Abstract
BACKGROUND
The majority of patients in need of a hematopoietic-cell transplant do not have a matched related donor. Data are needed to inform the choice among various alternative donor-cell sources.
METHODS
In this retrospective analysis, we compared outcomes in 582 consecutive patients with acute leukemia or the myelodysplastic syndrome who received a first myeloablative hematopoietic-cell transplant from an unrelated cord-blood donor (140 patients), an HLA-matched unrelated donor (344), or an HLA-mismatched unrelated donor (98).
RESULTS
The relative risks of death and relapse between the cord-blood group and the two other unrelated-donor groups appeared to vary according to the presence of minimal residual disease status before transplantation. Among patients with minimal residual disease, the risk of death was higher in the HLA-mismatched group than in the cord-blood group (hazard ratio, 2.92; 95% confidence interval [CI], 1.52 to 5.63; P = 0.001); the risk was also higher in the HLA-matched group than in the cord-blood group but not significantly so (hazard ratio, 1.69; 95% CI, 0.94 to 3.02; P = 0.08). Among patients without minimal residual disease, the hazard ratios were lower (hazard ratio in the HLA-mismatched group, 1.36; 95% CI, 0.76 to 2.46; P = 0.30; hazard ratio in the HLA-matched group, 0.78; 95% CI, 0.48 to 1.28; P = 0.33). The risk of relapse among patients with minimal residual disease was significantly higher in the two unrelated-donor groups than in the cord-blood group (hazard ratio in the HLA-mismatched group, 3.01; 95% CI, 1.22 to 7.38; P = 0.02; hazard ratio in the HLA-matched group, 2.92; 95% CI, 1.34 to 6.35; P = 0.007). Among patients without minimal residual disease, the magnitude of these associations was lower (hazard ratio in the HLA-mismatched group, 1.28; 95% CI, 0.51 to 3.25; P = 0.60; hazard ratio in the HLA-matched group, 1.30; 95% CI, 0.65 to 2.58; P = 0.46).
CONCLUSIONS
Our data suggest that among patients with pretransplantation minimal residual disease, the probability of overall survival after receipt of a transplant from a cord-blood donor was at least as favorable as that after receipt of a transplant from an HLA-matched unrelated donor and was significantly higher than the probability after receipt of a transplant from an HLA-mismatched unrelated donor. Furthermore, the probability of relapse was lower in the cord-blood group than in either of the other groups.
The preferred donor for patients who are in need of an allogeneic hematopoietic-cell transplant remains an HLA-identical sibling. Such a donor is not available for the majority (approximately 70%) of patients, and alternative donor sources are necessary.1 At the Fred Hutchinson Cancer Research Center, the first alternative choice for patients who do not have an HLA-identical sibling has been an unrelated donor who has been matched with the patient at the allele level for HLA-A, HLA-B, HLA-C, HLA-DRB1, and HLA-DQB1 (a so-called 10/10 match, or an HLA-matched unrelated donor). However, approximately 50% of white patients who do not have an HLA-identical sibling will also not find an HLA-matched unrelated donor, and the percentage is higher in some nonwhite racial and ethnic groups; thus there is a need for alternative donor choices.1
One such option is an unrelated donor who is mismatched for a single allele at HLA-A, HLA-B, HLA-C, HLA-DRB1, or HLA-DQB1 (a so-called 9/10 unrelated donor, or an HLA-mismatched unrelated donor). Another option is an unrelated umbilical cord–blood donor. Cord blood can be used with greater allowance for HLA disparity, and thus sources of cord-blood transplants are available for nearly all patients. Advances in cord-blood transplantation, including the increased availability of cord-blood units with a high cellular content, the use of double cord-blood grafts to increase the cell dose, and numerous ex vivo expansion methods, have further increased the potential application of this donor source.2–7
These advances make the use of cord blood from unrelated donors a possible alternative stem-cell source when an HLA-identical sibling or a 10/10 unrelated donor is not available. In this retrospective study, we compared outcomes after receipt of a transplant from a cord-blood donor with outcomes after receipt of a transplant from an HLA-matched or HLA-mismatched unrelated donor in patients with acute leukemia or the myelodysplastic syndrome who underwent a first myeloablative allogeneic transplantation at our institution. We sought not only to compare outcomes among the groups but also to determine whether the magnitude of the difference between the donor sources in the risk of treatment failure with respect to various outcomes varied according to the presence or absence of minimal residual disease before transplantation.
