Abstract
The visible burrow system (VBS) utilizes the natural social behavior of rodents to model chronic social stress. Classically, when male and female rats are housed together in the VBS a dominance hierarchy rapidly forms with one dominant (DOM) and three subordinate (SUB) males. SUB animals show signs of chronic social stress, including loss of body weight and elevated basal corticosterone. This study furthered examined differences among the SUB population. Quantitative observations across numerous VBS colonies within the Sakai Lab suggest that there is variability in the effects of stress on the SUB population, specifically that some animals may experience more severe effects of chronic social stress than others. To further examine this observation, SUB animals were classified as OMEGA if they received a disproportionate amount of their colonies’ wounds. OMEGA animals received more wounds to their body compared to SUB (P<0.0001) and lost significantly more weight throughout the stress period compared to all other VBS-housed animals (group × time interaction P<0.0001). Following VBS housing it was determined the OMGEA also lost lean body mass (P<0.01 vs. controls and DOM), are hyporesponsive to an acute restraint challenge (P<0.01 vs all other groups) and show depressive-like behavior during a forced swim test. Furthermore, expression of neuropeptide Y within the amygdala, known for anxiolytic properties following chronic stress, was elevated among OMEGA (group × region interaction P<0.001). Together these observations suggest that an additional phenotype exists among the SUB animals within a VBS colony and represents the variability of the effects of chronic social stress.
Keywords: visible burrow system, chronic social stress, body composition, amygdala, neuropeptide Y
Introduction
The Visible Burrow System (VBS) is a model of chronic social stress, which has been extensively studied in relation to behavioral, physiological, endocrine, neurochemical, and metabolic consequences of stress exposure (1–9). A classic VBS colony consists of four male and two female rats. Upon colony formation one male rat emerges as the dominant (DOM) and shows clear phenotypic behaviors and characteristics. These include residing in and defending the large open surface chamber of the VBS, guarding the tunnel openings that lead to other smaller chambers, offensive attack behaviors, minimal wounding, and minimal body weight loss. Subordinate (SUB) animals, by definition, have a contrasting phenotype including significant weight loss, higher basal corticosterone, a defensive wound pattern, and habitation of the smaller enclosed chambers. SUB animals are thought to experience chronic social stress during the 2-week VBS housing period, although it is likely DOM animals experience some level of stress maintaining their status within the hierarchy.
SUB animals adapt to the VBS by avoidance of the DOM, remaining in the smaller chambers, and even altering their meal patterns (6). However, qualitative observations across the countless VBS colonies established in the Sakai Lab suggest differences within the SUB population may exist. Within any given VBS colony, often one SUB appeared to receive a disproportionality high number of wounds suggesting continual engagement with the DOM or other SUB long past the establishment of the hierarchy. Given this, we sought to characterize the animals that receive a disproportionate number of wounds during VBS housing and hypothesize that these animals display differential behavior and exhibit more severe effects of chronic social stress.
We conducted experiments in which traditional VBS dominance hierarchies were formed. SUB animals were then further grouped and classified as OMEGA based on a disproportionally high number of wounds received within their assigned colony and were compared to the DOM and remaining SUB. Changes in body weight, body composition, and food intake were compared between groups to assess effects of social stress. We further examined the meal taking strategy of these animals during VBS housing and assessed consequences of chronic social stress with an acute restraint challenge and a forced swim test post-VBS. Finally, to explore potential central mechanisms involved in the regulation of behavior during chronic social stress we assessed amygdalar neuropeptide Y (NPY) expression. NPY is classically known for its role in the homeostatic regulation of food intake within the hypothalamus (10), however within the amygdala, NPY has anxiolytic effects (11, 12).
Methods
Animals
Male and female Long-Evans rats (90-days old; Harlan; Indianapolis, IN) were individually housed for 4 weeks, the last week of which they were habituated to a standard powdered chow diet (5% fat, 3.46 kcal/g Teklad Lab Animal Diets #7012) used in the VBS. All protocols, animal handling, and treatment were approved by the Institutional Animal Care and Use Committee of the University of Cincinnati. Animals were maintained in a temperature- and humidity- controlled room on a 12:12-h light-dark cycle (lights off at 1800h).
Male rats were body-weight matched (to within 25g) to form standard VBS colonies of 4 males and 2 females. Controls (CON) consisted of 8 males that were weight-matched to one of the VBS colonies and individually housed with one adult female during the VBS stress period.
Colonies were run in 2 cohorts, each with 4 colonies. All 8 colonies underwent behavioral evaluation, wound counting, and body weight assessment. Cohort 1 underwent body composition analysis, an acute restraint stress test and meal pattern analysis (N= 4 CON, 4 DOM, 7 SUB, 5 OMEGA). Cohort 2 completed a forced swim test after VBS housing (N= 4 CON, 4 DOM, 8 SUB, 4 OMEGA). See below for further description. Prior to VBS housing, animals from cohort 1 were briefly anesthetized with isoflurane and implanted with a unique subcutaneous microchip behind their ears (Trovan, Electronic Identification Devices; Santa Barbara, CA) allowing for identification and monitoring of feeding behavior while group housed.
