 on the dod-8 promoter in worms. It has been reported that MTL-1/metallothionein is a heavy metal-binding protein that is involved in metal homeostasis [36] and that DOD-8/17 β-hydroxysteroid dehydrogenase is localized to lipid droplets, which are highly specialized for lipid storage [37]. Thus, this study suggests that CK2 downregulation may reduce stress resistance and increase fat accumulation through controlling DAF-16 activity in worms.
In summary, we uncovered a novel mechanism for regulation of lifespan in C. elegans. CK2 downregulation led to shortened longevity, reduced physical ability and stress resistance, and increased lipofuscin accumulation. Importantly, CK2 activity decreased in aged worm. CK2 functioned between AKT and DAF-16 and regulated expression of DAF-16 target genes in worms. Because DAF-16 plays an essential role in the aging process, elucidating the molecular mechanism by which CK2 regulates the transcription activity of DAF-16 will extend understanding of aging processes.
MATERIALS AND METHODS
C. elegans strains and culture
C. elegans N2 (wild-type) strain, strains carrying mutant alleles daf-16(mu86), age-1(hx546), and akt-1(mg144), and reporter strains sod-3::gfp, dod-11::gfp, dod-8::gfp, mtl-1::gfp, and daf-16::gfp (CF1724, daf-2 mutant) were obtained from the Caenorhabditis Genetics Center. Worms were grown at 20 or 21 °C on nematode growth medium (NGM) agar plates with Escherichia coli strain OP50 as a food source. For some experiments, NAC was prepared in dH2O and added into NGM media to a final concentration of 3 or 6 µM.
RNAi experiments
E. coli HT115 expressing double-stranded kin-10 RNA was from the C. elegans ORFeome RNAi library. To inactivate kin-10 function, eggs from gravid adults were placed on HT115-seeded NGM plates and allowed to hatch at 21 °C. Expression of double-stranded RNA was induced by treatment with 1 mM isopropyl 1-thio-β-D-galactopyranoside. Worms hatched from eggs were fed on HT115-seeded NGM plates until L4 stage. To synchronize the worms, L4 larvae were then placed on HT115-seeded NGM plates supplemented with 5 µM 2′fluoro-5′deoxyuridine (FUdR; Sigma, MO), which prevents progeny production, and allowed to grow to day 1 or 8 of adulthood. E. coli HT115 carrying the empty L4440 vector was used as control RNAi. For some experiments (Supplemental Figures 2 and 4), we used worms fed with kin-10 RNAi at the L4 larval stage for 1 day to exclude the effect of kin-10 knockdown on worm development.
Lifespan measurement
Lifespan assays were performed as described previously [38]. Synchronized L4 larvae were placed on HT115-seeded NGM plates containing FUdR. Surviving worms were counted daily and were moved to new HT115-seeded NGM plates. Death was scored as the absence of a response to slight touch using a thin platinum wire. Three independent experiments were performed, with approximately 60 worms for each experimental group.
CK2 activity assay
Worms were lysed by sonication in lysis buffer [50 mM Tris-HCl (pH 8.0), 20 mM NaCl, 1 mM MgCl2, 1 mM EDTA, 1% Nonidet P-40, 0.5 mM PMSF, 1 µg/mL aprotinin, 1 µg/mL leupeptin, 1 µg/mL pepstatin, 1 mM sodium orthovanadate, 1 mM sodium pyrophosphate, and 4 mM p-nitrophenyl phosphate]. The particulate debris was removed by centrifugation at 12,000 × g. The volumes of the supernatants were adjusted for equal protein concentration. A standard assay for CK2 phosphotransferase activity was conducted in a reaction mixture containing 20 mM Tris-HCl (pH 7.5), 120 mM KCl, 10 mM MgCl2, and 100 µM [γ-32P]ATP in the presence of 1 mM synthetic peptide substrate (RRREEETEEE) in a total volume of 30 µl at 30 °C. Worm lysates were added to initiate the reactions and incubated for 15 min. The reaction was stopped by the addition of trichloroacetic acid to a final concentration of 10%. The mixture was then centrifuged, after which, 10 µl of supernatant was applied to P-81 paper. The paper was washed in 100 mM phosphoric acid, and radioactivity was measured by scintillation counting.