METHODS
PATIENTS
We included in this series all the patients with acute myeloid or lymphoid leukemia or the myelodysplastic syndrome who received a first allogeneic hematopoietic-cell transplant from an unrelated donor at our center between January 2006 and December 2014 (582 patients), with the source of stem cells being cord blood, bone marrow, or peripheral blood. All cord-blood grafts were matched for four, five, or six loci at HLA-A and HLA-B (at the antigen level) and at the allele level for HLA-DRB1. In the unrelated-donor groups, which included bone marrow and peripheral-blood stem-cell sources from adult volunteer donors, patients were matched with the donor at the allele level for HLA-A, HLA-B, HLA-C, HLA-DQB1, and HLA-DRB1 (HLA-matched group) or were mismatched for a single allele (HLA-mismatched group). In the HLA-matched and HLA-mismatched groups, the stem-cell source was determined largely according to treatment protocol and in some cases according to donor or patient preference. HLA-matched unrelated donors were generally selected as the primary source if patients did not have an HLA-matched related donor. For patients without an HLA-matched unrelated donor, the choice of a cord-blood graft or an HLA-mismatched unrelated-donor graft was determined according to an algorithm that was based on clinical protocols that were available at the time of enrollment and on the priority of such protocols (at our institution). Because the choice was not randomized, the possibility of bias cannot be excluded, but bias was not systematic.
Ten-color multiparameter flow cytometry was performed on bone marrow aspirates that were obtained from all the patients as a routine baseline assessment before transplantation in order to assess disease status and the presence or absence of minimal residual disease. Assessment of acute myeloid leukemia and acute lymphoid leukemia by means of flow cytometry was identical, although a different immunophenotypic panel was used.8 Any level of residual disease was considered to indicate positivity for minimal residual disease.9
STUDY DESIGN AND OVERSIGHT
The first and last authors designed the study, made the decision to submit the manuscript for publication, and vouch for the completeness and accuracy of the data and the study analysis. All the patients provided written informed consent in accordance with the provisions of the Declaration of Helsinki. Separate approval was obtained from the institutional review board at the Fred Hutchinson Cancer Research Center for patients’ records to be retrospectively reviewed for this analysis, and all the patients who were included in the analysis provided written informed consent for the use of their medical records for research.
STATISTICAL ANALYSIS
The demographic characteristics of the patients were compared with the use of the chi-square test (categorical variables) or analysis of variance (continuous variables). Unadjusted estimates of the probability of overall survival were computed with the use of the Kaplan–Meier method.10 Unadjusted probabilities of relapse, death without relapse, and acute and chronic graft-versus-host disease were summarized with the use of cumulative-incidence estimates.11 Adjusted estimates that were based on results from regression models were obtained by assuming that the patients in the unrelated-donor groups had the same demographic characteristics, on average, as those in the cord-blood group.12 The cause-specific hazards of treatment failure for each end point were compared between the cord-blood group and each of the unrelated-donor groups with the use of Cox regression. These models were adjusted for various factors from among the following: age of the patient, severity of disease, year of transplantation, presence or absence of minimal residual disease, and use or nonuse of high-dose total-body irradiation as part of the conditioning regimen. We tested the assumption of proportional hazards by using cumulative sums of Martingale residual plots and Kolmogorov-type supremum tests (based on 1000 simulations).
The results from a randomized trial examining bone marrow versus peripheral-blood mobilized stem cells in the context of transplantation from an unrelated donor showed similar outcomes in the two groups for all end points examined, with the exception of chronic graft-versus-host disease and engraftment.13 Therefore, the source of stem cells in the HLA-matched group and the HLA-mismatched group in our study was not considered for any end point other than chronic graft-versus-host disease, in which the HLA-matched group and the HLA-mismatched group were each separated into bone marrow and peripheral-blood subgroups. We assessed interactions between donor source and outcome by fitting appropriate factors in Cox regression models. Because the power to detect a significant interaction (at the 0.05 level of significance) is much lower than the power to detect a main effect, we used a more liberal threshold of significance to determine which interactions were worthy of being included in our models. Interactions tests that yielded a P value of less than 0.15 were considered to be important enough to warrant inclusion in a regression model.
