Abstract
Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) and Gulf War Illness (GWI) are debilitating diseases with overlapping symptomology and there are currently no validated tests for definitive diagnosis of either syndrome. While there is evidence supporting the premise that some herpesviruses may act as possible triggers of ME/CFS, the involvement of herpesviruses in the pathophysiology of GWI has not been studied in spite of a higher prevalence of ME/CFS in these patients. We have previously demonstrated that the deoxyuridine triphosphate nucleotidohydrolases (dUTPase) encoded by Epstein-Barr virus (EBV), human herpesvirus-6 (HHV-6), and varicella-zoster virus (VZV) possess novel functions in innate and adaptive immunity. The results of this study demonstrate that a significant percentage of patients with ME/CFS (30.91–52.7%) and GWI (29.34%) are simultaneously producing antibodies against multiple human herpesviruses-encoded dUTPases and/or the human dUTPase when compared to controls (17.21%). GWI patients exhibited significantly higher levels of antibodies to the HHV-6 and human dUTPases than controls (p = 0.0053 and p = 0.0036, respectively), while the ME/CFS cohort had higher anti-EBV-dUTPase antibodies than in both GWI patients (p = 0.0008) and controls (p < 0.0001) as well as significantly higher anti-human dUTPase antibodies than in controls (p = 0.0241). These results suggest that screening of patients’ sera for the presence of various combinations of anti-dUTPase antibodies could be used as potential biomarkers to help identify/distinguish patients with these syndromes and better direct treatment.
Keywords: Chronic Fatigue Syndrome, Epstein-Barr virus, Human herpesvirus 6, varicella-zoster virus, deoxyuridine triphosphate nucleotidohydrolase, antibodies
Introduction
Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is a chronic multisystem illness of unconfirmed etiology [Institute of Medicine, 2015]. In over half of cases, ME/CFS onset is associated with acute “flu-like” symptoms [Klimas et al., 2015]. There are multiple reports in the literature suggesting a role for viruses, particularly human herpesvirus-6 (HHV-6) [Ablashi et al., 2000; Komaroff, 2006; Chapenko et al., 2012] and Epstein-Barr virus (EBV) [Cameron et al., 2010; Loebel et al., 2014] in ME/CFS. However, the data concerning a causal relationship between a virus and ME/CFS has not been conclusively demonstrated and remains a challenge because of the heterogeneity of the patient population and the realization of possibly multiple etiologies. Surprisingly, none of these studies have approached the possibility that virus encoded proteins rather than the viruses themselves may act as drivers of/contribute to the pathophysiological alterations observed in a subset of patients with ME/CFS.
Approximately one-third of the veterans returning from the Operation Desert Storm conflict, were afflicted with a chronic multisystem disorder known as Gulf War Illness (GWI) [Fukuda et al., 1998]. Characterized by severe and debilitating symptoms including fatigue, musculoskeletal pain and cognitive problems, GWI has no known cure and requires long-term treatment and monitoring [Eisen et al, 2005]. Multiple hypotheses regarding the etiology of these symptoms soon emerged, as these servicemen were subjected to a number of potentially hazardous conditions, including infectious agents, medical prophylaxis, pesticides, depleted uranium, oil well fire smoke, chemical and biological warfare agents and psychological stressors [Steele et al., 2012].
GWI cases exhibit a significant overlap in symptoms with ME/CFS, which include long-term fatigue, sleep disturbances or non-restorative sleep, as well as immune and cognitive dysfunction [Whistler et al., 2009; Institute of Medicine, 2013; Parkitny et al., 2015]. There are currently no validated biomarkers for definitive diagnosis of either syndrome. Thus, there is an urgent need to identify triggers and drivers of ME/CFS and GWI and to understand the molecular mechanisms by which they contribute to the development or progression of these illnesses so more efficient diagnostic tools and effective therapeutics can be developed.
We have previously demonstrated that the deoxyuridine triphosphate nucleotidohydrolase (dUTPase) encoded by EBV, HHV-6 and VZV possess novel immunoregulatory functions by triggering the activation of toll-like receptor (TLR) 2, which leads to the activation of NF-κB and subsequent modulation of downstream genes involved in chronic inflammation, effector T-cell function and neurotransmitter function [Glaser et al., 2006; Ariza et al., 2009, 2013, 2014]. We have also shown that the EBV-encoded dUTPase is secreted in exosomes, which function as intracellular messengers, induces the secretion of the pro-inflammatory TH1/TH17 cytokines, alters T- and NK cell function in vitro, and induces anxiety and sickness behavior in mice [Padgett et al., 2004; Aubrecht et al., 2014]. Perhaps more importantly, was the finding of prolonged and significantly elevated neutralizing antibodies against the EBV-encoded dUTPase, which inversely correlated with ME/CFS symptomology in a small subset of patients with ME/CFS [Lerner et al., 2012].
In this study, we examined, using a newly developed ELISA assay, longitudinal samples from a cohort of patients with ME/CFS enrolled in the “Good Day Bad Day” study and single serum samples from other cohorts of patients diagnosed with GWI or ME/CFS as well as gender, age and ethnicity matched controls. The samples were examined for the presence of IgG antibodies against the HHV-6, EBV and VZV encoded dUTPases as well as the human nuclear dUTPase. The ELISA assay is more advantageous than the previously employed neutralization assays in that it is more rapid, allows for the detection of a wide assortment of antibodies directed against the proteins rather than just those that neutralize enzymatic activity. This is important in the case of dUTPases such as HHV-6, which lack functional enzymatic activity [Ariza et al., 2014]. Altogether, our data is consistent with previous findings supporting aberrant immune responses and a loss in immune competence in ME/CFS and GWI patients. This could result in lytic/abortive replication of these herpesviruses and subsequent expression of virus-encoded dUTPases, which in turn may contribute to the immunopotentiation of the disease process.
Materials and Methods
Purification of recombinant dUTPase proteins
Subcloning and purification of the recombinant herpesviruses’ dUTPase proteins (HHV-6, EBV and VZV), as well as, the human nuclear dUTPase were performed as we have described previously [26–30]. While the HHV-6A U45 gene was cloned from the HHV-6A strain GS, the recombinant protein exhibits greater than 94% identity to the HHV-6B strain Z29 U45 gene. Therefore, it is unlikely that the U45 recombinant protein can distinguish between these strains. The terminology HHV-6 will be employed. All recombinant dUTPase protein preparations were tested for the presence of contaminants as described previously [Glaser et al., 2006; Ariza et al., 2009, 2013, 2014] and were free of detectable levels of LPS, peptidoglycan (SLP-HS), DNA or RNA. Protein concentration was determined using the Qubit fluorimeter (Invitrogen Carlsbad, CA). The purified recombinant dUTPase proteins used in these studies were stored at −80°C until further use.