Measurement of pharyngeal pumping rate
Synchronized (day 1 and 8 of adulthood) worms were transferred to OP50-lawn on NGM plates, and the pharyngeal pumping rates of individual worms were recorded by counting the number of pharyngeal contractions for 1 min under a dissection microscope.
Lipofuscin assays
Intestinal lipofuscin was assayed in synchronized (day 1 and 8 of adulthood) worms. The autofluorescence of lipofuscin was measured using a fluorescence microscope (ZEISS AxioCam MRc, Germany) with an excitation/emission wavelength of 350 nm/470 nm. The relative fluorescence intensity was quantified using the ImageJ software (National Institutes of Health, MD) to determine the lipofuscin levels.
Nile red staining
Nile red staining was performed as previously described [39], with minor modifications. Briefly, synchronized (day 1 and 8 of adulthood) worms were grown on HT115-seeded NGM plates containing 75 ng/mL of Nile red (Sigma, MO) for 3 days. Worms were mounted on a 2% agarose pad and visualized with a fluorescence microscope (Carl Zeiss AxioScope A1) at 10× magnification. The data were quantified using the ImageJ software by determining the average pixel fluorescence intensity. The relative fluorescence of the whole body was determined densitometrically using the Image-Pro Plus version 6.0 software (Media Cybernetics, MD).
Measurement of mean velocity and maximum velocity
The worms’ mean velocity and maximum velocity were measured as previously described [40]. Briefly, synchronized (day 1 and 8 of adulthood) worms were transferred to a physical assay plate (NGM plate without bacterial lawn), and their movements were recorded immediately. The recording system comprised a stereomicroscope (Nikon SMZ 745T), a charge-coupled device camera (TUCSEN TCH-5.0), and imaging software (TUCSEN ISCapture). The recording period was 30 s at a rate of 30 frames per second. The locomotion velocity was expressed as millimeter per second [the distance (mm) between displaced centroids per second]. Recorded images were analyzed by ImageJ and wrMTrck (plugin for ImageJ: www.phage.dk/plugins). The locomotion velocity data were imported into an Excel spreadsheet. The peak locomotion velocity observed in the 30 s period was used as the maximum velocity.
Stress resistance assays
Thermotolerance assays were performed as previously described [41]. Briefly, HT115-seeded NGM plates with synchronized (day 1 and 8 of adulthood) worms were shifted from 21 °C to 35 °C. After the temperature shift, animal survival was scored for touch-provoked movement every 2 h. For the oxidative stress resistance assay, synchronized (day 8 of adulthood) worms were transferred to HT115-seeded NGM plates containing 3 mM of H2O2. After 5 h of incubation, worm viability was scored as touch-provoked movement every 4 h. Data were analyzed and plotted as described for the lifespan assays.
Measurement of intracellular ROS
Synchronized (day 1 and 8 of adulthood) worms were washed with M9 buffer (22 mmol/L KH2PO4, 22 mmol/L Na2HPO4, 85 mmol/L NaCl, and 1 mmol/L MgSO4) and then transferred to 2 mL of M9 buffer containing 6 µM dichlorofluorescein diacetate (DCFDA, Invitrogen, Carlsbad, CA) or 3 µM dihydroethidium (DHE, Invitrogen, Carlsbad, CA). After incubation for 60 min at 37 °C in the dark, the worms were rinsed with M9 buffer, and digital images were obtained with a Leica DM IRB inverted microscope (Leica Microsystems, Germany) equipped with a CoolSNAP HQ camera (Roper Scientific, NJ) operated by the Metamorph Image Software (Universal Imaging Corporation, PA).
Reporter gene assay
Synchronized (L4 larva) reporter worms dod-8::gfp, dod-11::rfp, mtl-1::rfp, and sod-3::gfp were transferred to HT115-seeded NGM plates. After 1 day, the fluorescence of GFP or RFP was measured using a fluorescence microscope (ZEISS AxioCam MRc, Germany) at an excitation/emission wavelength of 490 nm/525 nm. The relative fluorescence intensity was quantified using the ImageJ software to determine GFP levels.
Statistical analysis
The statistical significance of differences in the data was analyzed by one-way ANOVA with the SPSS package program. The results were considered significant if the value of P was less than 0.05. The Duncan's multiple-range test was performed if differences between groups were identified as α = 0.05.
