Abstract
Mood disorders such as major depressive disorder (MDD) affect a significant proportion of the population. Although progress has been made in the development of therapeutics, a large number of individuals do not attain full remission of symptoms and adverse side effects affect treatment compliance for some. In order to develop new therapies, there is a push for new models that better reflect the multiple risk factors that likely contribute to the development of depressive illness. We hypothesized that early life stress would exacerbate the depressive-like phenotype that we have previously observed in socially subordinate (SUB), adult male rats in the visible burrow system (VBS), a semi-natural, ethologically relevant environment in which males in a colony form a dominance hierarchy. Dams were exposed to chronic variable stress (CVS) during the last week of gestation, resulting in a robust and non-habituating glucocorticoid response that did not alter maternal food intake, body weight or litter size and weight. As adults, one prenatal CVS (PCVS) and one non-stressed (NS) male were housed in the VBS with adult females. Although there were no overt differences between PCVS and NS male offspring prior to VBS housing, a greater percentage of PCVS males became SUB. However, the depressive-like phenotype of SUB males was not exacerbated in PCVS males; rather, they appeared to better cope with SUB status than NS SUB males. They had lower basal plasma corticosterone than NS SUB males at the end of VBS housing. In situ hybridization for CRH in the PVN and CeA did not reveal any prenatal treatment or status effects, while NPY expression was higher within the MeA of dominant and subordinate males exposed to the VBS in comparison with controls, but with no effect of prenatal treatment. These data suggest that prenatal chronic variable stress may confer resilience to offspring when exposed to social stress in adulthood.
1. Introduction
Mood disorders affect a significant segment of society, reducing the quality of life and placing a significant burden on the workforce, economy and health care systems (Valladares et al. 2008, Kessler 2012, Lam et al. 2016). The etiology of affective disorders are largely unknown, and characterized by symptoms that can vary greatly by individual. Altered serotonergic transmission and hypothalamic-pituitary-adrenal (HPA) axis hyperactivity are the most widely accepted hallmarks of Major Depressive Disorder (MDD), but these findings are far from universal (Lopez et al. 1998, Nestler et al. 2002, McEwen 2005, de Kloet et al. 2007). The majority of available pharmacological therapies target the serotonergic system, and while these help many, it is estimated that up to one third of individuals fail to achieve full remission of symptoms (Mathys and Mitchell 2011, Al-Harbi 2012, Ionescu et al. 2015, O’Leary et al. 2015). It is clear that much more research is required to tackle this problem, and some investigators have turned their focus to more nuanced models of depression in laboratory animals. Since preexisting risk factors are thought to underlie stress-sensitivity and the susceptibility to mood disorders, some question the validity of previously utilized paradigms that often used naive, or “normal,” rodents to model aspects of human illness and have proposed that more “sophisticated” models are necessary to tease out the mechanistic underpinnings of depressive disorders (Cryan and Holmes 2005, Blanchard et al. 2013, Stewart and Kalueff 2015, Czeh et al. 2016). The general consensus in the field is that there is no single cause of affective disorders, and no “silver bullet” for treatment. One’s susceptibility likely arises through the interaction of multiple genetic and/or environmental factors, and chronic stress exposure may act as a particularly robust trigger. Indeed, certain stressful experiences are thought to engender a vulnerability to depressive illness and stress is frequently reported to precede or exacerbate depressive episodes (Kendler et al. 1999, Hammen 2005, Roca et al. 2013).
In social animals (including humans), some of the most common and salient stressors encountered are social in nature (Bjorkqvist 2001, Tamashiro et al. 2005, Huhman 2006). In their natural environment, rats form “societies” and social interactions can be a significant source of stress, which can be utilized to study chronic social stress (Barnett 1958, Barnett 1963, Koolhaas et al. 1997, Tamashiro et al. 2005). A seminatural social environment can be recreated in the laboratory using the visible burrow system (VBS), a housing system with a large open surface area connected to a series of tunnels and chambers. Male rats housed with females in the VBS quickly establish a dominance hierarchy, and socially subordinate males (SUBs) develop a behavioral, neurochemical and neuroendocrine profile that resembles some symptoms similar to MDD (Blanchard et al. 1993, Blanchard et al. 1995, Lucas et al. 2004). The “standard” VBS colony consists of 4 males and 2 females housed over a period of 2 weeks. Typically, the dominance hierarchy is established during the first day or two, with one male becoming and remaining dominant throughout the period of housing. Previous VBS studies have demonstrated that SUB males have enhanced HPA axis activity and that this corresponds with elevated CRH mRNA expression within the paraventricular nucleus of the hypothalamus (PVN) and the central nucleus of the amygdala (CeA). Occasionally, one subordinate male will be the main recipient of attacks in the burrow, and is referred to as the OMEGA; OMEGA males incur more wounds, more severe weight loss and exhibit depressive-like behaviours at the end of the 2 wk VBS housing period (Melhorn et al. 2017).
A female-biased VBS design can also be used, with 4 females and 2 males housed in the VBS. In our experience, the subordinate phenotype is further enhanced when two males are housed with four females, perhaps because there is only one male recipient of the dominant male’s aggression (Tamashiro et al. 2004). These males often resemble the OMEGA male of a traditional VBS, an experience increased wounding and more severe weight loss although depressive-like behaviours have not been assessed in these males.
Other environmental conditions also contribute to adult stress reactivity, and early life is a particularly sensitive period that can shape the health, coping style and physiological responses across the lifespan (de Kloet et al. 2005, Cottrell and Seckl 2009, Lupien et al. 2009, Rinaman et al. 2011, Entringer et al. 2015). To this end, we designed a study to assess whether early life stress would alter adult stress responses and behaviors within the VBS. Our hypothesis was that prenatal stress would increase the likelihood of becoming socially subordinate. We also assessed the combined and individual effects of prenatal stress and adult social stress, and hypothesized that the behavioral and physiological characteristics of SUB would be exaggerated by prenatal stress exposure. Finally, we hypothesized that changes in neurotransmission and CRH and NPY expression within limbic structures may mediate these effects.
2. Methods
2.1 Animals
All animal procedures were approved by the University of Cincinnati Institutional Animal Care and Use Committee and were performed in accordance with guidelines established by the NIH Guide for the Care and Use of Laboratory Animals. Timed pregnant female Long-Evans rats (Harlan, Indianapolis, IN) arrived at the University of Cincinnati animal facility on gestational day 2 (GD 2). The females were singly housed in conventional cages (18 × 24.5 × 18 cm) within a temperature- and humidity- controlled room with a 12h: 12h light: dark cycle (lights on from 0600 to1800 h), except when noted otherwise. All females were provided ad libitum access to chow (Harlan Teklad Diet 7012) and water. Body weight and food intake were recorded daily throughout gestation, and females were assigned to prenatal chronic variable stress (PCVS, n= 9 dams/litters) or no-stress (NS, n= 8 dams/litters) conditions at GD 14; groups were balanced according to maternal body weight and food intake.