ELISA assay
ELISAs were performed essentially as described previously [Lai et al., 2012]. Briefly, 96-microtiter well plates (Nunc-Immuno Plate MaxiSorp Surface) were coated overnight at 4 °C with recombinant dUTPase protein at 2.5 μg/ml in phosphate buffered saline (PBS). Plates were washed on a Biotek ELx50 plate washer three times with PBS/0.05 % Tween 20 (200 μl) and blocked with blocking buffer (PBS/2.5 % BSA; 100 μl) at room temperature (RT). All serum samples were used at a 1:800 dilution in blocking buffer and incubated for 2 h at RT in duplicate. Plates were washed three times with PBS/0.05% Tween 20 (200 μl) followed by incubation with anti-human-IgG horseradish peroxidase (HRP)-conjugated secondary antibody (Sigma Chemical Co. St. Louis MO) at 1:1000 dilution in blocking buffer at RT for 1 h. Plates were washed six times with PBS/0.05 % Tween 20 and incubated for 15 minutes with 100 μl of tetramethylbenzidine (Invitrogen, Carlsbad, CA). A volume of 50 μl H2SO4 (2 M) was added to stop the reaction and plates were read at 490 and 690 nm for background on a Lab Systems Multiskan MCC/340 plate reader using the Genesis v3.05 Life Sciences Ltd software. The background from the 490 nm uncoated wells and PBS-BSA (negative controls) were subtracted from the mean absorbance of the coated wells. ROC curves were constructed to determine optimal threshold points for each antigen using serum obtained from a healthy control donor. Intra-assay variation was determined to be less than 0.02 while inter-assay variation ranged from 0.005 for human to 0.049 for VZV. A positive reaction was defined as a serum sample that led to a signal 3-times over the background OD of the control serum (HHV-6: 0.124 ± 0.045; EBV: 0.104 ± 0.025; VZV: 0.256 ± 0.016; human dUTPase: 0.017 ± 0.005). For the studies involving sera from the Good Day Bad Day study, if any of the four samples from a patient were positive for an anti-dUTPase antibody that individual was considered positive for that particular dUTPase.
The recombinant dUTPase-specific ELISAs were validated using dUTPase-specific antibodies to the EBV, HHV-6 dUTPases and the human nuclear dUTPase and no cross-reactivity was observed. Because of the low protein homology among the herpesviruses’ dUTPases (maximum amino acid identity between EBV dUTPase, HHV-6 dUTPase and VZV dUTPase is 24%), it is very unlikely that these dUTPases will bind to each other.
ME/CFS patients
The patients in this study met the Canadian Clinical Case Definition (CCC) [Carruthers et al., 2003] and the Fukuda criteria [Fukuda et al., 1994] and exhibited fatigue, post-exertional malaise, sleep dysfunction and pain; two or more neurological/cognitive manifestations and one or more symptoms from two of the categories of autonomic, neuroendocrine and immune manifestations, as well as illness persisting for at least six months. All study subjects provided written informed consent and the protocol was approved by the Institutional Review Board for Human Subjects at NOVA Southeastern University.
ME/CFS exclusion criteria
Following the recommendations of Reeves et al., 2003, all ME/CFS subjects, selected by their medical history and physical examination, had no history of heart disease, COPD, malignancy, or other systemic disorders including the listed psychiatric exclusions that would be exclusionary for a diagnosis of ME/CFS.
Patients taking medications that have the potential to act as significant immunomodulators were excluded including gamma globulin, steroids and any cytokine or cytokine-blocking therapy. Medications with modest immunologic impact (e.g. anti-depressants) were maintained without change in dosage throughout the study periods.
ME/CFS longitudinal good day bad day study subjects
In 74 subjects with ME/CFS, baseline and 18 month blood samples and clinical data were collected. Two additional blood samples were collected from each subject, one on a day the subject self-described feeling “relatively good” and another one when the subject was having “bad day” (Table 1). There was a minimum of a three-month period between the collection of sera from a good day and a bad day.
Table 1.
Demographics of Study Subjects
| Longitudinal: 4 samples per subject over 18 months | |||
|---|---|---|---|
| ME/CFS GDBD | N | % of Total | |
| Gender | Male | 10 | 14% |
| Female | 64 | 86% | |
| Total | 74 | 100% | |
| ME/CFS GDBD | N | % of Total | |
| Ethnicity | Asian | 1 | 1% |
| Black | 4 | 5% | |
| Hispanic | 14 | 19% | |
| White | 51 | 70% | |
| More than One | 4 | 5% | |
| Total | 74 | 100% | |
| Age | Average | 49.18919 | |
| Median | 49.5 | ||
| Std. Devi | 10.95405 | ||
| Single samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ME/CFS | N | % of Total | GWI | N | % of Total | HC | N | % of Total | |||
| Gender | Male | 20 | 36% | Gender | Male | 50 | 87% | Gender | Male | 79 | 52% |
| Female | 35 | 64% | Female | 8 | 13% | Female | 72 | 48% | |||
| Total | 55 | 100% | Total | 58 | 100% | Total | 151 | 100% | |||
| ME/CFS | N | % of Total | GWI | N | % of Total | HC | N | % of Total | |||
| Ethnicity | Asian | 1 | 2% | Ethnicity | Asian | 2 | 4% | Ethnicity | Asian | 2 | 1% |
| Black | 3 | 5% | Black | 22 | 38% | Black | 23 | 15% | |||
| Hispanic | 19 | 35% | Hispanic | 18 | 31% | Hispanic | 66 | 44% | |||
| White | 32 | 58% | White | 16 | 27% | White | 60 | 40% | |||
| More than One | 0 | 0% | More than One | 0 | 0% | More than One | 0 | 0% | |||
| Total | 55 | 100% | Total | 58 | 100% | Total | 151 | 100% | |||
| Age | Average | 45.42 | Age | Average | 44.09 | Age | Average | 46.876 | |||
| Median | 44 | Median | 44 | Median | 47 | ||||||
| Std. Devi | 8.439 | Std. Devi | 5.977 | Std. Devi | 8.606 | ||||||
ME/CFS single sample study subjects
A single baseline serum sample from 55 ME/CFS subjects in our biobank was selected for testing. There was no overlap between these single sample ME/CFS patients and those in the ME/CFS Good Day Bad Day group. Demographic details of these patients are given in Table 1.
Gulf war illness cohort
Inclusion criteria for GWI was derived from Fukuda et al. [16], and consisted in identifying veterans deployed to the theater of operations between August 8, 1990 and July 31, 1991, with one or more symptoms present after 6 months from at least 2 of the following: fatigue; mood and cognitive complaints; and musculoskeletal complaints. Subjects were in good health prior to 1990 and had no current exclusionary diagnoses. Medications that could have impacted immune function were excluded as were supplements. The use of the Fukuda definition in GWI is supported by Collins et al., 2002. A single baseline serum sample from 45 GWI subjects in our biobank was selected for this study. Demographic details of these patients are given in Table 1.