SUPPLEMENTARY MATERIALS FIGURES
Acknowledgments
We would like to thank S. J. Lee (Pohang University of Science and Technology) for helpful discussions. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2015R1A2A2A01004593) to Y.B. and by Institute for Basic Science (IBS-R013-D1) to H.N.
Authors’ contributions
The study design was funded by Y.B. Y.B. and H.N. interpreted the experiments and wrote the paper. J.L. performed assays for CK2 activity, Nile red staining, and stress resistance. J.H.P. and J.H. performed the motility assay. J.W.P. and D.K. performed the experiments using NAC. J.H.P. performed the remaining experiments.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 2.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 3.Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118:75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin K, Dorman JB, Rodan A, Kenyon C. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. doi: 10.1126/science.278.5341.1319. [DOI] [PubMed] [Google Scholar]
- 6.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 7.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 8.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 9.Klotz LO, Sánchez-Ramos C, Prieto-Arroyo I, Urbánek P, Steinbrenner H, Monsalve M. Redox regulation of FoxO transcription factors. Redox Biol. 2015;6:51–72. doi: 10.1016/j.redox.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu SW, Woo JH, Kim YH, Lee YS, Park JW, Bae YS. Down-regulation of protein kinase CKII is associated with cellular senescence. FEBS Lett. 2006;580:988–994. doi: 10.1016/j.febslet.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Kang JY, Kim JJ, Jang SY, Bae YS. The p53-p21Cip1/WAF1 pathway is necessary for cellular senescence induced by the inhibition of protein kinase CKII in human colon cancer cells. Mol Cells. 2009;28:489–494. doi: 10.1007/s10059-009-0141-9. [DOI] [PubMed] [Google Scholar]
- 12.Jeon SM, Lee SJ, Kwon TK, Kim K, Bae YS. NADPH oxidase is involved in protein kinase CKII down-regulation-mediated senescence through elevation of the level of reactive oxygen species in human colon cancer cells. FEBS Lett. 2010;584:3137–3142. doi: 10.1016/j.febslet.2010.05.054. [DOI] [PubMed] [Google Scholar]
- 13.Jang SY, Kim SY, Bae YS. p53 deacetylation by SIRT1 decreases during protein kinase CK2 downregulation-mediated cellular senescence. FEBS Lett. 2011;585:3360–3366. doi: 10.1016/j.febslet.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Kim JJ, Bae YS. Involvement of PI3K-AKT-mTOR pathway in protein kinase CKII inhibition-mediated senescence in human colon cancer cells. Biochem Biophys Res Commun. 2013;433:420–425. doi: 10.1016/j.bbrc.2013.02.108. [DOI] [PubMed] [Google Scholar]
- 15.Park SY, Bae YS. Inactivation of the FoxO3a transcription factor is associated with the production of reactive oxygen species during protein kinase CK2 downregulation-mediated senescence in human colon cancer and breast cancer cells. Biochem Biophys Res Commun. 2016;478:18–24. doi: 10.1016/j.bbrc.2016.07.106. [DOI] [PubMed] [Google Scholar]
- 16.Ruzzene M, Pinna LA. Addiction to protein kinase CK2: a common denominator of diverse cancer cells? Biochim Biophys Acta. 2010;1804:499–504. doi: 10.1016/j.bbapap.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Faust RA, Gapany M, Tristani P, Davis A, Adams GL, Ahmed K. Elevated protein kinase CK2 activity in chromatin of head and neck tumors: association with malignant transformation. Cancer Lett. 1996;101:31–35. doi: 10.1016/0304-3835(96)04110-9. [DOI] [PubMed] [Google Scholar]
- 18.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene. 2001;20:3247–3257. doi: 10.1038/sj.onc.1204411. [DOI] [PubMed] [Google Scholar]
- 19.Stalter G, Siemer S, Becht E, Ziegler M, Remberger K, Issinger OG. Asymmetric expression of protein kinase CK2 subunits in human kidney tumors. Biochem Biophys Res Commun. 1994;202:141–147. doi: 10.1006/bbrc.1994.1904. [DOI] [PubMed] [Google Scholar]
- 20.Hu E, Rubin CS. Casein kinase II from Caenorhabditis elegans Properties and developmental regulation of the enzyme; cloning and sequence analyses of cDNA and the gene for the catalytic subunit. J Biol Chem. 1990;265:5072–5080. [PubMed] [Google Scholar]