2.2 Prenatal stress
The prenatal stress paradigm utilized chronic variable stress (PCVS), adapted from the model developed by Koenig et al. (Koenig et al. 2005, Lee et al. 2007). Dams were exposed to 2–3 unpredictable stressors per day during the last week of gestation (GD 14–21). Stressors included exposure to cold stress (4h at 4°C), forced swim (15 min in room temperature water), 8h of overcrowding with other pregnant females, overnight housing with wet bedding, 8h of housing in a mouse cage, one hour of cage agitation on an orbital shaker at 100 rpm, 60 min of acute restraint and one 24-h light period (Table 1). No-stress (NS) females were handled on a daily basis, but not exposed to the stressors of the PCVS paradigm. Prior to onset of the prenatal treatments on GD 14, a small sample of blood (less than 50 μl) was collected from the tail tip, to assess baseline plasma corticosterone (CORT) levels. All blood samples were taken in the morning between approximately 900 and 1100h. Following the final stressor on GD 21, PCVS and NS females had another small sample of blood was collected from the tip of the tail to determine plasma CORT levels. The blood samples were taken approximately 10 min after removal of PCVS females from the 15 min forced swim. Females were then returned to their home cages and were left undisturbed until litters were born.
Table 1.
Prenatal stress schedule (n= 8 NS, 9 PCVS). Prenatal stress was applied daily from gestation day 14 to 21.
| Day | Morning | Mid-day | Afternoon | |
|---|---|---|---|---|
| 14 | Restraint | Swim | Restraint | |
| 15 | Overcrowding | |||
| 16 | Swim | Restraint | ||
| 17 | Cold room | Overnight lights | ||
| 18 | Restraint | Shaker | Wet bedding | |
| 19 | Cold room | |||
| 20 | Swim | Mouse cage | ||
| 21 | Restraint | Swim | ||
Dams were checked at least 3 times per day (morning, midday, and just before lights out) for litters. Postnatal day (PND) 0 was defined as the day (during the light cycle) when a litter was first observed. On postnatal day 1 (PND 1), dams and litters were briefly removed from the home cage to determine litter size, weight and sex ratio. Litter size ranged from 7–15 pups. Large litters were culled to 12 pups. When possible, litters were culled in a way to maintain an equal number of males and female pups. A total of 6 litters (3 litters/prenatal treatment) were culled. Litters were reared according to standard UC facility procedures and left undisturbed except for weekly weighing and cage changes. Male pups were weaned on PND 21 or 22 and housed in same sex/treatment groups of 2 or 3. Groups were housed in conventional rat caging in a temperature- and humidity- controlled animal facility, with adlibitum access to standard chow and water. Pups were weighed on a weekly basis, and singly housed at 65 days of age.
2.3 Pre-VBS restraint stress test
At 86–87 days of age, male offspring were subjected to an acute restraint stress test. At approximately 1000 h, each male was quickly removed from its home cage and placed in a clear, ventilated Plexiglas tube, with a length and diameter of 21.5 cm × 6.3. Approximately 50 μl of blood was collected from the tip of the tail to assess basal (T0) plasma corticosterone (CORT). A second sample (50 μl) was taken 60 min into the restraint stress test (T60). After the T60 sample was collected, the rat was released from the restraining tube and returned to its home cage for a 60 min recovery period. Following this recovery period, a final 50 μl blood sample (T120) was quickly collected in order to assess CORT recovery from restraint. Blood was collected into sterile EDTA-treated tubes and placed on ice until they could be centrifuged; aliquots of plasma were stored at −20°C until the assay was performed.
2.4 Visible burrow system (VBS)
At approximately 100 d of age (99–107 d), males were assigned to the visible burrow system (VBS) or control (CON) housing. CON housing consisted of housing single males with a female in conventional caging within the animal facility as described previously, with ad libitum access to standard chow (Harlan Teklad 7012) and water.
The VBS system has been previously described in detail (Choi et al. 2006, Nguyen et al. 2007, Tamashiro et al. 2007, Smeltzer et al. 2012); the following is a brief description of the VBS apparatus. Each VBS apparatus consists of one large open surface area (SFC) and smaller chambers interconnected by tunnels (Fig.1). The SFC is maintained on a 12 h light cycle, illuminated by a 15-W incandescent light from 0600 h to 1800 h. The SFC was surrounded by 125 cm tall opaque walls to limit the amount of light leaving the area. The room was maintained on a 24-h dark cycle so that chambers and tunnels were constantly dark, simulating a semi-natural “burrow-like” environment that rats normally live in. The tunnels and tops of each chamber were made of clear Plexiglas and infrared lights were used to enable overhead video recording in the dark. A food hopper and water bottle were located in each chamber and the open surface area, to provide ad libitum access to chow (Harlan Teklad 7012) and water. One PCVS male and 1 NS male were housed in each VBS with 4 vendor-procured females (Harlan, Indianapolis, IN). Video cameras recorded 6 h of behavior per day, beginning at the onset of the dark cycle (1800 h). The first 15 min of each 6 h recording (90 min/day) were analyzed on D0, 2 and 11. Location within the burrow was recorded, as were the number of aggressive, defensive and reproductive behaviors. A photo of the unique pied coat pattern of each rat was taken prior to VBS housing in order to identify individual rats from video recordings, and an identifying number was marked on the tail with a non-toxic permanent marker to identify the animals during routine body condition assessments. IDs were arbitrarily assigned so that the investigator was blind to prenatal treatment.
Figure 1.
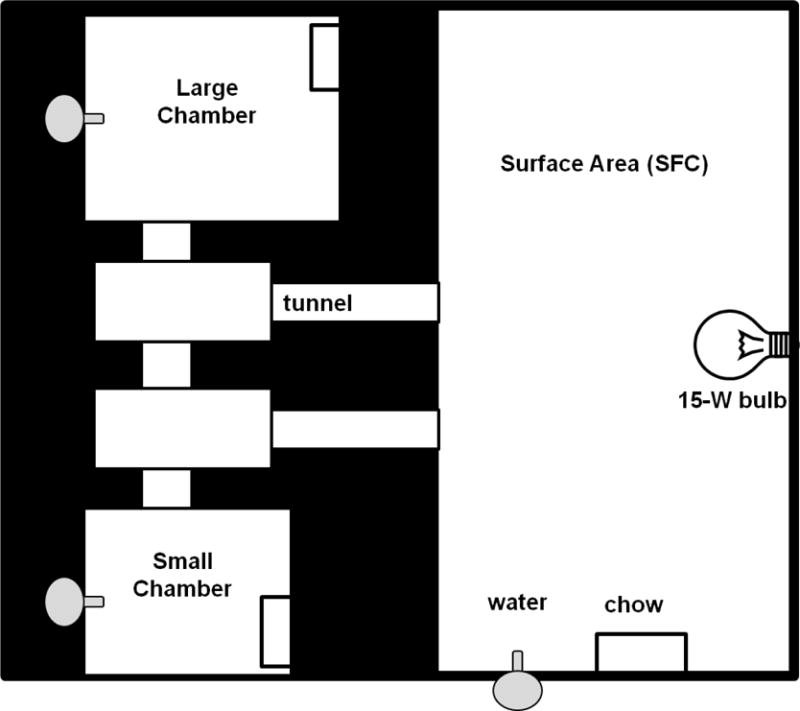
Schematic of the visible burrow system (VBS). The chambers and surface area contained standard chow and water. Chambers and tunnels were kept under constant darkness, whereas the open surface area was under a 12:12 light cycle.