Healthy controls
Healthy controls were self-defined as healthy, sedentary (no regular exercise program, sedentary employment), and matched to ME/CFS or GWI cases by age (± 5 years), gender, and race/ethnicity (see Table 1).
Herpesvirus serology
The average age of the subjects in this study ranged from 44.09 to 49.5 years and since 70–95% of the adult population has been infected with EBV, HHV-6 and VZV, standard herpesviruses serological assays were not performed.
Statistical analysis
GraphPad Prism 6 software (GraphPad Software, La Jolla, CA) was used for all statistical analyses. For the longitudinal ME/CFS GDBD samples, Pearson’s correlation coefficient was determined. A p value of < 0.05 was used to demonstrate a significant correlation between the expression of the various antibodies within the groups examined. For the GWI study, while only one serum sample was available per patient, the means of the various groups cross-sectional were compared using one-way analysis of variance (ANOVA). Values of p < 0.05 were considered statistically significant. To compare two groups an unpaired t test was employed. Values of p < 0.05 were considered statistically significant.
Results
Evaluation of humoral immune responses to herpesviruses and human dUTPase proteins in ME/CFS good day bad day longitudinal sera
To determine whether there was evidence supporting the hypothesis that herpesviruses-dUTPase proteins were expressed in patients with ME/CFS, we tested longitudinal sera from 74 ME/CFS Good Day-Bad Day (GDBD) patients (Table 1), for antibody responses against EBV, HHV-6 and human nuclear encoded dUTPases by ELISA.
As shown in Table 2, a spectrum of humoral reactivities to the various dUTPases was observed in the ME/CFS GDBD cohort. Of the 74 patients examined, approximately 34% were negative for antibodies to HHV-6, EBV- and the human nuclear encoded dUTPase proteins. While some patients expressed antibodies to only HHV-6 (2.7%) or EBV (5.41%) encoded dUTPases, the majority (48.65%) co-expressed antibodies to both HHV-6 and EBV-encoded dUTPases (Table 2). Furthermore, 55.40% and 54.06% of the total ME/CFS GDBD cohort were positive (single, or double) for HHV-6 and EBV anti-dUTPase antibodies, respectively. While a few patients were positive only for anti-human dUTPase antibodies (6.76%), somewhat surprising was the observation that a significant proportion of the ME/CFS GDBG cohort co-expressed anti-human and anti-HHV-dUTPase antibodies (31.08%) (Table 2).
Table 2.
Grouping of ME/CFS Good Day Bad Day Patients based upon presence of anti-HHV-6, EBV and human dUTPase antibodies*.
| dUTPase Ab Subgroups | N | Percentage (%) of total |
|---|---|---|
| Negative | 24 | 32.43 |
| HHV-6 only | 2 | 2.70 |
| EBV only | 4 | 5.41 |
| Human only | 5 | 6.76 |
| HHV-6 + EBV | 15 | 20.27 |
| HHV-6 + Human | 2 | 2.70 |
| EBV + Human | 1 | 1.35 |
| HHV-6 + EBV + Human | 21 | 28.38 |
Repetitive longitudinal serum samples (n = 4 samples/patient and a total of 74 patients) were examined for anti-HHV-6, EBV and human dUTPase antibodies by ELISA as described in Materials and Methods. Data represent the average of n≥4 measurements/sample for each recombinant protein at each time point (baseline, good day, bad day, end). A positive reaction was defined as a serum sample that led to a signal 3- times over the background OD of the control serum. A patient was considered positive for Abs to a specific dUTPase if a positive reaction occurred at any time point.
There was no direct correlation between the levels of anti-dUTPase antibodies and patients’ self-determination of whether they were having a good day or a bad day. Twenty-four patients, who were negative for anti-dUTPase antibodies, exhibited no significant change in antibody levels during the test period (Fig. 1A patient 029, and 1B patient 109 are examples). Of the fifty patients who exhibited a statistically significant increase (p <0.05) in the level of one or multiple anti-dUTPase antibodies, 6 (12%) patients exhibited no significant fluctuations in the antibody levels during the test period (Fig. 1C patient 022, and 1D patient 023 are examples). Ten patients (20%) exhibited high levels of antibodies at baseline with a decline over the test period (Fig. 1E patient 055), while 7 patients (14%) exhibited the highest level of antibodies at the conclusion of the test period. Twenty-seven patients (54%) exhibited a peak of anti-dUTPase antibody either on the bad day or on the good day and this was followed by an increase of antibodies at the end of the trial (Fig. 1F–H patients 054, 106 and 114, respectively). Interestingly, there was a statistically significant correlation between the presence of anti-HHV-6 and human dUTPase antibodies in two patients and all three anti-dUTPase antibodies in 21 patients (Pearson correlation coefficient, p = <0.05).
Fig. 1. Anti-dUTPase antibody patterns in ME/CFS good day bad day patients.
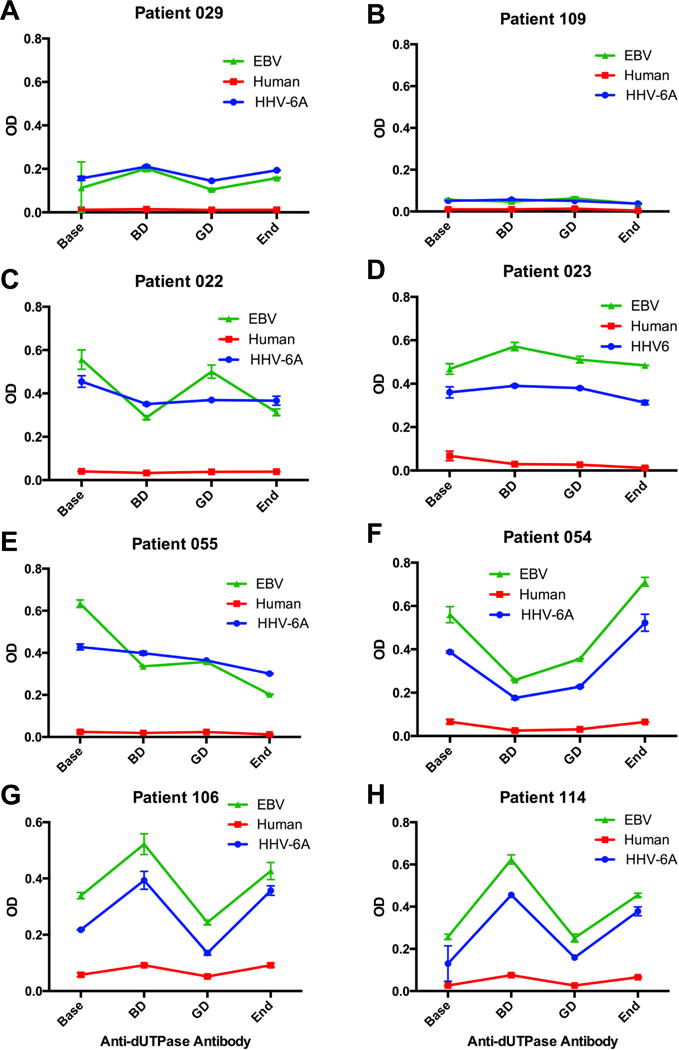
Representative examples of anti-dUTPase antibody patterns observed in longitudinal samples of individual ME/CFS GDBD subjects. (A, B) Patients 029 and 109: negative for dUTPase antibodies. (C) Patient 022: positive for anti-HHV-6 and EBV encoded dUTPase antibodies; (D) Patient 023: positive for anti HHV-6, EBV and human nuclear dUTPase antibodies. (E) Patient 055: positive for anti-HHV-6 and EBV encoded dUTPase antibodies. (F–H) Patients 054, 106 and 114: positive for anti HHV-6, EBV and human nuclear dUTPase antibodies. Levels of anti-dUTPase antibodies were determined by dUTPase protein-specific ELISA, as described in Material and Methods. Data represent the mean ± SD of an n ≥4.