Behavioral analyses
Each colony was continuously housed for 14 days in a VBS modified for recording individual food intake (for details see (6)). Behavior was digitally recorded for all colonies using an infrared light source and camera mounted above each VBS apparatus. Recordings were taken for the first 6 hours of lights out (1800h) every other day throughout the 2 weeks of VBS housing.
Videos were evaluated for the first 15 minutes of every hour for each of the 6 hours of each day recorded and analyzed for specific offensive (biting, boxing, lateral attack, on-top-of, chasing), defensive behaviors (on-the-back and flight) and other behaviors such as tumbling ((1, 7, 9). Time spent in the open surface area was noted.
Wound counts and body weight measures were recorded every other day of VBS housing (~1100h). Males would be removed from the colony one-by-one, quickly weighed and wounds counted from the head, back, lower back and tail. All males were returned to the colony at the same time. This was all done under red light, and animals were out of their colonies for 5–10 minutes. During analysis of the wound counts it was observed that within any given colony the amount of wounding was variable. To correct for this we expressed the data as a percent of the total wounds received in that colony (the sum of all four males’ total wounds).
Body composition
Adipose and lean mass was measured using an EchoMRI (Echo Medical Systems, Houston, TX) body composition analyzer prior to and immediately following VBS housing. Body weight and body composition data are expressed as change from baseline.
Food intake and meal pattern analysis
The food intake monitoring system and meal pattern analyses applied to this study have been described elsewhere ((6, 13)). Briefly, each VBS was equipped with 3 microchip-scale systems (one in each of the VBS chambers). A food cup filled with powered chow was placed on each scale with an access tunnel positioned above. Each tunnel was equipped with a beam sensor and RFID reader which was activated when an animal placed their head in the tunnel to access food thus recording their unique microchip ID. Both the tunnel and scale were connected to a central analyzer which recorded time and duration of entry and changes in food cup weight.
Data were recorded for 22h periods and meal patterns were analyzed as previously reported ((6, 13)). Ingestive bouts were considered valid at a consumption rate of 0.50g/min and meals were established by combining ingestive bouts with an interbout interval of <5min. VBS colonies in cohort 2 had an identical tunnel-scale set up, but the tunnels were not equipped with beam sensors or RFID readers. CON animals’ meal patterns and food intake were not monitored during this study, but have been previously reported (6).
Acute restraint challenge and forced swim test (FST)
VBS colonies in cohort 1 underwent an acute restraint challenge on day 13 of VBS housing. Colonies in cohort 2 were subjected to a forced swim test immediately following VBS stress.
The acute restraint test began at 1000h, males were removed from their assigned housing and placed directly into Plexiglas restraint tubes (length 21.5cm, inner diameter 6.3cm). A small blood sample (~50ul) via tail nic was immediately obtained and animals remained restrained for 60 minutes at which time another blood sample was taken. Then, animals were removed from the restraint tube and placed in individual cages to recover with only water available. After 60 minutes a final sample was obtained. All samples were collected in heparinized microcentrifuge tubes, processed and stored at −20°C until analyzed. Total corticosterone was measured by radioimmunoassay using a commercially available kit (CORT DA; MP Biomedicals, Solon, OH).
The FST occurred on day 14 of VBS housing. FST chambers were constructed out Plexiglas and measured 46cm high, 21cm wide. Each chamber was surrounded by opaque material so each rat could not observe another’s behavior. Water temperature was between 33 and 37°C. Males were placed 4 at a time into individual FST chambers and video recorded for 15 minutes. Climbing, swimming, diving and immobile behaviors were evaluated at 15 second intervals for each animal.
In situ hybridization of Neuropeptide Y (NPY)
The definition of the OMEGA phenotype was applied to other colonies in order to assess mRNA expression of NPY in the amygdala (N=6 CON, 3 DOM, 4 SUB, 4 OMEGA). These animals were of the same age to all other animals reported in this manuscript and displayed VBS-weight loss patterns appropriate to their hierarchical status (i.e. OMEGAs lost the most amount of weight).
Animals were sacrificed by rapid decapitation following 14 days of VBS stress. Brains were immediately removed, flash frozen, and stored at −20°C until coronally sectioned (14 μm, Leica cryostat). Tissue was mounted on Fisherbrand Superfrost-Plus charged glass slides (Hampton, NH) and stored at −20°C until further analysis. NPY in situ hybridization methods are detailed elsewhere (6). Briefly, slides were hybridized with the NPY riboprobe (1.0 × 106 cpm/50ul buffer), combined with hybridization buffer (50% dextran sulfate, 5× hybridization stock, formamide, fish sperm (ssDNA), tRNA and DTT) and covered with glass cover-slips before being placed into hybridization chambers which were moist with 50% formamide and incubated overnight at 55°C. The following morning slides were post-treated following the removal of the coverslips beginning with a wash in 2× standard saline citrate (SCC). Next, slides were incubated in RNase A for 20 minutes at 37°C, washed numerous times in 0.2X SSC, once in 65°C 0.2X SSC for 1 hour, dehydrated through an ethanol series and air-dried.