RESULTS
PATIENTS
Among the 582 patients included in this retrospective analysis, 344 received a transplant from an HLA-matched unrelated donor (107 patients received a bone marrow transplant, and 237 a peripheral-blood transplant), 98 received a transplant from an HLA-mismatched unrelated donor (28 received a bone marrow transplant, and 70 a peripheral-blood transplant), and 140 received a transplant from a cord-blood donor (Table 1). All the patients in the cord-blood group received a double cord-blood graft except for 16 (11%), who received a single donor graft. In addition, 39 patients (28%) received an ex vivo expanded cord-blood unit as part of their graft, combined with an unmanipulated cord-blood unit.
Table 1.
Demographic and Clinical Characteristics of the Patients.*
| Characteristic | Cord-Blood Group (N = 140) | HLA-Matched Group (N = 344) | HLA-Mismatched Group (N = 98) |
|---|---|---|---|
| Age — yr† | |||
| Median | 29 | 40 | 45 |
| Range | 1–64 | 1–67 | 2–64 |
| Weight — kg† | |||
| Median | 70 | 76 | 77 |
| Range | 9–112 | 13–173 | 12–142 |
| Female sex — no. (%) | 68 (49) | 150 (44) | 45 (46) |
| Race — no. (%)†‡ | |||
| White | 64 (46) | 294 (85) | 76 (78) |
| Other | 76 (54) | 50 (15) | 22 (22) |
| Positive serostatus for cytomegalovirus — no. (%) | 86 (61) | 178 (52) | 47 (48) |
| Diagnosis — no. (%) | |||
| Acute myeloid leukemia | 73 (52) | 175 (51) | 52 (53) |
| Acute lymphoid leukemia | 51 (36) | 106 (31) | 28 (29) |
| Myelodysplastic syndrome | 16 (11) | 63 (18) | 18 (18) |
| Disease risk — no. (%)§ | |||
| Low or intermediate | 93 (66) | 276 (80) | 77 (79) |
| High or very high | 47 (34) | 68 (20) | 21 (21) |
| Conditioning regimen — no. (%)† | |||
| Fludarabine, cyclophosphamide, and total-body irradiation at a dose of 1320 cGy | 97 (69) | 0 | 0 |
| Treosulfan, fludarabine, and total-body irradiation at a dose of 200 cGy | 43 (31) | 64 (19) | 7 (7) |
| Busulfan with either cyclophosphamide or fludarabine | 0 | 127 (37) | 54 (55) |
| Cyclophosphamide and total body irradiation at a dose of 1200 or 1320 cGy | 0 | 153 (44) | 37 (38) |
| GVHD prophylaxis — no. (%)† | |||
| Cyclosporine and mycophenolate mofetil | 140 (100) | — | — |
| Tacrolimus and methotrexate | — | 268 (78) | 98 (100) |
| Other | — | 76 (22) | — |
| Presence of minimal residual disease — no./total no. (%) | 45/137 (33) | 104/331 (31) | 35/90 (39) |
The chi-square test was used for categorical variables, and analysis of variance for continuous variables. GVHD denotes graft-versus-host disease.
P<0.001 for the difference in the distribution of characteristics among the three groups.
Race was self-reported.
Disease risk was categorized as low, intermediate, high, or very high, as previously described.14 P<0.01 for the difference in the distribution of characteristics among the three groups.
Patients who received transplants from cord-blood donors were, on average, younger than the patients in the other two groups, were more likely to be nonwhite (76 of 140 [54%]), and were more likely to be seropositive for cytomegalovirus (86 of 140 [61%]). The proportions of patients with acute leukemia were similar across the three groups, but a higher proportion of patients in the cord-blood group than in either unrelated-donor group had high-risk or very-high-risk disease. 14 The percentage of patients with minimal residual disease at the time of transplantation was similar in all groups: 45 of 137 patients (33%) in the cord-blood group, 104 of 331 (31%) in the HLA-matched group, and 35 of 90 (39%) in the HLA-mismatched group. Data on residual-disease status were missing for 24 patients.
Patients who received total-body irradiation at a dose of 1200 or 1320 cGy as part of their conditioning regimen, which also included fludarabine and cyclophosphamide, were combined into a high-dose total-body irradiation subgroup. There was a correlation between age and conditioning regimen. The median age of the patients who received high-dose total body irradiation was 23.8 years in the HLA-matched group, 31.9 years in the HLA-mismatched group, and 21.2 years in the cord-blood group. The median ages of the patients who received regimens that did not contain high-dose total body irradiation were 49.7 years, 51.0 years, and 50.4 years, respectively.