Antibody responses to herpesviruses and human nuclear dUTPase proteins in GWI, ME/CFS and healthy control sera
Because of the overlapping symptoms observed between patients with GWI and ME/CFS [Kipen et al., 1999; McCauley et al., 2002], the higher incidence of ME/CFS in patients with GWI and the lack of information concerning the etiology of GWI, we next examined the potential involvement of herpesviruses in the pathophysiology of GWI. To determine whether there was evidence supporting the hypothesis that herpesviruses-dUTPase proteins were expressed in patients with GWI, the anti-dUTPase antibody profile to HHV-6, EBV and VZV encoded dUTPases as well as the human dUTPase were determined in a single serum sample from 58 patients diagnosed with GWI and compared to the antibody profiles obtained in patients diagnosed with ME/CFS (n = 55) or healthy controls (n = 151). As shown in Table 3, a wide-spectrum of anti-dUTPase antibodies were identified in patients (GWI; ME/CFS).
Table 3.
Grouping of GWI and ME/CFS patients based upon anti-HHV-6, EBV, VZV and Human dUTPase antibodies*.
| dUTPase Ab Subgroup | GWI Cohort N (% of total) |
ME/CFS Cohort N (% of total) |
Control Cohort N (% of total) |
|---|---|---|---|
| Negative | 29 (50) | 25 (45.45) | 87 (57.6) |
| HHV-6 only | 12 (20.69) | 12 (21.82) | 20 (13.25) |
| EBV only | 0 (0) | 0 (0) | 15 (9.93) |
| VZV only | 0 (0) | 0 (0) | 2 (1.32) |
| Human only | 0 (0) | 1 (1.82) | 1 (0.67) |
| HHV-6 + EBV | 3 (5.20) | 10 (18.18) | 13 (8.60) |
| HHV-6 + VZV | 1 (1.72) | 0 (0) | 2 (1.32) |
| HHV-6 + Human | 2 (3.45) | 1 (1.82) | 0 (0) |
| EBV + VZV | 0 (0) | 0 (0) | 1 (0.67) |
| EBV + Human | 0 (0) | 0 (0) | 0 (0) |
| VZV + Human | 0 (0) | 0 (0) | 0 (0) |
| HHV-6 + EBV+ VZV | 2 (3.45) | 0 (0) | 4 (2.65) |
| HHV-6 + EBV + Human | 4 (6.90) | 4 (7.27) | 2 (1.32) |
| EBV + VZV+ Human | 0 (0) | 0 (0) | 0 (0) |
| HHV-6 + EBV + VZV + Human | 5 (8.62) | 2 (3.64) | 4 (2.65) |
| Total | 58 | 55 | 151 |
A single serum sample from each patient was examined for anti-HHV-6, EBV, VZV and human dUTPase antibodies by ELISA as described in Materials and Methods. Data represent the average of n≥4 measurements/sample for each recombinant protein. A positive reaction was defined as a serum sample that led to a signal 3-times over the background OD of the control serum.
As shown in Fig. 2, there were statistically significant differences in the expression of anti-dUTPase antibodies between the GWI, ME/CFS and control cohorts based upon unpaired t test analyses. The GWI cohort exhibited a statistically significant increase in anti-HHV-6 (p = 0.0053; Fig. 2A), anti-VZV (p = 0.032; Fig. 2B) and anti-human (p = 0.0036; Fig. 3C) dUTPase antibodies when compared to controls while the ME/CFS cohort exhibited a statistically significant increase in anti-EBV (p = 0.0001; Fig. 2D) and anti-human (p = 0.0241; Fig. 2C) dUTPase antibodies when compared to controls. Interestingly, the only statistically significant difference between the GWI and ME/CFS cohorts was an elevation in anti-EBV dUTPase antibodies in the ME/CFS cohort (p = 0.0008; Fig. 2D) and an elevation of anti-VZV dUTPase antibodies in the GWI cohort (p = 0.0441; Fig. 2B).
Fig. 2. Composite of the antibody response to the dUTPase encoded by EBV, HHV-6, VZV and human dUTPase in GWI and ME/CFS cohorts.
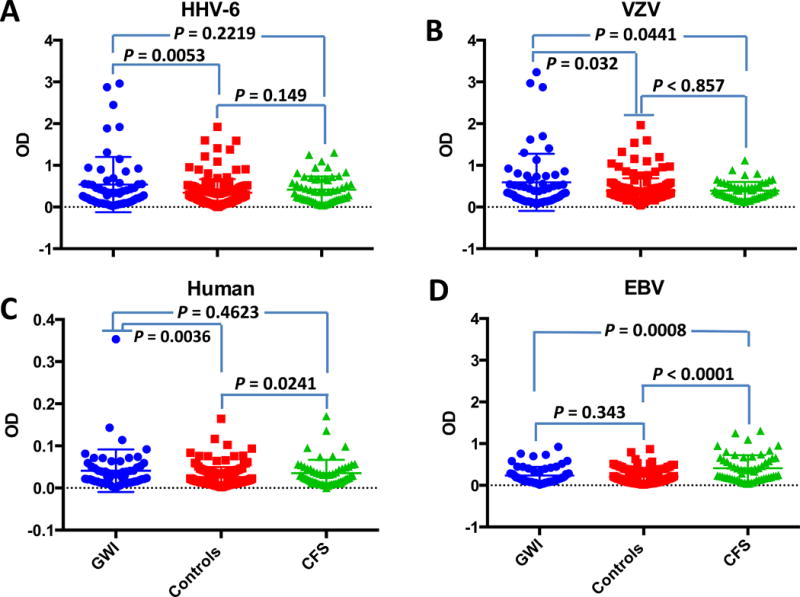
Graphs represent a composite of the anti-dUTPase antibody response of 58 GWI patients, 55 ME/CFS patients and 151 controls against (A) HHV-6 dUTPase, (B) EBV-dUTPase, (C) human nuclear dUTPase and (D) VZV dUTPase. The data represent the mean ± SD of an n ≥4. A p value < 0.05 was considered significant.