Image analysis
Hybridized slides were exposed to Kodak BioMax MR film for 4–6 days and subsequently developed. To further identify the amygdalar nuclei a thionin stain was applied. The riboprobe treated slide were soaked in Citrisolv for 5 minutes, 100% ethanol for 2 minutes and Citrisolv again for 3 minutes, then hydrated through an abbreviated ethanol series (100%, 95% and 70%) followed by purified water. Slides were dipped in thionin stain for up to 2 minutes, dehydrated and cover-slipped.
Each stained slide section was over-laid on top of the corresponding section on the developed film and an image was captured displaying the mRNA message and tissue fibers. Without moving the film, the slide was removed and a second image was captured of just the X-ray film in the same orientation. On the combined digital image, regions of interest for the central, medial and basolateral nuclei of the amygdala were identified using anatomical markers based on the Paxinos and Watson rat brain atlas (14). These regions were transferred to the same location on the second image and analyzed by subtracting the non-hybridized tissue (background) from the hybridized signal within the same brain section and data expressed as corrected gray level (CGL) (Scion Image, Alpha 4.0.3.2; Scion Corporation, Frederick, MD). Twenty-four brain sections were analyzed per region per animal. Average CGL values were calculated in series for each of the three brain regions and the highest average value was used for that individual animal. 14C standards were developed with each film and analyzed for CGL to confirm that all measured gray levels were within the liner range of the film.
Statistics
Data are expressed as mean ± SEM unless otherwise noted. One-way ANOVA models with Bonferroni correction or linear mixed models capable of accounting for repeated measures were used to evaluate differences between groups and output measures repeated at the subject level (e.g. behaviors assessed in the FST). Statistical analyses were done using STATA (13.1, College Station, TX).
Results
Classification of OMEGA
Classic dominance hierarchies were formed in each of the 8 VBS colonies. SUB animals were further grouped based on wounds received (% of total wounds within their assigned colony) with animals receiving a disproportional amount of wounds classified as OMEGA. This classification was further confirmed by reviewing the wounds received during the hierarchy maintenance phase (d7-14 of VBS housing), a time in which agonistic interactions tend to subside and the hierarchy is normally stable (6), with OMEGA animals continuing to have a disproportional amount of wounds compared to other males in their colony. On average, DOM received less than 1% of the colony’s total wounds, where OMEGA received 42% of the total wounds. This was true in 7 of the 8 colonies evaluated, with 2 animals in the 8th colony receiving an equally high percentage of wounds (34% and 38%, respectively) (Table 1). Compared to the other SUB, these animals did not differ in the time they spent in the open surface area of the VBS (the chamber classically inhabited and guarded by the DOM) or in number of agonistic interactions, but did receive significantly more defensive wounds to their body compared to the DOM and other SUB (Table 1).
Table 1.
VBS Behavior and Wound Counts
| DOM | SUB | OMEGA | P-value | |
|---|---|---|---|---|
| # offensive behaviors1 | 56 ± 30 | 6.9 ± 8.5$$ | 7.8 ± 4.8$$ | P<0.00015 |
| # defensive behaviors1 | 0.5 ± 0.8 | 6.9 ± 6.2 | 11 ± 15 | NS5 |
| % Time in Surface Chamber2 | 46 ± 14 | 9.6 ± 9.3$$ | 11 ± 13$$ | P<0.00015 |
| % of wounds received3 | 0.8 ± 0.7 | 28 ± 6.4$$ | 42 ± 5.0$$# | P<0.00015 |
| Wound pattern: Head4 | 1.7 ± 2.3 | 1.1 ± 1.2 | 1.2 ± 1.3 | |
| Wound pattern: Body4 | 0.1 ± 0.1 | 10 ± 3.1$$ | 16 ± 3.9$$# | P<0.00016 |
| Wound pattern: Tail4 | 0.4 ± 0.4 | 2.7 ± 2.4$ | 4.7 ± 4.4$$ |
Data are mean ± SD.
Total count of behaviors observed during video evaluation (first 15min of every hour for first 6 hours of lights out every other day of VBS housing).
Average % time spent in the surface chamber during video evaluation.
Average % of wounds received during VBS housing, calculated on a colony-by-colony basis (wound count evaluations were done every other day of chronic social stress period).
Average number of wounds received per day of VBS housing.
One-way model with Bonferroni correction for multiple comparisons.
Group × Location Interaction by general linear mixed model. N= 8(DOM), 15(SUB), 9(OMEGA);
P<0.0001 vs DOM,
P<0.05 vs. DOM,
P<0.0001 vs. SUB.
Body weight and composition
DOM, SUB and OMEGA lost a significant amount of body weight compared to CON during VBS housing (group × day interaction P<0.0001). Although SUB maintained a greater weight loss than DOM during VBS housing (P<0.05 starting at day 5), OMEGA lost and maintained a significantly higher percent body weight loss compared to all other groups (P<0.05 beginning on day 7) (Figure 1A).
Figure 1. Body weight and body composition.