OVERALL MORTALITY
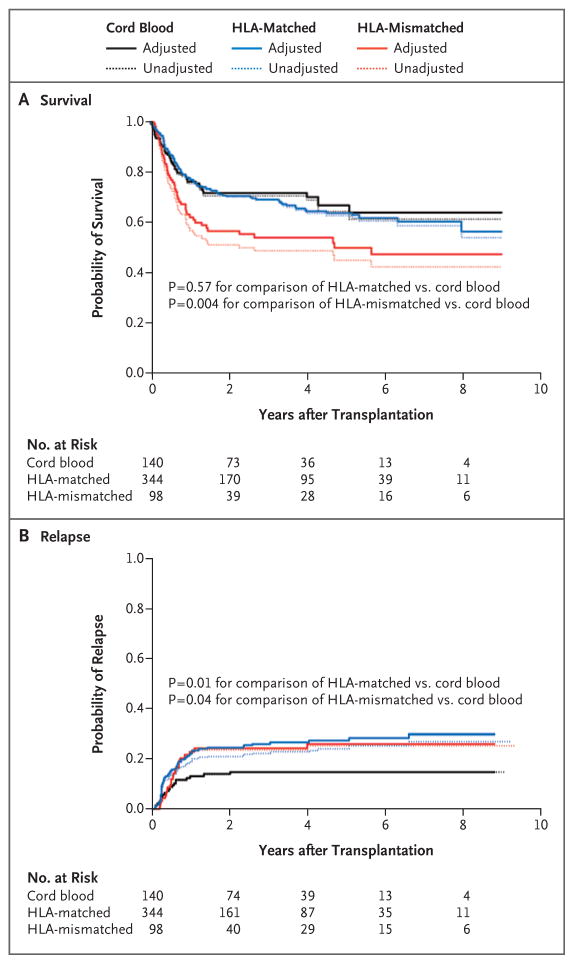
There were 44 deaths in the cord-blood group, 116 in the HLA-matched group, and 52 in the HLA-mismatched group. The unadjusted and adjusted estimates of the probability of survival in the three groups are shown in Figure 1A. At 4 years, the unadjusted estimate of the rate of survival was 71% (95% confidence interval [CI], 62 to 77) in the cord-blood group, 63% (95% CI, 57 to 68) in the HLA-matched group, and 49% (95% CI, 38 to 58) in the HLA-mismatched group.
Figure 1. Unadjusted and Adjusted Estimates of Overall Survival and Relapse.
Adjusted estimates are to be interpreted as the expected outcome if the HLA-matched and HLA-mismatched groups were the same, on average, as the cord-blood group with respect to disease severity, age of the patient, year of transplantation, and presence or absence of minimal residual disease. The hazard ratio for death in the HLA-matched group versus the cord-blood group was 1.12 (95% CI, 0.77 to 1.63; P = 0.57), and the hazard ratio in the HLA-mismatched group versus the cord-blood group was 1.91 (95% CI, 1.23 to 2.98; P = 0.004). The hazard ratio for relapse in the HLA-matched group versus the cord-blood group was 1.95 (95% CI, 1.16 to 3.27; P = 0.01), and the hazard ratio in the HLA-mismatched group versus the cord-blood group was 1.97 (95% CI, 1.04 to 3.73; P = 0.04).
The adjusted risk of death was higher in the HLA-mismatched group than in the cord-blood group (hazard ratio, 1.91; 95% CI, 1.23 to 2.98; P = 0.004) and was similar in the HLA-matched group and the cord-blood group (hazard ratio, 1.12; 95% CI, 0.77 to 1.63; P = 0.57). However, the results of a test for interaction between donor source and minimal residual disease status suggested a trend toward significance in the comparison of the difference between the HLA-mismatched group and the cord-blood group according to minimal residual disease status (P = 0.08 for interaction), whereas the difference between the HLA-matched group and the cord-blood group varied significantly according to minimal residual disease status (P = 0.04 for interaction).