Fig. 3. Proposed mechanism(s) by which HHV-6, EBV and VZV reactivation can drive ME/CFS and GWI symptomology.
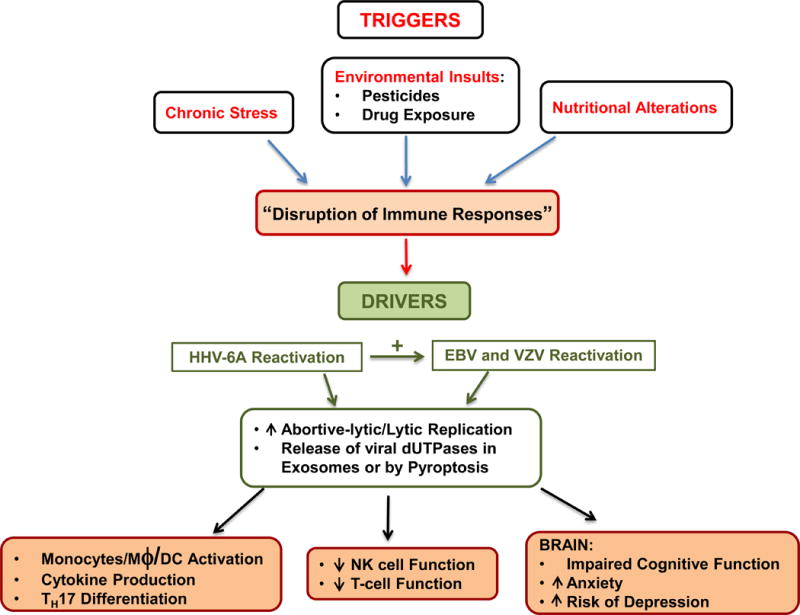
Abortive/lytic reactivation of HHV-6, EBV and/or VZV results in the expression of the early gene product dUTPase and subsequent release either through pyroptosis (localized effect) or in exosomes (systemic effect). Binding of virus dUTPase to TLR2 on target cells (DCs, PBMCs, NK, T-cells) leads to NF-κB activation, followed by induction/secretion of pro-inflammatory cytokines and the up-regulation of genes involved in inflammation, TH17 differentiation, modulation of neurotransmitters and genes involved with brain function.
Collectively, these results demonstrate a significant increase in IgG antibodies to EBV, HHV-6, VZV and human nuclear dUTPases suggesting that certain epitopes in the herpesviruses and human nuclear dUTPase proteins may be eliciting an immunological response in some patients with ME/CFS and/or GWI.
Discussion
ME/CFS and GWI are chronic multisystem illnesses with overlapping symptoms including fatigue, neurocognitive dysfunctions and immune activation (elevation of inflammatory markers and elevated cytokine levels). This is further complicated by the fact that GWI patients have an increased prevalence of chronic fatigue compared to the general population [Kipen et al., 1999; McCauley et al. 2002]. Furthermore, there are currently no biomarkers available that are clinically useful for differential diagnoses. Attempts to identify the etiological agents/triggers responsible for these multisystem illnesses have not been successful. Likewise, while numerous studies have demonstrated immune dysfunction in these patients including alterations in cytokine patterns and decreased NK cell function, the factors that act as drivers, which power and maintain these multisystem illnesses are unknown.
Our study demonstrates that a subgroup of patients diagnosed with either ME/CFS or GWI exhibited a statistically significant elevation in antibodies against the HHV-6, EBV and VZV encoded dUTPases, proteins that are only expressed during lytic or abortive lytic replication of these viruses, as well as the human nuclear dUTPase. These results suggest that in this subgroup of patients there is reactivation of multiple herpesviruses; a feature that is also observed in some transplant recipients [Shiley et al., 2010; Inazawa et al., 2015] and patients with drug-induced hypersensitivity syndrome (DISS/DRESS) [Shiohara et al., 2006; Picard et al., 2010; Chen et al., 2015]. Since GWI patients were exposed to a variety of chemical agents and environmental toxins a DIHS/DRESS-like reaction could contribute to the reactivation of herpesviruses, especially HHV-6 in these patients. While the mechanism(s) responsible for the simultaneous reactivation of these herpesviruses is unknown, it suggests a loss of immunological control primarily, at the CD8+ T-cell level. This is supported in part by a study demonstrating deficient EBV-specific T-cell responses in some ME/CFS patients [Loebel et al., 2014].
Our study demonstrates for the first time, the presence of autoantibodies against the human dUTPase in a subgroup of patients with ME/CFS and GWI. In our study 39.10% of the patients in the GDBD cohort, 14.55% and 18.6% of the ME/CFS and GWI patients, respectively possessed antibodies either alone or in combination with the anti-herpesviruses’ dUTPases when compared to the controls (4.64%). It is unlikely that the observed humoral response to the human dUTPase is due to cross-reactivity with the herpesviruses-encoded dUTPases since there is little homology between the homotrimeric human dUTPase and the monomeric herpesviruses dUTPases. Further antibody characterization studies using polyclonal antibodies against the EBV-encoded dUTPase revealed no reaction with the human dUTPase (data not shown). The human nuclear dUTPase, which is released from B cells in exosomes [Buschow et al., 2010], was identified as an autoantigen and potential biomarker for autoimmune hepatitis [Song et al., 2010]. Previous studies have demonstrated the presence of autoantibodies in patients with ME/CFS and in Gulf War veterans against numerous cellular components including nuclear envelope [Konstantinov et al., 1996], muscarinic cholinergic and β-adrenergic receptors [Yamamoto et al., 2012; Loebel et al., 2016] anchorage molecules [Maes et al., 2012], DNA [De Beéck et al, 2012], myelin basic protein and smooth and striated muscle tissue [Vojdani and Thrasher, 2004]. While additional studies are needed to determine the role, if any, of these autoantibodies in the disease process, our study suggest that antibodies against human, EBV, HHV-6 and VZV dUTPases may be useful as biomarkers to differentiate between patients with ME/CFS and GWI.