- 21.Wang X, Gupta P, Fairbanks J, Hansen D. Protein kinase CK2 both promotes robust proliferation and inhibits the proliferative fate in the C. elegans germ line. Dev Biol. 2014;392:26–41. doi: 10.1016/j.ydbio.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Gerstbrein B, Stamatas G, Kollias N, Driscoll M. vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell. 2005;4:127–137. doi: 10.1111/j.1474-9726.2005.00153.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P, Judy M, Lee SJ, Kenyon C. Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab. 2013;17:85–100. doi: 10.1016/j.cmet.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SW, Song YS, Lee SY, Yoon YG, Lee SH, Park BS, Yun I, Choi H, Kim K, Chung WT, Yoo YH. Downregulation of protein kinase CK2 activity facilitates tumor necrosis factor-α-mediated chondrocyte death through apoptosis and autophagy. PLoS One. 2011;6:e19163. doi: 10.1371/journal.pone.0019163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang C, Xiong CJ, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 27.Zhang R, Chen W, Adams PD. Molecular dissection of formation of senescence-associated heterochromatin foci. Mol Cell Biol. 2007;27:2343–2358. doi: 10.1128/MCB.02019-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EK, Kang JY, Rho YH, Kim YS, Kim DS, Bae YS. Silencing of the CKIIα and CKIIα’ genes during cellular senescence is mediated by DNA methylation. Gene. 2009;431:55–60. doi: 10.1016/j.gene.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Kim SY, Lee YH, Bae YS. miR-186, miR-216b, miR-337-3p, and miR-760 cooperatively induce cellular senescence by targeting α subunit of protein kinase CKII in human colorectal cancer cells. Biochem Biophys Res Commun. 2012;429:173–179. doi: 10.1016/j.bbrc.2012.10.117. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Kim SY, Bae YS. Upregulation of miR-760 and miR-186 is associated with replicative senescence in human lung fibroblast cells. Mol Cells. 2014;37:620–627. doi: 10.14348/molcells.2014.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bokov A, Chaudhuri A, Richardson A. The role of oxidative damage and stress in aging. Mech Ageing Dev. 2004;125:811–826. doi: 10.1016/j.mad.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Desjardins D, Cacho-Valadez B, Liu JL, Wang Y, Yee C, Bernard K, Khaki A, Breton L, Hekimi S. Antioxidants reveal an inverted U-shaped dose-response relationship between reactive oxygen species levels and the rate of aging in Caenorhabditis elegans. Aging Cell. 2017;16:104–112. doi: 10.1111/acel.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozden O, Park SH, Kim HS, Jiang H, Coleman MC, Spitz DR, Gius D. Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging (Albany NY) 2011;3:102–107. doi: 10.18632/aging.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jeffery J, Jörnvall H. Sorbitol dehydrogenase. Adv Enzymol. 1988;61:47–106. doi: 10.1002/9780470123072.ch2. [DOI] [PubMed] [Google Scholar]
- 36.Swindell WR. Metallothionein and the biology of aging. Ageing Res Rev. 2011;10:132–145. doi: 10.1016/j.arr.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su W, Wang Y, Jia X, Wu W, Li L, Tian X, Li S, Wang C, Xu H, Cao J, Han Q, Xu S, Chen Y, et al. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc Natl Acad Sci USA. 2014;111:11437–11442. doi: 10.1073/pnas.1410741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SJ, Hwang AB, Kenyon C. Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Curr Biol. 2010;20:2131–2136. doi: 10.1016/j.cub.2010.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh SP, Niemczyk M, Zimniak L, Zimniak P. Fat accumulation in Caenorhabditis elegans triggered by the electrophilic lipid peroxidation product 4-hydroxynonenal (4-HNE) Aging (Albany NY) 2008;1:68–80. doi: 10.18632/aging.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hahm JH, Kim S, DiLoreto R, Shi C, Lee SJ, Murphy CT, Nam HG. C. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat Commun. 2015;6:8919. doi: 10.1038/ncomms9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olsen A, Vantipalli MC, Lithgow GJ. Checkpoint proteins control survival of the postmitotic cells in Caenorhabditis elegans. Science. 2006;312:1381–1385. doi: 10.1126/science.1124981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