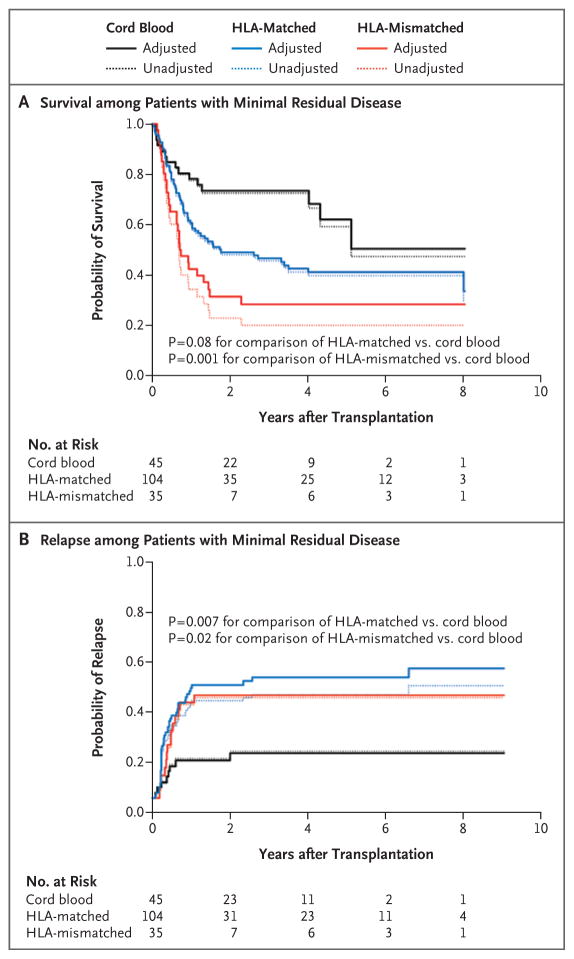
Table 2 summarizes the results of a regression model for overall mortality according to the presence or absence of minimal residual disease. Among patients with minimal residual disease, the cord-blood group had a significantly higher probability of survival than the HLA-mismatched group and had a probability of survival that was at least as good as that in the HLA-matched group (Table 2 and Fig. 2A). However, the risk of death in the HLA-mismatched group, as compared with the cord-blood group, that was seen among patients with minimal residual disease (hazard ratio, 2.92) was higher than the one observed among patients without minimal residual disease (hazard ratio, 1.36). Survival in the HLA-matched group was at least as good as that in the cord-blood group in the absence of minimal residual disease (Table 2). There was no suggestion that the relative differences in mortality between the cord-blood group and either unrelated-donor group varied according to the use or nonuse of high-dose total-body irradiation as part of the conditioning regimen (P = 0.90 for interaction in the HLA-matched group vs. the cord-blood group; P = 0.74 for interaction in the HLA-mismatched group vs. the cord-blood group). There was no evidence against the assumption of proportional hazards in the comparison of the cord-blood group with either unrelated-donor group (see the Supplementary Appendix, available with the full text of this article at NEJM.org).
Table 2.
Adjusted Cox Regression Models for Analyses of Death and Relapse, According to Minimal Residual Disease Status.*
| Outcome | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Death | ||
|
| ||
| Patients without minimal residual disease | ||
| Cord-blood group | 1.00 | — |
| HLA-matched group | 0.78 (0.48–1.28) | 0.33 |
| HLA-mismatched group | 1.36 (0.76–2.46) | 0.30 |
|
| ||
| Patients with minimal residual disease | ||
| Cord-blood group | 1.00 | — |
| HLA-matched group | 1.69 (0.94–3.02) | 0.08 |
| HLA-mismatched group | 2.92 (1.52–5.63) | 0.001 |
|
| ||
| Relapse | ||
|
| ||
| Patients without minimal residual disease | ||
| Cord-blood group | 1.00 | — |
| HLA-matched group | 1.30 (0.65–2.58) | 0.46 |
| HLA-mismatched group | 1.28 (0.51–3.25) | 0.60 |
|
| ||
| Patients with minimal residual disease | ||
| Cord-blood group | 1.00 | |
| HLA-matched group | 2.92 (1.34–6.35) | 0.007 |
| HLA-mismatched group | 3.01 (1.22–7.38) | 0.02 |
The analyses were adjusted for age (as a cubic polynomial), severity of disease, year of transplantation (as a continuous linear variable), use or nonuse of high-dose total-body irradiation, and presence or absence of minimal residual disease (in cases in which the interaction with donor group was not deemed to be important). Data on minimal residual disease status were missing for 3 patients in the cord-blood group, for 13 in the HLA-matched group, and for 8 in the HLA-mismatched group.
Figure 2. Unadjusted and Adjusted Estimates of Overall Survival and Relapse among Patients with Minimal Residual Disease.