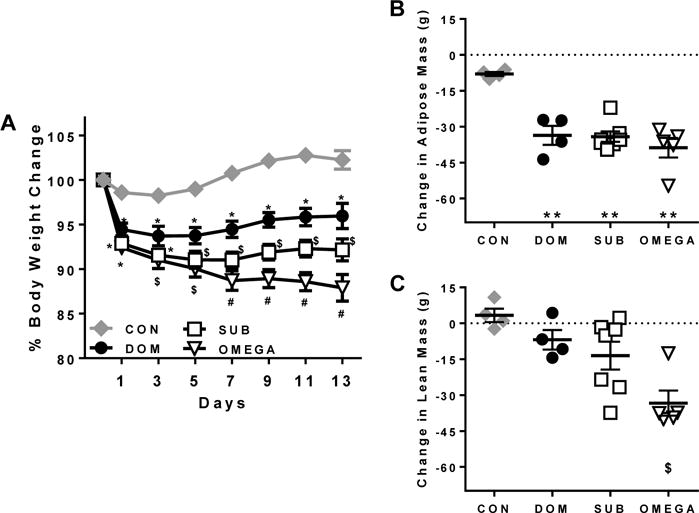
A) Dominant (DOM) and subordinate (SUB) animals displayed classic VBS-weight loss patterns compared to controls (CON) with SUB animals sustaining greater weight loss compared to DOM starting on day 5 and throughout the chronic social stress period. OMEGA lost the most weight during VBS-housing with significantly greater reductions compared to DOM beginning on day 3 and to SUB beginning on day 7. B) Animals housed in the VBS lost a significant amount of adipose tissue as a result of chronic social stress compared to CON. C) OMEGA lost significantly more lean tissue during VBS-housed compared to CON and DOM. N= 8(CON), 8(DOM), 15(SUB), 9(OMEGA) for % body weight change from baseline; N= 4(CON), 4(DOM), 7(SUB), 5(OMEGA) for change in adipose and lean tissue (post-vbs – baseline). Data are mean ± SEM. *P<0.01 vs. CON, **P<0.001 vs. CON, $P<0.05 vs. CON and DOM, #P<0.05 vs. CON, DOM and SUB.
Similar to previous reports, all groups lost a significant amount of adipose tissue during VBS exposure compared to CON (P<0.0001), however OMEGA also had a dramatic loss of lean tissue during VBS housing (P<0.05 vs DOM and CON) (Figure 1B&C).
Food intake and meal patterns
OMEGA animals consumed significantly less food on average per day during VBS housing compared to DOM (P=0.02). This was due to taking fewer meals per day than the DOM (P=0.02). Interestingly all groups consumed meals of similar size during the chronic social stress period (Table 2).
Table 2.
Average daily food intake and meal patterns during chronic social stress.
| DOM | SUB | OMEGA | P-value1 | |
|---|---|---|---|---|
| Food intake, g | 13.5 ± 1.73 | 10.5 ± 1.32 | 5.85 ± 0.66$ | 0.02 |
| Meal number | 14.3 ± 1.27 | 9.76 ± 1.22 | 7.22 ± 1.29$ | 0.02 |
| Meal size, g | 0.93 ± 0.10 | 1.11 ± 0.10 | 0.94 ± 0.10 | NS |
Data are mean ± SEM.
one-way model with Bonferroni correction for multiple comparisons. N= 4(DOM), 7(SUB), 5(OMEGA);
P<0.0001 vs. DOM.
We further examined meal patterns within each chamber of the VBS. A significant group × chamber interaction for daily average food intake (P=0.04) confirms that SUB and OMEGAs consume less food than the DOM in this surface chamber (P<0.05). In fact, SUB and OMEGA take meals across the chambers in a stepwise pattern, with the most meals consumed in the smallest chamber, an intermediate amount in the large chamber and very few in the surface chamber (P<0.05 for both groups large vs small chamber and large vs. surface chamber). No differences were found in the size of meal taken (Table 3).
Table 3.
Meal patterns within VBS chambers.
| Small | Large | Surface | P-value1 | |
|---|---|---|---|---|
| Meal Number | ||||
| DOM | 2.10 ± 1.74 | 3.99 ± 1.35 | 4.60 ± 1.26 | |
| SUB | 5.44 ± 0.73$ | 2.71 ± 0.81^ | 1.50 ± 0.59$^ | 0.0009 |
| OMEGA | 5.17 ± 0.72$ | 1.50 ± 0.69^ | 0.63 ± 0.28$^ | |
| Meal Size, g | ||||
| DOM | 0.64 ± 0.07 | 0.82 ± 0.13 | 1.01 ± 0.09 | |
| SUB | 1.19 ± 0.14 | 0.88 ± 0.19 | 0.95 ± 0.19 | NS |
| OMEGA | 1.10 ± 0.20 | 0.60 ± 0.07 | 0.70 ± 0.27 | |
Data are mean ± SEM.
Group × chamber interaction by general linear mixed model. N= 4(DOM), 7(SUB), 5(OMEGA);
P<0.05 vs. DOM within same chamber,
P<0.05 vs. small chamber within same hierarchical status.