Numerous studies have suggested a potential role for HHV-6 and/or EBV as triggers for ME/CFS [Ablashi et al., 2000; Chapenko et al., 2012; Loebel et al., 2014; Cameron et al., 2010] and limited studies have suggested the involvement of VZV in ME/CFS [Shapiro, 2009; Tsai et al., 2014]. Conversely, there has only been a single study on GWI patients and herpesviruses suggesting that there was reactivation of multiple herpesviruses (HHV-6, EBV and VZV) in these patients [Vojdani and Thrasher, 2004]. Our studies on ME/CFS are unique in that they focused on determining whether there was an increase in antibodies against a class of dUTPase proteins that have been shown to regulate immune and neurocognitive functions and thus, have the ability/potential to drive/exacerbate the pathophysiological alterations that occur in the course of ME/CFS. Furthermore, we wanted to determine whether a similar antibody profile is observed in patients with GWI, who have a higher prevalence of ME/CFS and who exhibit similar symptomology. The data from this study support the hypothesis that in some patients with ME/CFS and/or GWI there is a complex interaction between various herpesviruses resulting in increased expression of the virus-encoded dUTPases, which in turn drives the disease process. Our working model (Fig. 3), which is a modification of the latent viral immune inflammatory response (LVIIR) model for multisystem illnesses including GWI and ME/CFS [Maloney et al., 2013], shows how disruption of normal immune homeostasis allows for the increased abortive reactivation of the herpesviruses and release of virus-encoded dUTPases by pyroptosis or in exosomes. While the presence of antibodies to multiple herpesviruses dUTPases as well as the human dUTPase might be useful as potential biomarkers, these antibodies might not be protective unless they blocked the interaction of the dUTPase with TLR2. This may explain the lack of correlation between the presence of antibody and disease symptomology. Additional studies are required to address this possibility as well as to determine whether there is a relationship between disease severity and expression of multiple herpesviruses’ encoded dUTPases. This hypothesis, however, is supported by clinical studies demonstrating improvement of symptomology in a subset of patients following long-term therapy with valganciclovir, a potent inhibitor of herpesvirus replication, and relapse upon discontinuation of treatment [Kogelnik et al., 2006; Montoya et al., 2009; 2012 Lerner and Beqaj, 2011; Watt et al., 2012]. Similar studies have not been performed with GWI patients, although long-term valcyclovir treatment of military personnel with chronic pain associated with atypical herpesvirus reactivation improved symptoms [Maloney et al., 2009].
In summary, we have shown that some ME/CFS and GWI patients exhibit a statistically significant increase in IgG antibodies to EBV, HHV-6, VZV and human nuclear dUTPases relative to controls suggesting that the dUTPases encoded by these herpesviruses are expressed at sufficient quantities in these patients to trigger a humoral immune response. This study provides further evidence that the herpesviruses-encoded dUTPases may be involved in the pathophysiology in a subset of patients with ME/CFS and GWI. The data from this study also support the hypothesis that in some patients with ME/CFS and/or GWI there is a complex interaction between various herpesviruses resulting in increased expression of the virus-encoded dUTPases and warrants further studies to address whether the presence of various combinations of antibodies to the herpesviruses encoded dUTPases could be used as potential biomarkers with clinical application for directing the use of specific long-term antiviral chemotherapy. (3484 words max 4000)
Acknowledgments
This work was supported by grants from the National Institutes of Health: R01 A1084898 to MVW and MEA. R01 NS090200; 2R56AI065723 and R21 AI099809 to MAF; R01 AR057853 to NGK; Department of Defense GW08015 to NGK. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Authors’ contributions
MEA and MVW conceived and designed the study, performed data analysis, drafted the manuscript and revised it. MAF, NGK participated in study design, collected samples, helped draft the manuscript and revised it. PH performed the experiments, participated in data analysis and preparation of the manuscript. ZB collected samples and participated in the preparation of the manuscript. All authors have read and approved the final manuscript.
Ethical information
All study subjects provided written informed consent and the protocol was approved by the Institutional Review Board for Human Subjects at NOVA Southeastern University.
Competing interests: The authors declare they have no competing interests.
References
- Ablashi DV, Eastman HB, Owen CB, Roman MM, Friedman J, Zabriskie JB, Peterson DL, Whitman JE. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J Clin Virol. 2000;16:179–191. doi: 10.1016/s1386-6532(99)00079-7. [DOI] [PubMed] [Google Scholar]
- Ariza ME, Glaser R, Kaumaya PTP, Jones C, Williams M. The Epstein-Barr Virus (EBV)-encoded dUTPase activates NF-κB through the TLR2 and MyD88-dependent signaling Pathway. J Immunol. 2009;182:851–859. doi: 10.4049/jimmunol.182.2.851. [DOI] [PubMed] [Google Scholar]
- Ariza ME, Rivailler P, Glaser R, Chen M, Williams MV. Epstein-Barr virus encoded dUTPase containing exosomes modulate innate and adaptive immune responses in human dendritic cells and peripheral blood mononuclear cells. PLoS ONE. 2013;8(7):e69827. doi: 10.1371/journal.pone.0069827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza ME, Glaser R, Williams MV. Human herpesviruses encoded dUTPases: A family of proteins that modulate dendritic cells function and innate immunity. Frontiers in Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00504. Article 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubrecht TG, Weil ZM, Ariza ME, Williams M, Glaser R, Sheridan J, Nelson RJ. Epstein-Barr virus (EBV)-encoded dUTPase and chronic restraint induce impaired learning and memory and sickness responses. Physiology & Behavior. 2014;137:18–24. doi: 10.1016/j.physbeh.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenu EW, van Driel ML, Staines DR, Ashton KL, Hardcastle SL, Keane J, Tajouri L, Peterson D, Ramos SB, Marshall-Gradisnik SM. Longitudinal investigation of natural killer cells and cytokines in chronic fatigue syndrome/myalgic encephalomyelitis. J Translat Med. 2012;10:88. doi: 10.1186/1479-5876-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschow SI, van Balkom BWM, Alberts M, Heck AJR, Wauben M, Stoorvogel M. MHC class-II associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol Cell Biol. 2010;88:851–856. doi: 10.1038/icb.2010.64. [DOI] [PubMed] [Google Scholar]