Males were introduced to the VBS on D0, at the onset of the dark cycle (1800 h). The next morning (D1) and on every other day thereafter (D3, 5, 7, 9, 11), rats were removed from the VBS and placed into their home cage for a short period between 0900 and 1100 h in order to record body weight, assess body condition and perform routine maintenance on the VBS. On these days, CON males were weighed as well, and were handled in the same manner as required for body condition assessment of VBS males. Body condition was determined based on weight loss, coat condition and wounding patterns that occur as a result of agonistic interactions between males. Any rat that lost more than 20% of their starting body weight, exhibited signs of severe distress or had severe wounding were removed from the study. No animals had to be removed from the VBS based on these criteria; however, one PCVS- SUB male died unexpectedly during VBS housing.
2.4 Determination of social status
Dominant (DOM) and subordinate (SUB) social status was determined using previously established behavioral criteria (Blanchard et al. 1993, Blanchard et al. 1995, Lucas et al. 2004). In general, DOMs spend more time in the open surface area (SFC) of the VBS, and engage in more reproductive and aggressive behaviors (lateral attack, chasing, biting, pinning and standing over conspecifics). SUBs spend more time in the tunnels and chambers than the SFC. They also exhibit more risk assessment behaviors (stretch-attend postures, hesitation) before entering chambers, tunnels or the open surface area. SUBs engage in fewer aggressive behaviors and more defensive behaviors (fleeing, hiding and assuming a submissive, supine position in response to challenge). Body condition is also a reliable index of social status within the VBS. Both DOMs and SUBs lose weight during the first days of VBS housing, but SUBs lose significantly more weight, and this weight loss is maintained through the duration of VBS housing. SUBs also incur more wounds as a result of agonistic interactions with DOMs. These wounds are concentrated towards the rump and tail, and ventral surfaces of the SUBs, consistent with flight and submissive behaviors. The coat of DOMs is well groomed and there are few, if any, wounds; when there are, they are primarily located on the face and head, likely resulting from a defensive bite from a cornered SUB.
2.5 VBS restraint stress test
On D13 of VBS or CON housing, a restraint stress test was performed as previously described in 2.3. Briefly, at approximately 1000 h, males were quickly removed from their housing (individual CON cage or the VBS), placed into a ventilated restrainer and blood samples taken from a tail nick for basal (T0), restraint (T60) and 60 min recovery (T120) plasma CORT determination. Following the restraint stress test, males were weighed and body condition was noted before being returned to VBS or CON housing.
2.6 Termination of the experiment
On D14, VBS and CON males were sacrificed by rapid decapitation. Brains were quickly removed and frozen on powdered dry ice and stored at −80°C until processing for in situ hybridization. Trunk blood was collected in EDTA-treated tubes and centrifuged for 20 min at 3000 rpm. Aliquots of plasma were stored at −20°C until assays were performed. Thymus, adrenals and testes were removed from each rat, weighed and recorded as both absolute weight and weight/kg body weight.
2.7 Hormone assays
Plasma corticosterone (CORT) of dams and adult male offspring (prior to and following exposure to VBS or CON housing) was determined using a commercially available radioimmunoassay (RIA) kit (Corticosterone DA, MP Biomedicals, Solon, OH). Plasma total testosterone was assessed at the end of the study using a commercially available RIA kit (Coat-A-Count, Siemens, Tarrytown, NY).
2.8 Tissue preparation
Brains were coronally sectioned at 14 μm using a Leica cryostat, and thaw-mounted onto silanized slides (Gold Seal Ultrastick, Thermo Scientific). Five sets of sections were generated per rat and were stored at −20°C. Prior to hybridization, sections were fixed in 4% paraformaldehyde, washed with 5 mM potassium-phosphate buffered saline (KPBS), acetylated in a 0.1M triethanolamine/0.25% acetic anhydride solution, washed with saline sodium citrate, dehydrated in a graded ethanol series and delipidated in chloroform.
2.9 In situ hybridization
Radiolabeled antisense rat NPY and CRH riboprobes (courtesy of James Herman, University of Cincinnati) were generated by in vitro transcription using 35S-labeled UTP. The NPY DNA construct was a 513 bp insert within a pCR4 TOPO vector. The plasmid was linearized using PvuII and transcribed with T3 polymerase. The CRH DNA construct was a 765 bp insert in a pGEM 3 vector linearized using HindIII and transcribed using T7 polymerase.
A commercially available in vitro transcription kit (Invitrogen MAXIscript, Ambion, Carlsbad, CA) was used to label riboprobes. Each transcription reaction was made by incubating 1.0 ug linearized DNA, 1.0 μl of ATP, CTP and GTP, 5.0 μl 35S-labeled UTP and 2.0 μl polymerase at 37°C for 1 h. Following the removal of free nucleotides using ammonium acetate and ethanol, radiolabel incorporation was determined by trichloroacetic acid (TCA) precipitation. Resuspended, purified probe was heated for 10 min at 65°C before being added to the hybridization solution. 50 μl of hybridization solution, consisting of 1.0 × 106 cpm of incorporated riboprobe, 1.5 μl single stranded DNA, 0.5 μl tRNA and 1.0 μl 1.0M dithiothreitol (DTT) in buffer solution (50% dextran sulfate, 5x hybridization stock and deionized formamide) was applied to each slide. Slides were coverslipped and placed in hybridization chambers humidified with 50% formamide and incubated overnight at 55°C. On the following day, approximately 20 h post-hybridization, slides were removed from chambers, coverslips were removed and slides were then washed in 2X SSC and transferred to a ribonuclease A (RNase A) solution (100 ug/ul) heated to 37°C for 30 min. Following RNase A incubation, slides were washed again in 2X SSC, rinsed several times in 0.2X SSC and transferred to 0.2X SSC heated to 65°C for 1 h. Slides were cooled at room temperature in 0.2X SSC and dehydrated through a graded ethanol series and allowed to air dry.
2.10 Image analysis
Hybridized slides were exposed to film (Kodak BioMax MR, Rochester, NY) for 3 days (MeA NPY), 7 days (PVN CRH) or 10 days (CeA CRH). Images from developed film were captured using a digital camera and lightbox connected to a computer with Scion Image frame grabber (Scion Corp., Frederick, MD). Brain regions of interest were identified using the Paxinos and Watson rat brain atlas (1998). NPY slides were stained with thionin in order to further assist in identification of amygdalar subnuclei. Briefly, slides were soaked in xylene for 5 min, rehydrated through a graded ethanol series, rinsed with deionized water and placed in thionin stain for approximately 2 min. Slides were then rinsed in water, dehydrated through a graded ethanol series and transferred to xylene. Slides were coverslipped with DPX mountant (Sigma Aldrich) and allowed to air dry.
Semi-quantitative analysis of mRNA expression was performed by densitometry. Each region of interest (ROI) was outlined using ImageJ software (NIH, Bethesda, MD), which calculated area and intensity of signal. The corrected gray level (CGL) was determined by subtracting the background signal (from an area within the same section not expressing the gene of interest) from this number. Integrated gray level (IGL) was then calculated by multiplying the CGL by the area of the ROI. All images containing the ROI were analyzed, and average IGL was determined for each area. Five sections per animal were averaged for PVN CRH expression, 5 for CeA CRH expression and 8 for NPY expression within the MeA. Amygdalar nuclei from each hemisphere were analyzed, and values reported reflect the average of left and right sides. 14C standards were developed with each film to ensure that densitometric values were within the linear range of the film.
