Abstract
Perineuronal nets (PNN) are aggregations of chondroitin sulfate proteoglycans surrounding the soma and proximal processes of neurons, mostly GABAergic interneurons expressing parvalbumin. They limit the plasticity of their afferent synaptic connections. In zebra finches PNN develop in an experience-dependent manner in the song control nuclei HVC and RA (nucleus robustus arcopallialis) when young birds crystallize their song. Because songbird species that are open-ended learners tend to recapitulate each year the different phases of song learning until their song crystallizes at the beginning of the breeding season, we tested whether seasonal changes in PNN expression would be found in the song control nuclei of a seasonally breeding species such as the European starling. Only minimal changes in PNN densities and total number of cells surrounded by PNN were detected. However, comparison of the density of PNN and of PNN surrounding parvalbumin-positive cells revealed that these structures are far less numerous in starlings that show extensive adult vocal plasticity, including learning of new songs throughout the year, than in the closed-ended learner zebra finches. Canaries that also display some vocal plasticity across season but were never formally shown to learn new songs in adulthood were intermediate in this respect. Together these data suggest that establishment of PNN around parvalbumin-positive neurons in song control nuclei has diverged during evolution to control the different learning capacities observed in songbird species. This differential expression of PNN in different songbird species could represent a key cellular mechanism mediating species variation between closed-ended and open-ended learning strategies.
Keywords: Perineuronal nets, European starling, zebra finch, canary, song control nuclei, parvalbumin, song plasticity
INTRODUCTION
In songbirds, also known as oscines, the learning and production of songs rely on specific brain regions known as the song control system that are analogous to human brain regions involved in language learning and production (Brainard & Doupe, 2002; Jarvis, 2004). Two pathways connect the four main forebrain song control nuclei. The motor pathway directly connects HVC (proper name, formerly known as high vocal center) to the premotor nucleus RA (the robust nucleus of the arcopallium) and is involved in song production (Nottebohm et al., 1976; Nottebohm, 2004; 2005). The anterior forebrain pathway connects these two nuclei indirectly through Area X of the basal ganglia and LMAN (the lateral magnocellular nucleus of the anterior nidopallium) that control song learning especially via the regulation of auditory feedback effects and the generation of song variability (Bottjer et al., 1984; Scharff & Nottebohm, 1991; Kao et al., 2005; Olveczky et al., 2005).
Songbirds learn their species-specific song during ontogeny through three successive phases called subsong, plastic song, and finally song crystallization when song becomes fully structured and stable (Marler, 1990; Williams, 2004). This stability of adult song varies between species (Beecher & Brenowitz, 2005; Brenowitz & Beecher, 2005). The so-called closed-ended (Beecher & Brenowitz, 2005) sometimes called age-limited learners (Marler, 1987b) such as zebra finches (Taeniopygia guttata) do not modify their song at all during adulthood, after it crystallizes at 80–90 days post-hatch (Böhner, 1990). In contrast, open-ended learners modify their songs and/or the syllables they sing during adulthood across the annual cycle (Brainard & Doupe, 2002; Beecher & Brenowitz, 2005). There are however substantial differences in this adult vocal plasticity within these open-ended songbird species (Williams, 2004; Beecher & Brenowitz, 2005; Brenowitz & Beecher, 2005). Some only change the amount of song they produced during the breeding season (Leitner et al., 2001a; Phillmore et al., 2006) while others also modify their repertoire size and composition each year (Riters et al., 2000b) (for review see (Brenowitz & Beecher, 2005; Brenowitz, 2008)). Among species of this sort, some such as white-crowned sparrow modify their repertoire each year but the new syllables added to the repertoire are memorized during a sensitive period for learning during early ontogeny (Hough et al., 2000). In contrast, European starlings (Sturnus vulgaris) are clearly able to incorporate each year into their repertoire new syllables that they have heard only in adulthood (Chaiken et al., 1994) and they have been reported to learn new song elements during all phases of the annual cycle including during the mid to late summer when they are photorefractory (Böhner et al., 1990; Chaiken et al., 1994). Their song repertoire therefore increases with age (Eens et al., 1992; Bernard et al., 1996) and this increase in the singing repertoire is modulated by seasons and the associated changes in circulating testosterone (Van Hout et al., 2009; Van Hout et al., 2012). They are thus true open-ended learners. Domesticated canaries (Serinus canaria) also display seasonal variations in song rate and they modify their song repertoire annually (Nottebohm et al., 1986; Leitner et al., 2001a; Voigt & Leitner, 2008). However we still do not know definitely whether this change in repertoire reflects the memorization of new syllable during adulthood or the re-expression of syllables that had been heard during ontogenesis but were never produced as shown in white-crowned sparrows (Hough et al., 2000).