Adjusted estimates are to be interpreted as the expected outcome if the HLA-matched and HLA-mismatched groups were the same, on average, as the cord-blood group with respect to disease severity, age of the patient, and year of transplantation. The hazard ratio for death in the HLA-matched group versus the cord-blood group was 1.69 (95% CI, 0.94 to 3.02; P = 0.08), and the hazard ratio in the HLA-mismatched group versus the cord-blood group was 2.92 (95% CI, 1.52 to 5.63; P = 0.001). The hazard ratio for relapse in the HLA-matched group versus the cord-blood group was 2.92 (95% CI, 1.34 to 6.35; P = 0.007), and the hazard ratio in the HLA-mismatched group versus the cord-blood group was 3.01 (95% CI, 1.22 to 7.38; P = 0.02). Data on minimal residual disease status were missing for 3 patients in the cord-blood group, for 13 in the HLA-matched group, and for 8 in the HLA-mismatched group.
RELAPSE
There were 20 events of relapse in the cord-blood group, 79 in the HLA-matched group, and 24 in the HLA-mismatched group. Figure 1B summarizes the estimated probabilities of relapse in the three groups in both the unadjusted and adjusted analyses. The 4-year unadjusted estimate of the risk of relapse was 15% (95% CI, 9 to 21) in the cord-blood group, 24% (95% CI, 19 to 29) in the HLA-matched group, and 25% (95% CI, 16 to 34) in the HLA-mismatched group.
The adjusted risk of relapse was significantly higher in each unrelated-donor group than in the cord-blood group (hazard ratio in the HLA-matched group, 1.95; 95% CI, 1.16 to 3.27; P = 0.01; hazard ratio in the HLA-mismatched group, 1.97; 95% CI, 1.04 to 3.73; P = 0.04). However, as was the case with the risk of death, the relative risk of relapse between the HLA-matched group and the cord-blood group was weakly but not significantly affected by the presence of minimal residual disease (P = 0.12 for interaction). An interaction test in the comparison of the HLA-mismatched group with the cord-blood group did not meet our P-value threshold of 0.15 (P = 0.19 for interaction), but for completeness we present the results for relapse according to minimal residual disease status (Table 2).
Among patients with minimal residual disease, the risk of relapse after transplantation was significantly lower in the cord-blood group than in either unrelated-donor group (Fig. 2B). However, the risks of relapse in the two other unrelated-donor groups, as compared with the cord-blood group, that were seen among patients with minimal residual disease (hazard ratio in the HLA-matched group, 2.92; hazard ratio in the HLA-mismatched group, 3.01) were higher than the ones seen among patients without minimal residual disease (hazard ratios, 1.30 and 1.28, respectively) (Table 2). Similar to the differences seen with mortality, the relative differences in the rate of relapse between the cord-blood group and the two other unrelated-donor groups did not vary according to the use or nonuse of high-dose total-body irradiation (P = 0.82 for interaction in the HLA-matched group vs. the cord-blood group; P = 0.51 for interaction in the HLA-mismatched group vs. the cord-blood group). There was no evidence against the assumption of proportional hazards in the comparison of the cord-blood group with either unrelated-donor group (see the Supplementary Appendix).
MORTALITY WITHOUT RELAPSE
There were 29 deaths without relapse in the cord-blood group, 56 in the HLA-matched group, and 29 in the HLA-mismatched group. The probabilities of death without relapse in the three groups in the unadjusted and adjusted analyses are summarized in Figure S2 in the Supplementary Appendix. The 4-year unadjusted estimate of mortality without relapse was 18% (95% CI, 12 to 25) in the cord-blood group, 17% (95% CI, 13 to 21) in the HLA-matched group, and 28% (95% CI, 19 to 37) in the HLA-mismatched group. There was no significant difference in mortality without relapse between the cord-blood group and either unrelated-donor group (Table S3 in the Supplementary Appendix).
GRAFT-VERSUS-HOST DISEASE
The estimated probability of grade III or IV acute graft-versus-host disease15 was 18% (25 of 136 patients) in the cord-blood group, 14% (49 of 342) in the HLA-matched group, and 26% (25 of 98) in the HLA-mismatched group. The risk of grade III or IV acute graft-versus-host disease was numerically higher in the HLA-mismatched group than in the cord-blood group and lower in the HLA-matched group than in the cord-blood group, but neither difference was significant. The risk of chronic graft-versus-host disease16 did not differ significantly between the cord-blood group and any unrelated-donor group with the exception of the HLA-matched group of bone marrow donors, but the hazard ratios in the two bone marrow groups of unrelated donors (as compared with the cord-blood group) were similar, as were the hazard ratios in the two peripheral-blood groups of unrelated donors. (Details are provided in Table S3 in the Supplementary Appendix.)