2.11 Statistics
Interactions between the 2 prenatal treatments (PCVS and NS) and 3 outcomes/treatments for adults (CON, SUB and DOM) were assessed. Maternal plasma corticosterone levels, body weight and food intake were analyzed by 2-way repeated measures ANOVA (trt × day). Litter data (litter size, weight, sex ratio) were analyzed using t-tests. All litter data represent the average of the littermates; because the prenatal treatment was applied to the dam, pups were considered replicates or samples from the dam (Holson and Pearce 1992, Zorrilla 1997). As adults, multiple males from each litter were assigned to the CON treatment (PCVS- CON and NS-CON males that were singly housed with a female, without VBS exposure and subsequent social rank). Again, these data were pooled according to litter and the data reported refer to litter averages. Measures from each VBS-exposed male were not pooled since the postnatal treatment/social status was unique to each male. A chi-square analysis was used to assess whether pretreatment significantly affected social status in adulthood. Behavior, organ weights, basal hormone levels and mRNA expression were assessed by 2-way ANOVA, with prenatal treatment (PCVS and NS) and adult status (CON, DOM and SUB) as independent variables. Body weight changes were assessed using 2-way repeated measures ANOVA.
3. Results
3.1. Maternal and litter data
Prenatal stress had no effect on maternal body weight (Fig. 2A) or daily food intake (Fig. 2B) through the duration of gestation. As expected, no difference in maternal basal CORT was detected prior to treatment exposure (GD 14). On D 21, NS CORT levels were not significantly higher than basal levels assessed on GD). CORT levels of PCVS dams were significantly higher on GD 21 than on GD 14 (p< 0.001), and significantly higher than either day for NS dams (p< 0.001) (Fig. 2C).
Figure 2.
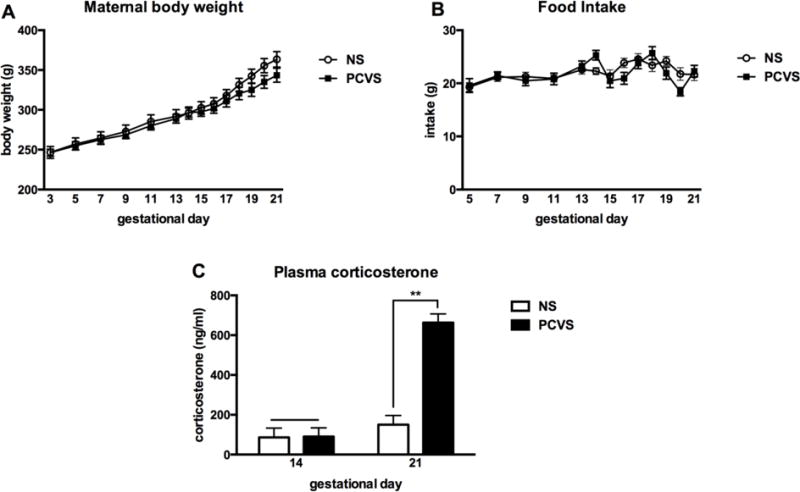
Effects of prenatal stress paradigm on maternal body weight, food intake and plasma corticosterone (n= 8 NS, 9 PCVS). Prenatal stress treatment began on gestation day (GD) 14. (A) Prenatal treatment did not significantly affect maternal body weight during gestation, nor did it affect food intake (B). (C) There was no difference in plasma corticosterone (CORT) before onset of PCVS treatment on GD 14. PCVS CORT was significantly higher on GD 21 than GD 14, and significantly higher than NS dams on GD 14 and GD 21. Data are expressed as mean +/− SEM. **p <0.01.
Litter size ranged from 7–15 pups (mean of 11.3 pups per litter). Treatment did not have a significant effect on litter size, birth weight or sex ratio (Table 2). Three litters from each treatment were culled to 12 pups due to their large size. Treatment did not have a significant effect on weekly body weights of males prior to and after weaning (data not shown).
Table 2.
Litter characteristics. PCVS treatment did not have a significant effect on litter size, sex ratio or birth weights (n= 8 NS litters, 9 PCVS litters). Data are expressed as mean +/− SEM.
| GROUP | LITTER SIZE (number of pups) |
SEX RATIO (male:female) |
AVG BIRTH WEIGHT (g) |
|---|---|---|---|
| NS | 11.25 +/− 0.96 | 0.45 +/− 0.07 | 7.32 +/− 0.23 |
| PCVS | 11.33 +/− 0.50 | 0.51 +/− 0.03 | 6.99 +/− 0.14 |
3.2. Pre-VBS restraint stress test
There was no significant effect of prenatal treatment on basal plasma CORT. Although time of sampling during an acute restraint test had a significant effect on CORT levels (i.e., Peak CORT levels were higher than basal or recovery levels), PCVS CORT levels did not differ from those of NS males (data not shown).
3.3. VBS social status
Of 24 VBSs, 18 male pairs formed a well-defined dominant and subordinate relationship. Six burrows were excluded from analyses because the males failed to form a clear DOM-SUB relationship fitting any of the previously set criteria. In these burrows, there were few or no wounds on either of the males, few agonistic interactions and little to no weight loss. There was no clear difference in the numbers of aggressive and defensive behaviors and no attempts to block access to territory and/or females were observed. NS males were significantly more likely to become DOM and PCVS males were more likely to be SUB when housed in the VBS. Of 18 burrows that formed a clear DOM/SUB pair, PCVS males became SUB in 14 burrows, whereas only 4 NS males became SUB (Fig. 3, p= 0.003).
Figure 3.
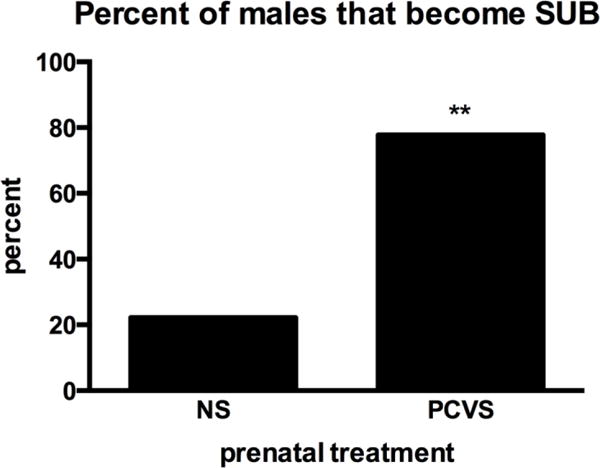
Percentage of males that became SUB (n= 18 VBS colonies). Significantly more PCVS males became SUB; Of 18 burrows, 14 PCVS males and 4 NS males were SUB (X2 analysis). ** p <0.01.
3.4. VBS behavior
Adult social status influenced time spent in the SFC of the VBS. DOMs spent a significantly greater percentage of time in the SFC than SUB males on D2 (p= 0.008). On D11, time spent in the SFC by DOMs approached, but did not reach, statistical significance (p= 0.052, Fig. 4). Prenatal treatment did not affect overall time spent in the SFC.
Figure 4.