In many temperate zone species, the seasonal changes in song rate, quality and repertoire are correlated with morphological changes in the song control regions (Nottebohm, 1981; Tramontin & Brenowitz, 2000; Brenowitz, 2008). For example, the volume of HVC, RA, and Area X increases seasonally in males at the onset of the reproductive period during the spring. These seasonal changes in volume reflect increases in neuronal size and spacing and in the case of HVC, changes in neuron numbers resulting largely from a positive effect of testosterone on the recruitment and survival of new RA-projecting neurons (Kirn & Nottebohm, 1993; Alvarez-Buylla & Kirn, 1997; Tramontin & Brenowitz, 2000; Brenowitz, 2004) even if singing activity (Li et al., 2000; Alvarez-Borda & Nottebohm, 2002; Alward et al., 2013; Alward et al., 2016) and social conditions (Balthazart et al., 2008) also seem to modulate this process (see (Brenowitz & Larson, 2015; Balthazart & Ball, 2016) for recent reviews). Many other dimensions of seasonal plasticity are also revealed when one examines other aspects of the song control system such as the density of the connections between different nuclei (Kirn & Nottebohm, 1993; De Groof et al., 2008).
Other forms of plasticity also exist in the brain. Perineuronal nets (PNN) are aggregations of chondroitin sulfate proteoglycans, hyaluronic acid and tenascin R surrounding the soma of some neurons and the proximal part of their processes (Deepa et al., 2006) and seem to be implicated in brain plasticity (Hensch, 2005a; b). PNN are mainly found around GABAergic interneurons expressing parvalbumin or calbindin, two calcium binding proteins (Hartig et al., 1995; Morris & Henderson, 2000). They restrict synaptic plasticity around these neurons and prevent dendritic growth (Karetko & Skangiel-Kramska, 2009). They also regulate ion exchanges through Kv3.1b potassium channel units (Hartig et al., 1999) and limit GABAergic neurons proliferation (Xu et al., 2014). In mammals the development of PNN in sensory regions is experience-dependent and their full development usually coincides with the end of the sensitive period for sensory learning in the relevant brain regions while their pharmacological removal restore brain and behavioral plasticity (Pizzorusso et al., 2002; Pizzorusso et al., 2006; Galtrey & Fawcett, 2007; Karetko & Skangiel-Kramska, 2009; Wang & Fawcett, 2012; Slaker et al., 2015)
Multiple lines of evidence suggest that PNN could be part of the seasonal plasticity of the brain. Indeed in juvenile zebra finches, PNN expression in HVC and RA increases in males at the end of the sensitive period for learning around 90 days post hatch (dph) but this increase is delayed if exposure of the juvenile males to a tutor is prevented, a manipulation that delays in parallel song crystallization (Balmer et al., 2009). Evidence was additionally presented indicating that the degradation of PNN induced by chondroitinase (ChABC) injections in the HVC of adult males allows birds to modify their song when exposed to a female while such modifications are never observed in adult zebra finches (Best et al., 2012). In addition, the percentage of neurons and particularly of parvalbumin-positive interneurons surrounded by PNN is significantly higher in male than in female HVC and RA, a difference possibly related to the fact that only males sing in this species (Meyer et al., 2014; Cornez et al., 2015). PNN could thus be responsible, at least in part, for the closing the sensitive period for song learning in zebra finches.
We thus wondered whether PNN could also play a role in adult brain plasticity associated with song modifications that occur during adulthood in some seasonal songbird species. We tested this idea in male European starlings (Sturnus vulgaris) a species that is known to be exquisitely sensitive to seasonal variation in the photoperiod (Dawson et al., 2001; Riters et al. 2002) and shows in response to photoperiodic manipulations major changes in the volume of song control nuclei (Bernard and Ball, 1995; Eens, 1997; Riters et al., 2002), in singing rate (Feare, 1984; Eens, 1997) and in the social conditions in which song is expressed (Eens, 1997; Riters et al., 2000). However, as mentioned before, male starlings modify (increase) their repertoire during their entire life (Eens et al., 1992; Bernard et al., 1996) and learn new song elements across the entire annual cycle (Böhner et al., 1990; Chaiken et al., 1994). Quantifying PNN expression across states typical of the different stages of the annual cycle thus allowed us to test whether seasonal brain plasticity includes changes in PNN expression and to provide initial indications on the specific aspects of song production or learning that relate to this form of neural plasticity. The ontogenetic studies in zebra finches described before (Balmer et al., 2009) did not indeed establish such specific links with song plasticity versus singing rate or song quality.