EFFECT OF MINIMAL RESIDUAL DISEASE ON MORTALITY AND RATE OF RELAPSE
The effect of minimal residual disease on outcome (for overall mortality and rate of relapse) varied, depending on the donor group examined. Table 3 summarizes the estimated effect of minimal residual disease (presence vs. absence) according to donor (from the same models for overall mortality and rate of relapse as shown above). Patients in the HLA-matched and HLA-mismatched unrelated-donor groups who had minimal residual disease had higher risks of death and relapse than those without minimal residual disease. However, no such association with mortality was seen in the cord-blood group; in this group, the risk of death was similar among patients with minimal residual disease and those without minimal residual disease (Table 3).
Table 3.
Effect of Minimal Residual Disease on Mortality and Risk of Relapse, According to Donor Group.*
| Donor Group | Overall Mortality | Risk of Relapse | ||
|---|---|---|---|---|
|
|
|
|||
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| HLA-matched group | 2.34 (1.59–3.45) | <0.001 | 3.23 (2.01–5.19) | <0.001 |
|
| ||||
| HLA-mismatched group | 2.33 (1.32–4.09) | 0.003 | 3.37 (1.39–8.15) | 0.007 |
|
| ||||
| Cord-blood group | 1.09 (0.57–2.08) | 0.80 | 1.43 (0.58–3.57) | 0.44 |
The hazard ratio represents the adjusted risk of treatment failure among patients with minimal residual disease as compared with those without minimal residual disease. The analyses were adjusted for age (as a cubic polynomial), severity of disease, year of transplantation (as a continuous linear variable), and use or nonuse of high-dose total-body irradiation. Data on minimal residual disease status were missing for 3 patients in the cord-blood group, for 13 in the HLA-matched group, and for 8 in the HLA-mismatched group.
DISCUSSION
Previous studies17–21 have compared outcomes regarding transplants from cord-blood donors with those from other donor sources. Because of the previously reported profound effect of the presence of minimal disease before transplantation on outcomes in the context of non–cord-blood transplantation, this variable was examined closely. As with data in all retrospective analyses, our data may be subject to bias because which patients received which treatment was the result of nonrandomized selection (e.g., clinical priority). With this caveat, the survival rate after receipt of a transplant from a cord-blood donor appeared to be higher than that after receipt of a transplant from an HLA-mismatched unrelated donor, largely owing to lower relapse rates and higher rates of survival among patients with minimal residual disease who received a transplant. Among patients without minimal residual disease, there was no evidence that the risk of relapse was greater in the cord-blood group than in either unrelated-donor group, nor was there any evidence of a higher risk of death in the cord-blood group than in the HLA-mismatched group. The risk of death among patients without minimal residual disease was lower in the HLA-matched group than in the cord-blood group, but the difference was not significant.
The finding of a significant P value for the interaction between minimal residual disease status and donor group indicates that the relative difference in outcome between patients with minimal residual disease and those without minimal residual disease varied according to donor group. In the HLA-matched and HLA-mismatched unrelated-donor groups, the presence of minimal residual disease before transplantation was associated with a higher risk of death and disease relapse after transplantation than was the absence of minimal residual disease, a finding similar to results described in previous reports. 9,22 However, in contrast to these previous reports, in the cord-blood group, the overall mortality and rate of relapse were similar among patients with minimal residual disease and those without minimal residual disease. These observations are not entirely consistent with previous reports regarding transplants from cord-blood donors,23–25 in which a higher risk of relapse and a lower rate of leukemia-free survival was seen among patients with minimal residual disease than among those without minimal residual disease.