Acute restraint stress test and forced swim test
Immediately following VBS exposure, male rats from cohort 1 were challenged with a novel 1-hour restraint stress test. A significant group × time interaction (P<0.001) confirmed that all groups mounted an appropriate stress response except for OMEGA who were hyporesponsive at the 60 minute mark (P<0.01 vs all other groups) (Figure 2A). Furthermore, although not significant, OMEGA had a higher basal plasma corticosterone level (101±44.7 ug/dL) compared to CON, DOM and SUB (40.3±10.5, 23.5±5.7 and 40.3±14.6 ug/dL, respectively).
Figure 2. Acute restraint stress test and forced swim test.
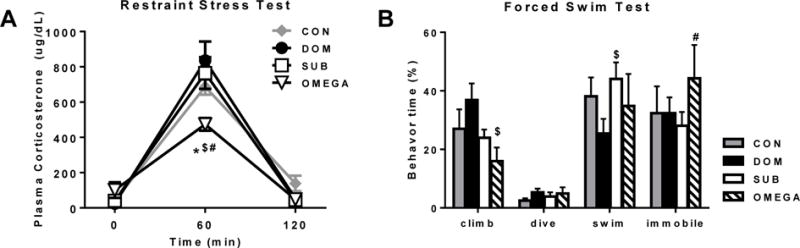
A) Following chronic social stress, OMEGA animals were hyporesponsive to an acute restraint stress challenge. B) During a forced swim test, OMEGA animals displayed less climbing behavior compared to dominants (DOM) and more immobility compared to subordinates (SUB). N=4(CON), 4(DOM), 7(SUB), 5(OMEGA) for restraint stress test; N= 4(CON), 4(DOM), 8(SUB), 4(OMEGA). *P<0.01 vs. CON, $P<0.01 vs. DOM, #P<0.05 vs. SUB.
VBS colonies from cohort 2 that did not undergo the restraint stress test were evaluated with a forced swim test directly following the social stress period. Although the interaction for activity × group only approached significance (P=0.055), OMEGA showed reduced climbing activity compared to DOM (P=0.02) and spent more time immobile compared to SUB (P=0.04) (Figure 2B).
Amygdalar NPY expression
Directly after VBS exposure OMEGA had increased NPY mRNA expression across all regions of the amygdala measured (group × region interaction P<0.001). Specifically, within the basolateral amygdala, OMEGA had higher NPY expression compared to all other groups (P<0.001) and within the central and medial nuclei OMEGA had higher NPY expression compared to SUB and CON (P<0.05) (Figure 3).
Figure 3. Neuropeptide Y mRNA expression in the amygdala after chronic social stress.
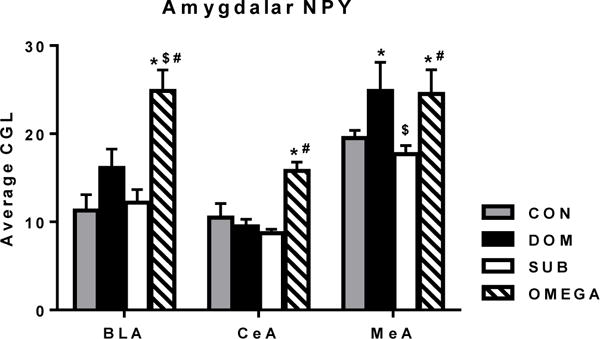
OMEGA animals had higher expression of neuropeptide Y (NPY) within the basolateral (BLA), central (CeA) and medial (MeA) nucleus of the amygdala. CGL=corrected gray level. *P<0.05 vs. CON, $P<0.01 vs. DOM, #P<0.01 vs. SUB.
Discussion
This study sought to characterize phenotypic differences within the SUB population of VBS colonies. To do this, classic VBS SUB populations were further stratified by the number of wounds received during VBS housing and stress-related outcomes were evaluated. Animals with high wound patterns, termed OMEGA, exhibited notable differences from other SUB animals in changes in body weight, body composition, and altered meal patterns, as well as differences in post-stress evaluations such as a suppressed glucocorticoid response, an increase in passive behaviors and elevated expression of NPY in the amygdala. Together these outcomes confirm that a sub-phenotype exists within the SUB population of the classic VBS hierarchy; and that these animals appear to experience differential effects of chronic social stress.
The behaviors that lead to the differential wounding patterns among SUB remain unclear. OMEGA, by definition, endured a disproportional amount of wounds, which were mostly located on the body suggesting more antagonistic interactions and specifically, more defensive behavior. However, observed interactions failed to show any difference in defensive behaviors among any of the groups resulting in a discrepancy of resulting data (i.e. OMEGA animals received more wounds, but did not express differential behavior). Given only segments of the VBS housing period were analyzed for behavior (i.e. the first 15 minutes of the first 6 hours of the dark cycle each day), it is possible differences in defensive behaviors went undetected. More extensive video analyses would yield more insight including the location of the interaction. Confrontations within the smaller VBS chambers could limit escape options yielding more severe defensive wounds. Furthermore, the status of the opposing animal could be helpful to understand if the wounds received by the OMEGA were inflicted by the DOM or other SUB. Although the identity of the opposing animal nor location of interactions are known, the meal pattern data can provide insight as it indicates time spent within a specific area of the VBS. OMEGA animals take the majority of their meals in the smaller chamber, in contrast DOM predominantly consume their meals in the open surface chamber, however also display eating bouts in both of the enclosed spaces. SUB consume an equal number of meals in the smaller chamber as OMEGAs. Therefore, it is possible that OMEGAs endured confrontations within the smaller chambers, perhaps from both the DOM and other SUB. Further video analyses are required to confirm these conjectures; specifically, if the OMEGA defends the smaller chamber in a similar manner that the DOM defends the surface area; if the OMEGA initiates confrontations (and with which animals) or if they are unable, or unwilling to avoid post-hierarchy formation interactions; and if the source of the OMEGA’s disproportional wounding pattern is from the DOM or other SUB or both.