- Cameron B, Flamand L, Juwana H, Middeldorp J, Naing Z, Rawlinson W, Ablashi D, Lloyd A. Serological and virological investigation of the role of the herpesviruses EBV, CMV and HHV-6 in post-infective fatigue syndrome. J Med Virol. 2010;82:1684–1688. doi: 10.1002/jmv.21873. [DOI] [PubMed] [Google Scholar]
- Carruthers BM, Jain AK, De Meirleir DL, Peterson DL, Klimas NG, Lerner AM, Bested AC, Flor-Henry P, Joshi P, Powles ACP, Sherkey JA, van de Sande MI. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J CFS. 2003;11(1):7–115. [Google Scholar]
- Chapenko S, Krumina A, Logina I, Rasa S, Chistjakovs M, Sultanova A, Viksna L, Murovaka M. Association of active human herpesvirus-6, -7 and parvovirus B19 infection with clinical outcomes in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Adv Virol. 2012;2012 doi: 10.1155/2012/205085. Article ID 205085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YC, Chiang HH, Cho YT, Chang CY, Chen KL, Yang CW, Lee YH, Chu CY. Human herpes virus reactivations and dynamic cytokine profiles in patients with cutaneous adverse drug reactions – a prospective comparative study. Allergy. 2015;70:568–575. doi: 10.1111/all.12602. [DOI] [PubMed] [Google Scholar]
- Collins JF, Donta ST, Engel CC, Baseman JB, Dever LL, Taylor T, Boardman KD, Martin SE, Wiseman AL, Feussner JR. The antibiotic treatment trial of Gulf War Veterans’ Illnesses: issues, design, screening, and baseline characteristics. Control Clin Trials. 2002;23(3):333–353. doi: 10.1016/s0197-2456(02)00192-7. [DOI] [PubMed] [Google Scholar]
- De Beéck KO, Vermeersch P, Verschueren P, Westhovens R, Mariën G, Blockmans DD, Bossuyt X. Antinuclear antibody detection by automated multiplex immunoassay in untreated patients at the time of diagnosis. Autoimmunity Rev. 2012;12:137–143. doi: 10.1016/j.autrev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Eisen SA, Kang HK, Murphy FM, Blanchard BS, Reda DJ, Henderson WG, Toomey R, Jackson LW, Alpern R, Parks BJ, Klimas N, Hall C, Pak HS, Hunter J, Karlinsky J, Battistone MJ, Lyons MJ. Gulf War Study Participating Investigators, Gulf War veterans’ health: medical evaluation of a US cohort. Ann Intern Med. 2005;142:881–890. doi: 10.7326/0003-4819-142-11-200506070-00005. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med. 1994;121:953–940. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Nisenbaum R, Stewart G, Thompson WW, Robin L, Washko RM, Noah DL, Barrett DH, Randall B, Herwaldt BL, Mawle AC, Reeves WC. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. J Am Medical Society. 1998;280:981–998. doi: 10.1001/jama.280.11.981. [DOI] [PubMed] [Google Scholar]
- Glaser R, Litsky ML, Padget DA, Baiocchi RA, Yang EV, Chen M, Yeh P-E, Green-Church KB, Caligiuri MA, Williams MV. The EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology. 2006;346:205–218. doi: 10.1016/j.virol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Inazawa N, Hori T, Hatakeyama N, Yamamoto M, Yoto Y, Nojima M, Suzuki N, Shimizu N, Tsutaumi H. Large-scale multiplex polymerase chain reaction assay for diagnosis of viral reactivations after allogeneic hematopoietic stem cell transplantation. J Med Virol. 2015;87:1427–1435. doi: 10.1002/jmv.24161. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine of the National Academies. Gulf War and Health: Treatment for a Chronic Multisystem Illness. The National Academies Press; Washington DC: 2013. [Google Scholar]
- Institute of Medicine of the National Academies. Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an illness. The National Academies Press; Washington DC: 2015. [Google Scholar]
- Kipen HM, Hallman W, Kang H, Fiedler N, Natelson BH. Prevalence of chronic fatigue and chemical sensitivities in Gulf registry veterans. Arch Environ Health. 1999;54:313–317. doi: 10.1080/00039899909602493. [DOI] [PubMed] [Google Scholar]
- Klimas NG, Ironson G, Carter A, Balbin E, Bateman L, Felsenstein D, Levine S, Peterson D, Chiu K, Allen A, Cunningham K, Gottschalk CG, Fletcher M, Horning M, Canning C, Komaroff AL. Findings from a clinical and laboratory database developed for discovery of pathogenic mechanisms in myalgic encephalomyelitis/chronic fatigue syndrome. Fatigue: Biomedicine, Health & Discovery. 2015;3:75–96. [Google Scholar]
- Kogelnik AM, Loomis K, Hoegh-Petersen M, Rossoa F, Hischier C, Montoya JG. Use of valganciclovir in patients with elevated antibody titers against human herpesvirus-6 (HHV-6) and Epstein−Barr virus (EBV) who were experiencing central nervous system dysfunction including long-standing fatigue. J Clin Virol. 2006;37(Suppl 1):S33–S38. doi: 10.1016/S1386-6532(06)70009-9. [DOI] [PubMed] [Google Scholar]
- Konstantinov K, von Mikecz A, Buchwald D, Jones JL, Gerace L, Tan EM. Autoantibodies to nuclear envelope antigens in chronic fatigue syndrome. J Clin Invest. 1996;98:1888–1896. doi: 10.1172/JCI118990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff AL. Is human herpesvirus-6 a trigger for chronic fatigue syndrome? J Clin Virol. 2006;37(Suppl 1):S39–S36. doi: 10.1016/S1386-6532(06)70010-5. [DOI] [PubMed] [Google Scholar]
- Lai OY, Chen H, Michaud HA, Hayashi G, Kuebler PJ, Hultman GK, Ariza ME, Williams MV, Batista MD, Nixon DF, Foerster J, Bowcock AM, Liao W. Protective effect of human endogenous retrovirus K dUTPase variants on psoriasis susceptibility. J Invest Dermatol. 2012;132:1833–1840. doi: 10.1038/jid.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AM, Ariza ME, Williams M, Jason L, Beqaj S, Fitzgerald JT, Lemeshow S, Glaser R. Antibody to Epstein-Barr Virus deoxyuridine triphosphate nucleotidohydrolase and deoxyribonucleotide polymerase in a chronic fatigue syndrome subset. PLoS ONE. 2012;7(11):e47891. doi: 10.1371/journal.pone.0047891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner AM, Beqaj S. A paradigm linking herpesvirus immediate-early gene expression apoptosis and myalgic encephalomyelitis chronic fatigue syndrome. Virus Adaptation Treatment. 2011;3:19–24. [Google Scholar]