Three groups of male starlings were exposed to different photoperiodic conditions that placed them in the different endocrine states similar to those encountered by starlings across the annual cycle and PNN were then quantified in the four prominent forebrain song control nuclei and in three auditory regions. Because little variation in PNN expression was detected, we asked whether this related to the extremely high vocal plasticity of this species. Comparison with the less plastic canaries (Serinus canaria, a seasonal open-ended learner) and with zebra finches (Taeniopygia guttata, a closed-ended learner) supported this hypothesis by demonstrating a clear correlation between the density of PNN in song control nuclei and the degree of vocal plasticity. Therefore we propose that differential expression of PNN might represent a component of the cellular mechanisms controlling differences in vocal plasticity among songbird species that exhibit either the closed-ended and open-ended pattern of song learning.
MATERIAL AND METHODS
Two separate experiments were performed with methods for brain analysis that are identical. We shall therefore present in a first step the specific in vivo procedures experienced by the birds before brain collection and then in a second step the ex vivo procedures used to analyze the brains.
In vivo procedures for experiment 1: Effects of photoperiodic conditions in starlings
Male European starlings (n=24) were wild caught during the winter and placed in collective cages in short days photoperiodic condition (8L:16D) in the Ball Laboratory at Johns Hopkins University, Baltimore MD. Birds were randomly assigned to one of three experimental groups. One group stayed for 12 weeks in short days (8L:16D) and constituted the photosensitive group (PSENS; n=8), one group stayed 8 weeks in 8L:16D and was then transferred for 4 weeks in long days (14L:10D) to form the photostimulated group (PSTIM; n=8; one brain was later lost in this group due to technical problems reducing sample size to 7 for the histological measures), while a final group of birds were immediately switched to 14L:10D where they stayed 12 weeks to form the photorefractory group (PREF; n=8). This provided on the day of brain collection 3 groups of birds representative of the 3 main photoperiodic conditions experienced across the year by European starlings (Dawson et al., 2001).
At the beginning of the experiment (birds in 8L:16D), laparotomies were performed under isoflurane anesthesia to measure the left testis size (width, W and length, L measured with calipers; volume, V calculated as V=4/3 π a2*b where a and b are half of the width and length respectively) and blood samples were collected from the wing vein to assay circulating testosterone concentrations. Blood samples were collected again just before brain collection at the end of the photoperiod manipulations in June 2013. Birds were then anesthetized with secobarbital (50mg/kg). They were perfused with NaCl 0.9% followed by paraformaldehyde (PFA) fixation (4% in phosphate buffer saline, PBS). Brains were dissected out of the skull and post-fixed for 24 hours in the same fixative. Finally, they were transferred into a 30% sucrose solution until they sank, frozen on dry ice and kept at −80°C until processed. Left testis size was measured again at that time. All procedures were approved by the Johns Hopkins University Animal Care and Use Committee (Protocol #: AV15A106).
In vivo procedures for experiment 2: Species comparison
All experimental subjects used in this experiment were in endocrine conditions that are similar to those observed in mature sexually active males. This was however achieved by different experimental means because these 3 species have different physiological characteristics and therefore need to be manipulated differentially to put them in similar endocrine conditions. All birds received food and water ad libitum throughout the experiment. All procedures were in agreement with the Belgian laws on animal experimentation and had been approved by the animal care committee at the University of Liège (Protocols number 926, 1396 and 1739).
Zebra finches
The brain of 6 adult males more than 160 days old that were born and reared in the large aviary of our captive colony containing adult and juvenile conspecifics from both sexes were used. They were throughout their life until brain collection in February/March 2014 exposed to tutor songs and social interactions as seen in wild conditions and maintained in a 13L:11D light cycle. In these conditions zebra finches breed continuously.
Birds were captured with a net in the aviary and anesthetized within less than 3 minutes with pentobarbital (0.05 ml Nembutal™ at 0.6 mg/ml). The heart was exposed and birds were perfused with 200ml phosphate buffer saline (PBS) followed by PFA 4% diluted in PBS. Brains were dissected out of the skull and post-fixed for 24 hours in the same fixative as described for experiment 1. Finally, they were transferred into a 30% sucrose solution until they sunk, frozen on dry ice and kept at −80°C until processed. The quantitative analysis of PNN and PV expression in these subjects and in matched females has been previously published (Cornez et al., 2015).