All VBS-housed animals lost weight during stress, however OMEGA sustain a greater than 10% weight loss on average throughout VBS housing, and more importantly lose a significant amount of lean tissue. The extensive body weight loss can be attributed to a reduction in food intake, specifically by reduced meal frequency, but loss of lean mass suggests chronic negative energy balance. It is unclear how the stress-induced reduction of energy stores in OMEGAs (or even potential elevation of pro-inflammatory factors due to extensive wounding) plays a role in current outcomes, but, it is clear that OMEGAs do not compensate for their chronic negative energy balance during the stress period suggesting other indirect controllers of ingestive behavior may be playing a role. For example, ingestive behavior begins with the initial evaluation of risk to seek a meal. The reduced meal frequency seen in OMEGAs could represent that this risk was often determined to be too high thus leading to reduced meal number and thus decreased food intake.
Although this is the first detailed examination of the SUB population, differences among SUB have been previously observed. Specifically, some SUB animals, but not all, have shown a lack of glucocorticoid response to a 1-hour restraint test (2, 15, 16). In fact, it was suggested that these animals were the most severely stressed as they lose the most body weight, have the lowest plasma level of testosterone and greatest extent of adrenal hypertrophy (17). The current study’s OMEGA classification supports and extends these findings. Furthermore, during a forced swim test, an evaluation used to assess depression, OMEGAs display more passive behaviors. This could suggest post-stress depression, however it is unclear if the state of these animals (e.g. reduced energy availability) is playing a role in their immobility.
Previous studies have shown that following VBS housing, SUB have increased corticotropin-releasing hormone (CRH) in the central amygdala (2). Corticotropin-releasing hormone (CRH), the initiating peptide of the HPA axis, plays an anxiogenic role within the amygdala and may contribute to the fight-or-flight response to a stressful stimulus (18–20). Intraventicular and intra-amygdalar administration of CRH decreases general activity, social interaction time, time spent on the open arms of the elevated plus maze and increases startle response, all measures of increased anxiety (18, 20, 21). In contrast, NPY, most well-known for its role in food intake within the hypothalamus, has anxiolytic properties within the amygdala (22–25) and can oppose the anxiogenic effects of CRH during time in which anxiety may be maladaptive such as during chronic stress (24, 26–31). For example, acute restraint increases anxiety, as measured on the elevated plus maze, and amygdalar NPY levels are reduced, but, following chronic restraint where there is no longer an anxiogenic effect on the elevated plus maze or an endocrine stress response, NPY is upregulated in the amygdala (31, 32).
The current study reports OMEGA animals have increased NPY mRNA expression following the chronic social stress period in all three of the amygdalar nuclei measured, potentially opposing the effect of increased CRH and promoting anxiolytic behaviors. Within the VBS, anxiolytic behaviors could be considered increased time in the open surface area, which could increase the OMEGA’s exposure to the DOM thus promoting more agonistic interactions. However, this was not clearly observed. Preferred location of food intake suggest that OMEGA spent very little time in the open chamber; but their disproportional wound pattern suggests increased agonistic social interaction. Anxiolytic behavior can also be measured through the increased acceptance of punished responding. The Vogel and Geller-Seifter punished responding paradigms deprive animals of water or food then expose them to a shock chamber either immediately following deprivation, or with training to press a lever. During testing each level press, which provides the previously deprived element, is paired with a footshock (21, 23, 33). An anxiolytic agent will increase the number of punished-responses for water or food meaning the animal accepts more punishment. Although this cannot be mimicked in the VBS, it is possible that within the VBS environment the OMEGA accepts more punishment and does not flee or alter his behavior, supporting the increase in wounds received by these animals.
As discussed, NPY within the amygdala may play a role in stress resiliency specifically through anxiolytic-like effects in social behavior (29), however OMEGA animals show signs of chronic social stress despite their elevated amygdalar NPY. Similarly, repeated restraint stress results in elevated NPY expression and a suppressed corticosterone response (31), however, direct administration of NPY to the amygdala did not have an effect on the HPA axis response (29). Furthermore, general central administration of NPY has mixed outcomes on plasma levels of corticosterone. Intraventricular NPY increases plasma corticosterone, where intranasal NPY reduces anxiety-like behavior and suppresses the corticosterone response following a stress challenge (34, 35). These outcomes suggest that NPY likely plays a role in modulating the HPA axis response to a stressor, but it is unclear how NPY within the amygdala may be involved.