- Loebel M, Strohschein K, Giannini C, Koelsch U, Bauer S, Doebis C, Thomas S, Unterwalder N, von Baehr V, Reinke P, Knops M, Hanitsch LG, Meisel C, Volk HD, Scheibenbogen C. Deficient EBV-specific B-and T-cell response in patients with chronic fatigue syndrome. PLoS ONE. 2014;9(1):e85387. doi: 10.1371/journal.pone.0085387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loebel M, Grabowski P, Heidecke H, Bauer S, Hanitsch LG, Wittke K, Meisel C, Reinke P, Volk HD, Fuge O, Mella O, Scheibenbogen C. Antibodies to β adrenergic and muscarinic cholinergic receptors in patients with Chronic Fatigue Syndrome. Brain Behavior Immunity. 2016;52:32–39. doi: 10.1016/j.bbi.2015.09.013. [DOI] [PubMed] [Google Scholar]
- Maes M, Mihaylova I, Kubera M, Leunis JC, Twisk FNM, Geffard M. IgM-mediated autoimmune responses directed against anchorage epitopes are greater in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) than in major depression. Metab Brain Dis. 2012;27:415–423. doi: 10.1007/s11011-012-9316-8. [DOI] [PubMed] [Google Scholar]
- Maloney SR, Jensen S, Gil-Rivas V, Goolkasian P. Latent viral immune inflammatory response model for chronic multisystem illness. Med Hypotheses. 2013;80:220–229. doi: 10.1016/j.mehy.2012.11.024. [DOI] [PubMed] [Google Scholar]
- Maloney S, Taber K, Jensen S, Gil-Rivas V, Goolkasian P, Mancil R, Buckler J. Chronic pain and atypical herpatic viral reactivation. Pract Pain Manage. 2009 Mar;:38–41. [Google Scholar]
- McCauley LA, Joos SK, Barkhuizen A, Shuell T, Tyree WA, Bourdette DN. Chronic fatigue in a population-based study of Gulf War veterans. Arch Environ Health. 2002;57:340–348. doi: 10.1080/00039890209601419. [DOI] [PubMed] [Google Scholar]
- Montoya JG, Kogelnik AM, Bhangoo M, Lunn MR, Flamand L, Merrihew LE, Watt T, Kubo JT, Paik J, Desai M. Randomized clinical trial to evaluate the efficacy and safety of valganciclovir in a subset of patient with chronic fatigue syndrome. J Med Virol. 2009;85:2101–2109. doi: 10.1002/jmv.23713. [DOI] [PubMed] [Google Scholar]
- Montoya JG, Neely MN, Gupta S, Lunn MR, Loomis KS, Prichett JC, Polsky B, Medveczky PG. Antiviral therapy of two patients with chromosomally-integrated human herpesvirus-6A presenting with cognitive dysfunction. J Clin Virol. 2012;55:40–45. doi: 10.1016/j.jcv.2012.05.016. [DOI] [PubMed] [Google Scholar]
- Padgett DA, Hotchkiss AK, Pyter LM, Nelson RJ, Yang E, Yeh P, Litsky M, Williams M, Glaser R. Epstein-Barr virus-encoded dUTPase modulates immune function and induces sickness behavior in mice. J Med Virol. 2004;74:442–448. doi: 10.1002/jmv.20196. [DOI] [PubMed] [Google Scholar]
- Parkitny L, Middleton S, Baker K, Younger J. Evidence for abnormal cytokine expression in Gulf War Illness: A preliminary analysis of daily immune monitoring data. BMC Immunology. 2015;16:57. doi: 10.1186/s12865-015-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D, Janela B, Descamps V, D’Incan M, Courville P, Jacquot S, Rogez S, Mardivirin L, Moins-Teisserenc H, Toubert A, Benichou J, Joly P, Musette P. Drug reaction with eosinophilia and systemic symptoms (DRESS): A multiorgan antiviral T cell response. Sci Transl Med. 2010;2(46):46ra62. doi: 10.1126/scitranslmed.3001116. [DOI] [PubMed] [Google Scholar]
- Reeves WC, Lloyd A, Vernon SD, Nancy Klimas K, Jason LA, Bleijenberg G, Evengard B, White PD, Nisenbaum R, Unger ER. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Services Research. 2003;3:25. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro JS. Does varicella-zoster virus infection of the peripheral ganglia cause chronic fatigue syndrome? Med Hypotheses. 2009;73:728–734. doi: 10.1016/j.mehy.2009.04.043. [DOI] [PubMed] [Google Scholar]
- Shiley KE, Blumberg E. Herpes Viruses in transplant recipients: HSV, VZV, Human HerpesViruses, and EBV. Infect Dis Clin N Am. 2010;24:373–393. doi: 10.1016/j.idc.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Shiohara T, Inaoka M, Kano Y. Drug-induced HypersensitivitySyndrome (DIHS): A reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergology Int. 2006;55:1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- Song Q, Liu G, Hu S, Zhang Y, Tao Y, Han Y, Zeng H, Huang W, Li F, Chen P, Zhu J, Hu C, Zhang S, Li Y, Wu L. Novel autoimmune hepatitis-specific autoantigens identified using protein microarray technology. J Proteome Res. 2010;9:30–39. doi: 10.1021/pr900131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L, Sastre A, Gerkovich MM, Cook MR. Complex factors in the etiology of Gulf War illness: wartime exposures and risk factors in veteran subgroups. Environ Health Perspect. 2012;120:112–118. doi: 10.1289/ehp.1003399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SY, Yang TY, Chen HJ, Chen CS, Lin WN, Shen WC, Kuo CN, Kao CH. Increased risk of chronic fatigue syndrome following herpes zoster: a population-based study. Eur J Clin Microbiol Infect Dis. 2014;33:1653–1659. doi: 10.1007/s10096-014-2095-x. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Thrasher JD. Cellular and humoral immune abnormalities in Gulf War veterans. Environ Health Perspect. 2004;112:840–846. doi: 10.1289/ehp.6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt T, Oberfoell S, Balise R, Lunn MR, Kar AK, Merrihew L, Bhangoo MS, Montoya JG. Response to valganciclovir in chronic fatigue syndrome patients with human herpesvirus 6 and Epstein–Barr virus IgG antibody titers. J Med Virol. 2012;84:1967–1974. doi: 10.1002/jmv.23411. [DOI] [PubMed] [Google Scholar]
- Whistler T, Fletcher MA, Lonergan W, Zeng XR, Laperrier A, Lin JM, Laperriere A, Vernon SD, Klimas NG. Impaired immune function in Gulf War Illness. BMC Medical Genomics. 2009;2:12. doi: 10.1186/1755-8794-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Ouchi Y, Nakatsuka D, Tahara T, Mizuno K, Tajima S, Onoe H, Yoshikawa E, Tsukada H, Iwase M, Yamaguti K, Kuratsune H, Watanabe Y. Reduction of [11C](+)3-MPB binding in brain of chronic fatigue syndrome with serum autoantibody against muscarinic cholinergic receptor. PLoS ONE. 2012;7(12):e51515. doi: 10.1371/journal.pone.0051515. doi:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]