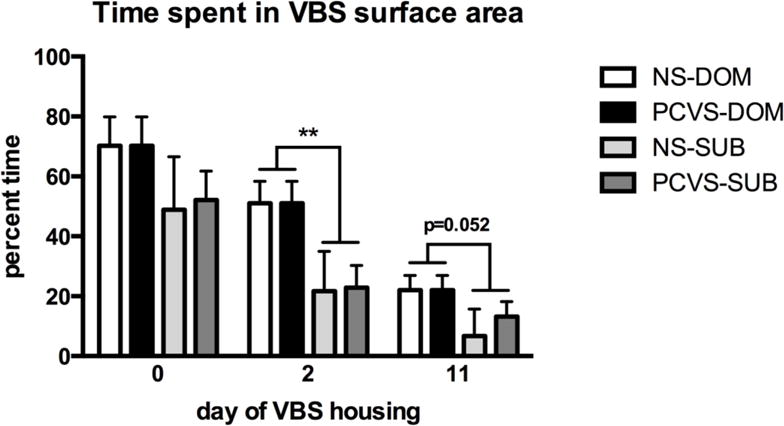
Percentage of time spent in the SFC area. DOM (n=18) spent significantly more time in the SFC area than SUB (n=18) on D2, but there was no effect of, or interaction with prenatal treatment. Data are expressed as mean +/− SEM. **p <0.01.
3.5. Hormone analyses
Following 13 d of social stress within the VBS, there was a main effect of VBS exposure on basal CORT levels; basal CORT levels were higher in DOMs and SUBs in comparison to CONs (p< 0.001). The interaction between prenatal stress and adult social status approached, but did not reach significance (p= 0.059). A post-hoc t-test was used to compare the effects of prenatal treatment on basal CORT levels of SUBs. This test indicated that NS-SUBs had a significantly higher basal CORT levels than PCVS-SUBs (p= 0.014, Fig. 5A). At the end of the study, no difference in plasma testosterone (T) was observed; there was no effect of prenatal treatment, social stress or interactions between prenatal treatment and social status on plasma T (Fig. 5B).
Figure 5.
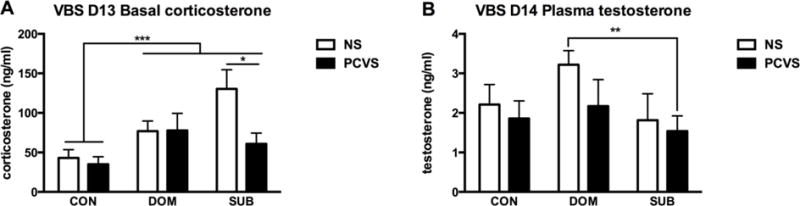
Plasma corticosterone and testosterone at the end of VBS or control housing. (A) There was a main effect of social status; a main effect of prenatal stress approached, but did not reach statistical significance (p= 0.059). Post hoc comparison of prenatal stress treatment and SUB status by t-test indicated a significantly higher basal CORT in NS-SUB males (n=4) compared to PCVS-SUBs (n=14). (B) There was no main effect of prenatal treatment or housing/social status on plasma testosterone, although a t-test indicated a significant difference between plasma testosterone of NS-DOM and PCVS-SUB males. Data are expressed as mean +/− SEM. *p <0.05, **p <0.01, ***p <0.001.
3.6. Body and organ weights
Social status had an overall significant effect on body weight change during VBS exposure with DOM males losing significantly less weight than SUBs on days 9, 11, 13 and 14 (Fig. 6A). When assessed by prenatal treatment, there was a significant difference between PCVS-SUB and NS-DOM on days 9, 11, 13 and 14 of the VBS (Fig. 6B). Despite losing a greater percentage of body weight than CVS-SUB, there was no difference between NS-SUB and PCVS-DOM during VBS housing, likely due to the small number of colonies (4 burrows). VBS exposure had a significant effect upon adrenal weights (p< 0.01), which were significantly heavier in DOMs and SUBs (p <0.05) than those of CONs (Fig 7A). Thymus weight was also affected by VBS exposure (p< 0.001). The thymus weights of SUBs and DOMs were significantly lower than CONs (p< 0.05). No interactions between prenatal treatment and adult social status on thymus weight were observed (Fig 7B).
Figure 6.
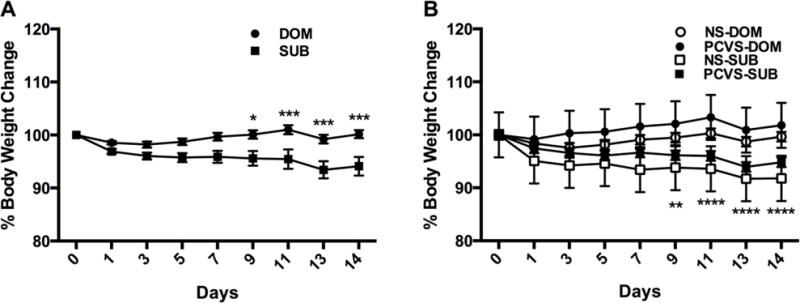
Body weights during VBS housing expressed as percentage of starting weight. A. DOM males weighed significantly more than SUB males on days 9, 11, 13 and 14 of the VBS. B. Body weights of DOM and SUB during VBS housing by prenatal treatment. There was a significant difference in percentage body weight change between NS-DOM and PCVS-SUB on days 9,11,13 and 14, but no difference between PCVS-DOM and NS-SUB. Data are expressed as mean +/− SEM. *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001.
Figure 7.
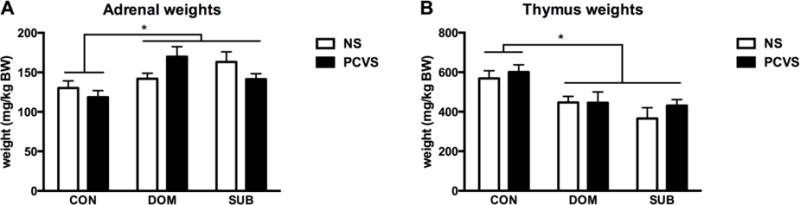
Adrenal and thymus weights at the end of VBS or control housing. (A) Adrenal weights are adjusted to BW (mg/kg) at the end of VBS housing. DOM and SUB adrenal weights were significantly higher than CON adrenal weight. PCVS-DOM (n=4) adrenal weight was heavier than PCVS-CON. (C) Thymus weights are adjusted to BW (mg/kg) at the end of VBS housing. Thymus weight of DOM (n=18) and SUB (n=18) was significantly less than that of CON after 14 days of social stress in the VBS. Data are expressed as mean +/− SEM. *p <0.05.
3.7. In situ hybridization
Neither prenatal treatment nor adult social status had an effect on PVN CRH expression (Fig. 8A). CRH mRNA expression within the CeA also did not differ by prenatal treatment or adult social status. No interactions between prenatal treatment and adult social status were observed (Fig. 8B). NPY mRNA expression within the MeA was significantly affected by VBS exposure (p< 0.05); both DOMs and SUBs had a greater expression of NPY mRNA within MeA than CONs. No effects of prenatal treatment or interactions between prenatal treatment and social status were detected (Fig. 8C).
Figure 8.