Furthermore, within the medial amygdala both DOM and OMEGA animals had elevated NPY expression. This region has been implicated in stress-induced body weight regulation (36) and the modulation of aggressive behaviors (37), the latter being of specific interest to the current study given it is likely, although not confirmed, that both DOM and OMEGA animals engage in more agonist behaviors-potentially with one another. However, it is not clear what role elevated amygdalar NPY may play in these processes.
In conclusion, clear differences exist between DOM, SUB and OMEGA animals within the VBS hierarchy. The characterization of the OMEGA animals highlight the variability of the effects of chronic social stress and introduce a phenotype that could be useful in elucidating what factors and mechanisms make an individual more susceptible to stress-related disease. Further investigation into the elevated amygdalar NPY in OMEGA animals is of interest, specifically does NPY within this region drive behavior within the VBS, or is it a result of VBS-stress.
Perspectives
Being a direct trainee of Randall’s was quite the experience. I hold many memories of the years I spent as a student in the Sakai Lab and feel extremely fortunate to have had the opportunity to be one of Randall’s graduate students, but to also be one of Randall’s friends.
Randall touched many people and had a tremendous impact on the scientific community. But, most importantly to me was the community that was built within his own lab both by Randall and because of Randall. The atmosphere Randall created lead to teamwork and the confidence to be independent. I will never forget the generosity, critique (specifically of my oral presentations) and unique support Randall bestowed upon me as his graduate student as it changed me as a person. Randall’s ability to bring people together is what I most admired about him, and even now that he is gone he continues to bring people together in a way no one else could.
Highlights.
Further phenotypes may exist within the classic VBS dominance hierarchy, specifically within the subordinate population suggesting variability in the effects of chronic social stress.
Animals that receive a disproportionate amount of wounds while housed in the visible burrow system exhibit more severe stress-related consequences such as loss of lean mass, a dysregulated hypothalamic-pituitary-adrenal axis, and show signs of depression.
Unlike other subordinates, the most severely stressed animals exhibited elevated neuropeptide Y expression in the amygdala, which may promote anxiolytic behavior.
Acknowledgments
Funding: This research was supported by the National Institutes of Health award number R01DK066596 (RRS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–34. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- 2.Albeck DS, McKittrick CR, Blanchard DC, Blanchard RJ, Nikulina J, McEwen BS, Sakai RR. Chronic social stress alters levels of corticotropin-releasing factor and arginine vasopressin mRNA in rat brain. J Neurosci. 1997;17(12):4895–903. doi: 10.1523/JNEUROSCI.17-12-04895.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanchard RJ, Dulloog L, Markham C, Nishimura O, Nikulina Compton J, Jun A, Han C, Blanchard DC. Sexual and aggressive interactions in a visible burrow system with provisioned burrows. Physiol Behav. 2001;72(1–2):245–54. doi: 10.1016/s0031-9384(00)00403-0. [DOI] [PubMed] [Google Scholar]
- 4.Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod. 2002;67(6):1750–5. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- 5.McKittrick CR, Magarinos AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36(2):85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 6.Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Meal patterns and hypothalamic NPY expression during chronic social stress and recovery. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R813–22. doi: 10.1152/ajpregu.00820.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen MM, Tamashiro KL, Melhorn SJ, Ma LY, Gardner SR, Sakai RR. Androgenic influences on behavior, body weight, and body composition in a model of chronic social stress. Endocrinology. 2007;148(12):6145–56. doi: 10.1210/en.2007-0471. [DOI] [PubMed] [Google Scholar]
- 8.Scott KA, Melhorn SJ, Sakai RR. Effects of Chronic Social Stress on Obesity. Curr Obes Rep. 2012;1(1):16–25. doi: 10.1007/s13679-011-0006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamashiro KL, Nguyen MM, Fujikawa T, Xu T, Yun Ma L, Woods SC, Sakai RR. Metabolic and endocrine consequences of social stress in a visible burrow system. Physiol Behav. 2004;80(5):683–93. doi: 10.1016/j.physbeh.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443(7109):289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 11.Davis M. Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci. 1997;9(3):382–402. doi: 10.1176/jnp.9.3.382. [DOI] [PubMed] [Google Scholar]