Our analysis was conducted with the use of retrospective data and is therefore subject to the attendant limitations. Although regression modeling was performed as a means of controlling for differences in the characteristics of the patients and their disease, such adjustment cannot account for all discrepancies in demographic and clinical characteristics between groups. A randomized trial is the only way to assess definitively the differences between cord-blood and unrelated-donor transplantation. Given the lack of such a trial, however, studies such as this are one means of providing guidance regarding difficult donor choices in situations in which an HLA-identical sibling is not available. This situation is particularly important for patients from nonwhite racial and ethnic minority groups, because it can be difficult to find an HLA-matched unrelated donor for such patients. Indeed, in our study, cord-blood donors were used more often than unrelated donors for nonwhite patients. Because cord blood can allow for greater HLA disparity, it is possible to find suitable donors for nearly all patients, regardless of their racial or ethnic group.1 Although the magnitude of effects was reasonably large in patients with minimal residual disease, particularly with regard to the comparison of transplants from HLA-mismatched unrelated donors with those from cord-blood donors, the numbers of patients in these groups were not large, and this factor, along with the number of comparisons that were made (without statistical adjustment) as well as the retrospective nature of this study, requires that our conclusions be made both with caution and with a call for further examination in the future.
In conclusion, our results showed that in patients with minimal residual disease, the use of cord blood as the donor source for hematopoietic-cell transplantation led to a higher rate of survival and a lower rate of relapse than the use of a transplant from an HLA-mismatched unrelated donor. Our data also showed that the risk of relapse was higher after receipt of a transplant from an HLA-matched unrelated donor than after receipt of a transplant from a cord-blood donor, and there was no evidence that among patients with minimal residual disease, the rate of survival was higher after receipt of a transplant from a matched unrelated donor than after receipt of a transplant from a cord-blood donor. In some cases, the time to transplantation can also be a critical determinant in the success of the entire transplantation process, and the ability to identify and procure a cord-blood donor rapidly can allow for a shorter time to transplantation.
Supplementary Material
Acknowledgments
We thank the patients and families who consented to the use of clinical research results; and Adrienne Papermaster, Heather Brammer, Denise Ziegler, MaryJoy Lopez, Ivy Riffkin, and Nancy Anderson for assistance in the preparation of an earlier version of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–48. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–22. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 3.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–75. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 4.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–85. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 5.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–66. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 6.Delaney C, Heimfeld S, Brashem-Stein C, Voorhies H, Manger RL, Bernstein ID. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–6. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–15. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood BL. Flow cytometric monitoring of residual disease in acute leukemia. Methods Mol Biol. 2013;999:123–36. doi: 10.1007/978-1-62703-357-2_8. [DOI] [PubMed] [Google Scholar]
- 9.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122:1813–21. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 11.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Storer BE, Gooley TA, Jones MP. Adjusted estimates for time-to-event end-points. Lifetime Data Anal. 2008;14:484–95. doi: 10.1007/s10985-008-9098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487–96. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 16.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. I. Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–54. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 18.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atsuta Y, Morishima Y, Suzuki R, et al. Comparison of unrelated cord blood transplantation and HLA-mismatched unrelated bone marrow transplantation for adults with leukemia. Biol Blood Marrow Transplant. 2012;18:780–7. doi: 10.1016/j.bbmt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631–8. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 21.Marks DI, Woo KA, Zhong X, et al. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2014;99:322–8. doi: 10.3324/haematol.2013.094193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar M, Wood BL, Radich JP, et al. Impact of minimal residual disease, detected by flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute lymphoblastic leukemia. Leuk Res Treatment. 2014;2014:421723. doi: 10.1155/2014/421723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachanova V, Burke MJ, Yohe S, et al. Unrelated cord blood transplantation in adult and pediatric acute lymphoblastic leukemia: effect of minimal residual disease on relapse and survival. Biol Blood Marrow Transplant. 2012;18:963–8. doi: 10.1016/j.bbmt.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruggeri A, Michel G, Dalle JH, et al. Impact of pretransplant minimal residual disease after cord blood transplantation for childhood acute lymphoblastic leukemia in remission: an Eurocord, PDWP-EBMT analysis. Leukemia. 2012;26:2455–61. doi: 10.1038/leu.2012.123. [DOI] [PubMed] [Google Scholar]
- 25.Tucunduva L, Ruggeri A, Sanz G, et al. Impact of minimal residual disease on outcomes after umbilical cord blood transplantation for adults with Philadelphia-positive acute lymphoblastic leukaemia: an analysis on behalf of Eurocord, Cord Blood Committee and the Acute Leukaemia working party of the European group for Blood and Marrow Transplantation. Br J Haematol. 2014;166:749–57. doi: 10.1111/bjh.12970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.