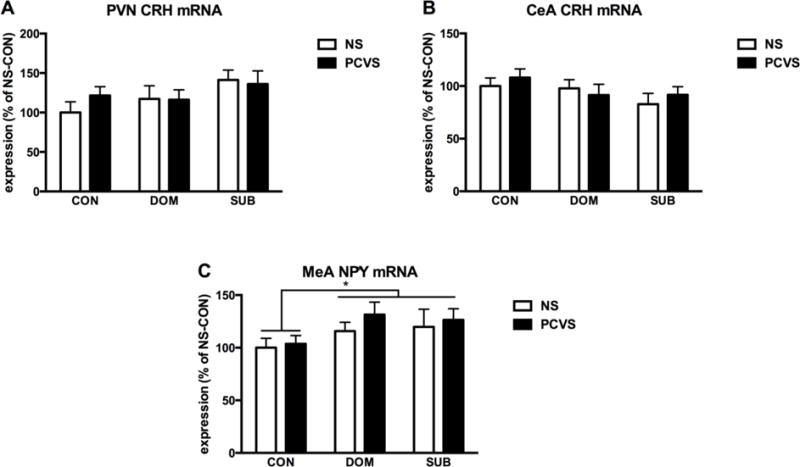
CRH and NPY mRNA expression within brain after 14 days social stress. (A) There was no effect of prenatal treatment or adult status on CRH mRNA expression within the PVN, and no interactions between the two. (B) There was no main effect of prenatal or adult status on CRH mRNA expression within the CeA, and no interactions between the two. (C) NPY mRNA expression was significantly higher in DOM and SUB compared to CON with no effect of prenatal condition. For all graphs, NS-CON is set to 100%. Data are expressed as mean +/− SEM. *p <0.05.
Discussion
Over the years of using the VBS model, our lab and others have tried, but have been unable to predict which animals will become subordinate. Social status has not been predicted by body weight, composition, plasma testosterone, or plasma corticosterone (Sakai and Tamashiro 2005, Nguyen et al. 2007). In these studies we tested the hypothesis that early life adversity would increase vulnerability to social subordination in adulthood. This part of the hypothesis was supported, as prenatally stressed males were significantly more likely to become subordinate within the VBS as adults. As our lab and others have previously demonstrated, chronic variable stress exposure during the last week of gestation induces a robust CORT response in dams, without significantly altering maternal food intake or weight gain or litter parameters (Koenig et al. 2005, Tamashiro et al. 2009). Furthermore, we did not observe any effects of prenatal stress on basal plasma CORT of adult male offspring, or in the response to acute restraint stress. There are many conflicting reports concerning the effects of prenatal stress on adult HPA axis activity and these are likely due to numerous factors, including rat strain, stressor type, sex of the offspring tested and age at testing (Weinstock et al. 1992, Maccari et al. 2003, Louvart et al. 2005, Richardson et al. 2006). Our failure to detect changes in HPA activity may be the result of the relatively mild nature of stressors employed and the strain of rat chosen for these studies. Prenatal CVS models typically reveal prolonged CORT responses following acute stress, but these studies primarily use Sprague-Dawley rats, whereas we used Long-Evans (Koenig et al. 2005, Lee et al. 2007). Other studies using Long-Evans rats have reported that prenatally stressed males do not exhibit alterations in basal CORT nor in response to acute stress (McCormick et al. 1995, Szuran et al. 2000).
Previous studies in the VBS and other paradigms of chronic social stress demonstrate that social stress leads to elevations in CRH within the paraventricular nucleus of the hypothalamus (PVN) and within the central nucleus of the amygdala, and that these elevations are greatest in SUBs (Albeck et al. 1997, McCormick et al. 2007, Perez-Tejada et al. 2013). However, although there were modest increases in CRH expression in these regions in VBS-exposed males, they did not reach significance in the current study. This makes sense, since we observed a “milder” SUB phenotype with only modest effects on HPA axis activity. However, we did observe some changes in HPA axis activity of SUBs on D13 of VBS exposure with that basal plasma CORT of NS-SUBs higher than that of PCVS-SUBs. The reason for this change in basal CORT is unclear, but could be related to serotonergic transmission. In an unpublished pilot study, we found that prenatal stress was associated with a significant reduction of serotonergic cell bodies within the mid-rostrocadual region of the dorsal raphe nucleus (DRN) and in the dorsomedial portion of the DRN (DRD) in particular. Neurons within this region are stress-sensitive and play a role in HPA activation and modulation of mood (Lowry et al. 2008). Although further investigation is necessary, recent studies have supported this hypothesis; prenatal stress has been associated with a reduction of TPH2 and serotonin immunoreactivity, as well as reductions in baseline firing of serotonergic neurons within the DRN (Van den Hove et al. 2014, Oosterhof et al. 2016). We hypothesize that our paradigm may induce subthreshold changes within the offspring; an acute stressor may not be sufficient to produce a clear effect on certain behavioral or physiological measures, but differences may be revealed in response to subsequent challenges, such as chronic social stress. Although there were changes in amygdala NPY mRNA expression that were associated with VBS exposure (DOMs and SUBs expressed higher levels of mRNA than CONs within the MeA), there was no interaction with prenatal treatment. NPY is thought to have anxiolytic-like effects within the amygdala that are thought to counteract the actions of CRH and to facilitate adaptation or resilience to stress (Heilig et al. 1994, Primeaux et al. 2005, Sajdyk et al. 2006, Sajdyk et al. 2008). However, these effects may depend on the type of stressor one is exposed to. Recently, Melhorn et al. (2017) demonstrated that OMEGA males, SUBs that experienced the most wounding and most severe body weight loss had significantly higher elevations of NPY mRNA within the amygdala that traditional SUBs. Whether this reflects a beneficial, but insufficient compensatory mechanism or a maladaptive one is unclear. Increases in a neuropeptide that increases exploratory behavior and entries into open areas may prove maladaptive to a socially subordinate male. Although we found that PCVS males were more likely to become socially subordinate than NS males, exposure to prenatal stress did not exacerbate the aforementioned phenotype of SUBs as we had hypothesized. Rather than potentiating the previously observed effects of subordination stress, PCVS-SUBs may in fact, be better able to cope with social stress. Although more likely to be SUB, the SUB phenotype was not as marked as in prior studies using vendor-procured rats. We used female-biased colonies (2 males, 4 females) in the current studies for two main reasons. Firstly, assessing the interactions between two males, one prenatally stressed and one non-prenatally stressed, makes for a clear DOM-SUB relationship without the possible confounding factors introduced by multiple social relationships. That is, while there is only one DOM in a 4 male VBS, there are 3 SUBs and the relationship among those 3 SUBs may also be hierarchical in nature making analysis much more complex. Secondly, we have previously found that female-biased VBSs produce more robust DOM and SUB phenotypes than the traditional 4 male, 2 female burrows. Historically, males within female biased colonies engage in a significantly higher number of agonistic interactions, and SUBs exhibit poor body condition and more pronounced weight loss than SUBs in traditional 4 male VBSs (Tamashiro et al. 2004).
Although the trends in body weight and body condition in this study were similar to those in previous studies, the effects of SUB status were much less pronounced in our current study. Although SUBs lost more weight than DOMs and CONs in the current studies, the effect did not nearly approach the weight loss typically observed in 2 male and 4 male VBSs (Tamashiro et al. 2004, Nguyen et al. 2007, Melhorn et al. 2010, Smeltzer et al. 2012). Similarly, fewer agonistic interactions and fewer wounds were observed in these colonies when compared with data from previous studies. Furthermore, of the 24 burrows run, 6 did not form clear DOM-SUB relationships. Although it is not uncommon to see a burrow in which a social hierarchy does not form, it is rare to see so many in one study, particularly in the case of female-biased colonies. In future studies a more detailed analysis of social behaviors is warranted. It is not clear whether prenatally stressed rats are less aggressive and/or avoid social interaction. It would also be interesting to investigate the effects that prenatal stress and subordination stress have on oxytocinergic systems based on some of the effects of prenatal stress that also correlate with schizophrenia. Koenig and colleagues reported that prenatally stressed males spend less time interacting with conspecifics in a social withdrawal paradigm, and this effect on social behavior can be reversed when oxytocin is directly injected into the CeA (Lee et al. 2007).