- 12.Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44(12):1239–47. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- 13.Melhorn SJ, Krause EG, Scott KA, Mooney MR, Johnson JD, Woods SC, Sakai RR. Acute exposure to a high-fat diet alters meal patterns and body composition. Physiol Behav. 2010;99(1):33–9. doi: 10.1016/j.physbeh.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1998. p. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58(1–2):113–21. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 16.Lucas LR, Celen Z, Tamashiro KL, Blanchard RJ, Blanchard DC, Markham C, Sakai RR, McEwen BS. Repeated exposure to social stress has long-term effects on indirect markers of dopaminergic activity in brain regions associated with motivated behavior. Neuroscience. 2004;124(2):449–57. doi: 10.1016/j.neuroscience.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 17.McKittrick CRB,DC. Behavioral and endocrine consequences of fluoxetine intervention in a model of social stress. Society for Neuroscience; Miami Beach, FL: 1994. [Google Scholar]
- 18.Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the “anxiogenic” response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103(2):227–32. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- 19.Britton KT, Morgan J, Rivier J, Vale W, Koob GF. Chlordiazepoxide attenuates response suppression induced by corticotropin-releasing factor in the conflict test. Psychopharmacology (Berl) 1985;86(1–2):170–4. doi: 10.1007/BF00431704. [DOI] [PubMed] [Google Scholar]
- 20.Dunn AJ, File SE. Corticotropin-releasing factor has an anxiogenic action in the social interaction test. Horm Behav. 1987;21(2):193–202. doi: 10.1016/0018-506x(87)90044-4. [DOI] [PubMed] [Google Scholar]
- 21.Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17(2):80–5. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- 22.Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic-like effects in animal anxiety models. Psychopharmacology (Berl) 1989;98(4):524–9. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- 23.Kask A, Harro J, von Horsten S, Redrobe JP, Dumont Y, Quirion R. The neurocircuitry and receptor subtypes mediating anxiolytic-like effects of neuropeptide Y. Neurosci Biobehav Rev. 2002;26(3):259–83. doi: 10.1016/s0149-7634(01)00066-5. [DOI] [PubMed] [Google Scholar]
- 24.Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur J Pharmacol. 1999;368(2–3):143–7. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- 25.Thorsell A, Michalkiewicz M, Dumont Y, Quirion R, Caberlotto L, Rimondini R, Mathe AA, Heilig M. Behavioral insensitivity to restraint stress, absent fear suppression of behavior and impaired spatial learning in transgenic rats with hippocampal neuropeptide Y overexpression. Proc Natl Acad Sci U S A. 2000;97(23):12852–7. doi: 10.1073/pnas.220232997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7(17):1645–59. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- 27.Gutman AR, Yang Y, Ressler KJ, Davis M. The role of neuropeptide Y in the expression and extinction of fear-potentiated startle. J Neurosci. 2008;28(48):12682–90. doi: 10.1523/JNEUROSCI.2305-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heilig M. The NPY system in stress, anxiety and depression. Neuropeptides. 2004;38(4):213–24. doi: 10.1016/j.npep.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Sajdyk TJ, Johnson PL, Leitermann RJ, Fitz SD, Dietrich A, Morin M, Gehlert DR, Urban JH, Shekhar A. Neuropeptide Y in the amygdala induces long-term resilience to stress-induced reductions in social responses but not hypothalamic-adrenal-pituitary axis activity or hyperthermia. J Neurosci. 2008;28(4):893–903. doi: 10.1523/JNEUROSCI.0659-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sajdyk TJ, Shekhar A, Gehlert DR. Interactions between NPY and CRF in the amygdala to regulate emotionality. Neuropeptides. 2004;38(4):225–34. doi: 10.1016/j.npep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Thorsell A, Carlsson K, Ekman R, Heilig M. Behavioral and endocrine adaptation, and up-regulation of NPY expression in rat amygdala following repeated restraint stress. Neuroreport. 1999;10(14):3003–7. doi: 10.1097/00001756-199909290-00024. [DOI] [PubMed] [Google Scholar]
- 32.Thorsell A, Svensson P, Wiklund L, Sommer W, Ekman R, Heilig M. Suppressed neuropeptide Y (NPY) mRNA in rat amygdala following restraint stress. Regul Pept. 1998;75–76:247–54. doi: 10.1016/s0167-0115(98)00075-5. [DOI] [PubMed] [Google Scholar]
- 33.Garner M, Mohler H, Stein DJ, Mueggler T, Baldwin DS. Research in anxiety disorders: from the bench to the bedside. Eur Neuropsychopharmacol. 2009;19(6):381–90. doi: 10.1016/j.euroneuro.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Serova LI, Tillinger A, Alaluf LG, Laukova M, Keegan K, Sabban EL. Single intranasal neuropeptide Y infusion attenuates development of PTSD-like symptoms to traumatic stress in rats. Neuroscience. 2013;236:298–312. doi: 10.1016/j.neuroscience.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 35.Song C, Earley B, Leonard BE. The effects of central administration of neuropeptide Y on behavior, neurotransmitter, and immune functions in the olfactory bulbectomized rat model of depression. Brain Behav Immun. 1996;10(1):1–16. doi: 10.1006/brbi.1996.0001. [DOI] [PubMed] [Google Scholar]
- 36.Solomon MB, Jones K, Packard BA, Herman JP. The medial amygdala modulates body weight but not neuroendocrine responses to chronic stress. J Neuroendocrinol. 2010;22(1):13–23. doi: 10.1111/j.1365-2826.2009.01933.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, He Z, Zhao C, Li L. Medial amygdala lesions modify aggressive behavior and immediate early gene expression in oxytocin and vasopressin neurons during intermale exposure. Behav Brain Res. 2013;245:42–9. doi: 10.1016/j.bbr.2013.02.002. [DOI] [PubMed] [Google Scholar]