In short, we found no cumulative effect of prenatal stress and subordination stress other than the proportion that became SUB and basal CORT following chronic stress exposure. Taken together, these findings suggest that prenatally stressed males may, in fact, be better able to deal with chronic social stress in adulthood, and may adopt different coping strategies within the burrow.
The current studies also highlight the importance of experimental design and interpretation of findings. Because animal models are used to test hypotheses about stress-related pathology, we often focus on adverse early life experiences with an eye towards maladaptation and without consideration of its potential adaptive value. From an evolutionary standpoint, the adverse intrauterine environment used in this paradigm may allow the offspring to better adapt to an adverse social environment in adulthood. Relatively few individuals become dominant within their respective societies, so it does not make sense for all members to be predisposed to become dominant. It is also misguided to assume that traits associated with dominance are always adaptive or “better.” Indeed, dominance has its own set of stressors, and in some cases, the dominant animal shows signs of greater stress than subordinates, particularly under conditions of social instability, including periods during which dominance is challenged (Sapolsky 1992, Abbott et al. 2003).
It seems unlikely that the effects of prenatal stress would only have negative effects. Instead, like stress responses in general, the main effect is to facilitate adaptation. Predisposition to social subordination may increase the likelihood that a rat does not challenge conspecifics, is more vigilant and avoids interactions when in a stressful environment, which clearly has adaptive value in context of survival. However, this propensity may also have negative effects if there is little threat and the rat continues to engage in these behaviors as they do present a burden on the system. Mild early life stress exposure has been known to engender resilience to stress in adulthood, but had largely fallen out of favor. However, the concept has experienced a resurgence in recent years and is the basis of the stress-mismatch and stress inoculation hypotheses, which posit that early life stress exposure may be maladaptive in a “normal” adult environment, but may reduce vulnerability in a similarly adverse (matching) adult environment (Schmidt 2011, Homberg 2012, Ashokan et al. 2016). Therefore, in the case or prenatal stress and adult chronic social stress, it appears that the ability to adapt to the current environment is more important than one’s social status.
In summary, we found that chronic variable prenatal stress did not induce overt changes in the phenotype of male offspring during the perinatal period or in adult males exposed to an acute, onetime stress exposure. However, we found that males exposed to stress in utero were significantly more likely to become socially subordinate when housed in a visible burrow system. Furthermore, these subordinate males did not develop an exaggerated SUB phenotype that has been hypothesized, despite using the 2-male, 4-female VBS housing model, which has exacerbated aggression of dominant males towards subordinate male in the past. They also had significantly lower plasma CORT levels than non-prenatally stress SUB males. These changes in behavior were not related to differences in NPY mRNA expression within the MeA or CRH mRNA expression within the PVN or CeA. Based on recent literature and our own unpublished experiments, we hypothesize that this effect on behavior and stress response in prenatally stressed males exposed to adult chronic social stress may reflect neurodevelopmental changes within the serotonergic system, and future studies should address this possibility.
Highlights.
Socially subordinate rats in the visible burrow system (VBS) model of social stress develop a depressive-like phenotype
Chronic variable stress during the last week of gestation caused a robust increase in maternal corticosterone without influencing maternal body weight, food intake, litter size or offspring birth weights
Prenatally stressed males were significantly more likely to become subordinate upon exposure to the VBS
Prenatally stressed subordinate males did not develop an exaggerated depressive-like phenotype, but rather appeared better able to cope in the VBS
Exposure to prenatal stress may impart resilience upon exposure to a stressful environment in adulthood.
Acknowledgments
Funding
This research was supported by NIH grants MH088230 (KAS) and DK068273 (RRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott DH, Keverne EB, Bercovitch FB, Shively CA, Mendoza SP, Saltzman W, Snowdon CT, Ziegler TE, Banjevic M, Garland T, Jr, Sapolsky RM. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43(1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- Al-Harbi KS. Treatment-resistant depression: therapeutic trends, challenges, and future directions. Patient Prefer Adherence. 2012;6:369–388. doi: 10.2147/PPA.S29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17(12):4895–4903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasubramanian Ashokan AM, Mitra R. Seeding Stress Resilience through Inoculation. Neural Plast. 2016;2016:4928081. doi: 10.1155/2016/4928081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. An analysis of social behaviour in wild rats. Proc Zool Soc Lond. 1958;130:107–152. [Google Scholar]
- Barnett SA. The Rat: A Study in Behaviour. Chicago, IL: Aldine Publishing Company; 1963. [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73(3):435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58(1–2):113–121. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Summers CH, Blanchard RJ. The role of behavior in translational models for psychopathology: functionality and dysfunctional behaviors. Neurosci Biobehav Rev. 2013;37(8):1567–1577. doi: 10.1016/j.neubiorev.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav. 2006;89(3):301–310. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov. 2005;4(9):775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- Czeh B, Fuchs E, Wiborg O, Simon M. Animal models of major depression and their clinical implications. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:293–310. doi: 10.1016/j.pnpbp.2015.04.004. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Derijk RH, Meijer OC. Therapy Insight: is there an imbalanced response of mineralocorticoid and glucocorticoid receptors in depression? Nat Clin Pract Endocrinol Metab. 2007;3(2):168–179. doi: 10.1038/ncpendmet0403. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Sibug RM, Helmerhorst FM, Schmidt MV. Stress, genes and the mechanism of programming the brain for later life. Neurosci Biobehav Rev. 2005;29(2):271–281. doi: 10.1016/j.neubiorev.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology. 2015;62:366–375. doi: 10.1016/j.psyneuen.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17(2):80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14(3):221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Homberg JR. The stress-coping (mis)match hypothesis for naturexnurture interactions. Brain Res. 2012;1432:114–121. doi: 10.1016/j.brainres.2011.11.037. [DOI] [PubMed] [Google Scholar]
- Huhman KL. Social conflict models: can they inform us about human psychopathology? Horm Behav. 2006;50(4):640–646. doi: 10.1016/j.yhbeh.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Ionescu DF, Rosenbaum JF, Alpert JE. Pharmacological approaches to the challenge of treatment-resistant depression. Dialogues Clin Neurosci. 2015;17(2):111–126. doi: 10.31887/DCNS.2015.17.2/dionescu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The costs of depression. Psychiatr Clin North Am. 2012;35(1):1–14. doi: 10.1016/j.psc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig JI, Elmer GI, Shepard PD, Lee PR, Mayo C, Joy B, Hercher E, Brady DL. Prenatal exposure to a repeated variable stress paradigm elicits behavioral and neuroendocrinological changes in the adult offspring: potential relevance to schizophrenia. Behav Brain Res. 2005;156(2):251–261. doi: 10.1016/j.bbr.2004.05.030. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Boer SF De, Rutter AJ De, Meerlo P, Sgoifo A. Social stress in rats and mice. Acta Physiol Scand Suppl. 1997;640:69–72. [PubMed] [Google Scholar]
- Lam RW, McIntosh D, Wang J, Enns MW, Kolivakis T, Michalak EE, Sareen J, Song WY, Kennedy SH, MacQueen GM, Milev RV, Parikh SV, Ravindran AV, C. D. W. Group Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 1. Disease Burden and Principles of Care. Can J Psychiatry. 2016;61(9):510–523. doi: 10.1177/0706743716659416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JF, Chalmers DT, Little KY, Watson SJ. A.E. Bennett Research Award. Regulation of serotonin1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: implications for the neurobiology of depression. Biol Psychiatry. 1998;43(8):547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- Louvart H, Maccari S, Darnaudery M. Prenatal stress affects behavioral reactivity to an intense stress in adult female rats. Brain Res. 2005;1031(1):67–73. doi: 10.1016/j.brainres.2004.10.025. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Evans AK, Heerkens J, Staub DR, Gasser PJ, Shekhar A. Serotonergic systems, anxiety, and affective disorder: focus on the dorsomedial part of the dorsal raphe nucleus. Ann N Y Acad Sci. 2008;1148:86–94. doi: 10.1196/annals.1410.004. [DOI] [PubMed] [Google Scholar]
- Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124(2):449–457. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maccari S, Darnaudery M, Morley-Fletcher S, Zuena AR, Cinque C, Reeth O Van. Prenatal stress and long-term consequences: implications of glucocorticoid hormones. Neurosci Biobehav Rev. 2003;27(1–2):119–127. doi: 10.1016/s0149-7634(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Mathys M, Mitchell BG. Targeting treatment-resistant depression. J Pharm Pract. 2011;24(6):520–533. doi: 10.1177/0897190011426972. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Merrick A, Secen J, Helmreich DL. Social instability in adolescence alters the central and peripheral hypothalamic-pituitary-adrenal responses to a repeated homotypic stressor in male and female rats. J Neuroendocrinol. 2007;19(2):116–126. doi: 10.1111/j.1365-2826.2006.01515.x. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84(1):55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Glucocorticoids, depression, and mood disorders: structural remodeling in the brain. Metabolism. 2005;54(5 Suppl 1):20–23. doi: 10.1016/j.metabol.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Melhorn SJ, Elfers CT, Scott KA, Sakai RR. A closer look at the subordinate population within the visible burrow system. Physiol Behav. 2017 doi: 10.1016/j.physbeh.2017.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R813–822. doi: 10.1152/ajpregu.00820.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nguyen MM, Tamashiro KL, Melhorn SJ, Ma LY, Gardner SR, Sakai RR. Androgenic influences on behavior, body weight, and body composition in a model of chronic social stress. Endocrinology. 2007;148(12):6145–6156. doi: 10.1210/en.2007-0471. [DOI] [PubMed] [Google Scholar]
- O’Leary OF, Dinan TG, Cryan JF. Faster, better, stronger: towards new antidepressant therapeutic strategies. Eur J Pharmacol. 2015;753:32–50. doi: 10.1016/j.ejphar.2014.07.046. [DOI] [PubMed] [Google Scholar]
- Oosterhof CA, Mansari M El, Merali Z, Blier P. Altered monoamine system activities after prenatal and adult stress: A role for stress resilience? Brain Res. 2016;1642:409–418. doi: 10.1016/j.brainres.2016.04.032. [DOI] [PubMed] [Google Scholar]
- Perez-Tejada J, Arregi A, Gomez-Lazaro E, Vegas O, Azpiroz A, Garmendia L. Coping with chronic social stress in mice: hypothalamic-pituitary-adrenal/sympathetic-adrenal-medullary axis activity, behavioral changes and effects of antalarmin treatment: implications for the study of stress-related psychopathologies. Neuroendocrinology. 2013;98(1):73–88. doi: 10.1159/000353620. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Cusick MC, York DA, Wilson MA. Effects of altered amygdalar neuropeptide Y expression on anxiety-related behaviors. Neuropsychopharmacology. 2005;30(9):1589–1597. doi: 10.1038/sj.npp.1300705. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Zorrilla EP, Mandyam CD, Rivier CL. Exposure to repetitive versus varied stress during prenatal development generates two distinct anxiogenic and neuroendocrine profiles in adulthood. Endocrinology. 2006;147(5):2506–2517. doi: 10.1210/en.2005-1054. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Banihashemi L, Koehnle TJ. Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiol Behav. 2011;104(4):632–640. doi: 10.1016/j.physbeh.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca M, Gili M, Garcia-Campayo J, Armengol S, Bauza N, Garcia-Toro M. Stressful life events severity in patients with first and recurrent depressive episodes. Soc Psychiatry Psychiatr Epidemiol. 2013;48(12):1963–1969. doi: 10.1007/s00127-013-0691-1. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Fitz SD, Shekhar A. The role of neuropeptide Y in the amygdala on corticotropin-releasing factor receptor-mediated behavioral stress responses in the rat. Stress. 2006;9(1):21–28. doi: 10.1080/10253890600557315. [DOI] [PubMed] [Google Scholar]
- Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28(4):893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai RR, Tamashiro KLK. Social hierarchy and stress. In: Steckler T, Kalin NH, Reul JMHM, editors. Handbook of stress and the brain. Vol. 15. Amsterdam, The Netherlands: Elsevier; 2005. pp. 113–132. [Google Scholar]
- Sapolsky RM. Cortisol concentrations and the social significance of rank instability among wild baboons. Psychoneuroendocrinology. 1992;17(6):701–709. doi: 10.1016/0306-4530(92)90029-7. [DOI] [PubMed] [Google Scholar]
- Schmidt MV. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology. 2011;36(3):330–338. doi: 10.1016/j.psyneuen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Smeltzer M, Scott K, Melhorn S, Krause E, Sakai R. Amylin blunts hyperphagia and reduces weight and fat gain during recovery in socially stressed rats. Am J Physiol Regul Integr Comp Physiol. 2012;303(6):R676–682. doi: 10.1152/ajpregu.00090.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeltzer M, Scott KA, Melhorn SJ, Krause EG, Sakai RR. Amylin blunts hyperphagia and reduces weight and fat gain during recovery in socially stressed rats. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00090.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AM, Kalueff AV. Developing better and more valid animal models of brain disorders. Behav Brain Res. 2015;276:28–31. doi: 10.1016/j.bbr.2013.12.024. [DOI] [PubMed] [Google Scholar]
- Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71(3–4):353–362. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Nguyen MM, Melhorn SJ, Ma LY, Woods SC, Sakai RR. Dynamic body weight and body composition changes in response to subordination stress. Physiol Behav. 2007;91(4):440–448. doi: 10.1016/j.physbeh.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Ma L Yun, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80(5):683–693. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26(1):27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. Prenatal Stress or High Fat Diet increases Susceptibility to Diet-Induced Obesity in Rat Offspring. Diabetes. 2009 doi: 10.2337/db08-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valladares A, Dilla T, Sacristan J. Depression: a social mortgage. Latest advances in knowledge of the cost of the disease. Actas Esp Psiquiatr. 2008 [PubMed] [Google Scholar]
- Van den Hove DL, Leibold NK, Strackx E, Martinez-Claros M, Lesch KP, Steinbusch HW, Schruers KR, Prickaerts J. Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur Neuropsychopharmacol. 2014;24(4):595–607. doi: 10.1016/j.euroneuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595(2):195–200. doi: 10.1016/0006-8993(92)91049-k. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]