Canaries
Five adult males of the Fife Fancy breed arrived in our animal facility in July 2014 at the age of one year and were kept in a collective cage located in a room containing other male and female canaries under short days photoperiodic conditions (8L:16D) for 16 weeks to induce a photosensitive state (Hurley et al. 2008). They were then transferred to individual cages in the same room. Birds had no visual contact but they could hear each other. They received food and water ad libitum.
Two weeks after isolation, 12mm Silastic™ implants (inner diameter: 0.76mm – outer diameter: 1.65mm; Degania Silicone, Israel) filled with crystalline testosterone (Fluka Analytical, Sigma-Aldrich) were placed subcutaneously in the back of three of these males while the other two birds received empty implants as a control. This procedure has been shown to induce singing rate and circulating testosterone concentrations typical of sexually mature males (Sartor et al., 2005; Boseret et al., 2006; Madison et al., 2015). Five weeks after implantation, in December 2014, birds were perfused with PFA and their brains were collected following the procedure described for zebra finches.
Starlings
Seven photostimulated adult male starlings that were also part of experiment 1 provided brain sections for this study. They were wild caught during the winter and placed in collective cages in short days photoperiodic condition (8L:16D). They stayed for 8 more weeks in the same photoperiodic condition to keep them in a photosensitive state and were then transferred in long days (14L:10D) during 4 weeks to place them in a reproductive state that corresponds to photostimulated sexually active males at the time of brain collection (see Experiment 1 for additional in vivo procedures).
Immunohistochemistry
All brain sections were processed and stained by immunohistochemistry following the same protocol. Brains were cut on a cryostat at 30 μm thickness in the coronal (canaries, starlings) or sagittal (zebra finches) plane and collected in 24-wells plates containing a Tris buffer saline solution (TBS 0.01M: 4 series of 3 wells for zebra finches (for each hemisphere) and canaries, 6 series of 4 wells for starlings. Sections were transferred into Eppendorf™ vials containing antifreeze solution, left one hour to equilibrate at +4°C and then stored at −20°C until immunostained.
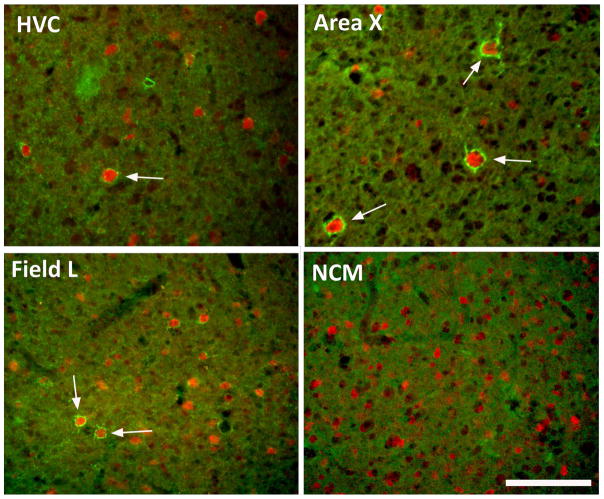
One full series for each bird was stained by immunohistochemistry for parvalbumin-positive cells (PV) and perineuronal nets (PNN). The protocol used is similar to the protocol previously used in zebra finches (Balmer et al., 2009; Meyer et al., 2014; Cornez et al., 2015). After equilibration at room temperature, sections were washed in TBS. Sections were then blocked in Normal Goat Serum solution (NGS) 5% diluted in TBS with 0.1% Triton-X-100 (TBST) for 30 minutes. They were incubated overnight at 4°C in a mixture of two primary antibodies diluted in TBST: a mouse monoclonal anti-chondroitin sulfate antibody (CS-56; C8035, 1:500, Sigma Aldrich) specific for the glycosaminoglycan portion of the chondroitin sulfate proteoglycans that are main components of the PNN of the extracellular matrix and a polyclonal rabbit anti-parvalbumin antibody (ab11427,1:1000, Abcam). Sections were then washed in TBS and incubated at room temperature in a mixture of secondary antibodies diluted in TBST. A goat anti-mouse IgG coupled with Alexa 488 (green, 1:100, Invitrogen) was used to visualize PNN staining and a goat anti-rabbit IgG coupled with Alexa 546 (red, 1:200, Invitrogen) was used to visualize PV cells (see Fig. 1 for representative images of sections from the starling brain). Finally, sections were washed, mounted on slides using TBS with gelatin and coverslipped with Vectashield containing DAPI (H-1500, Vector laboratories).
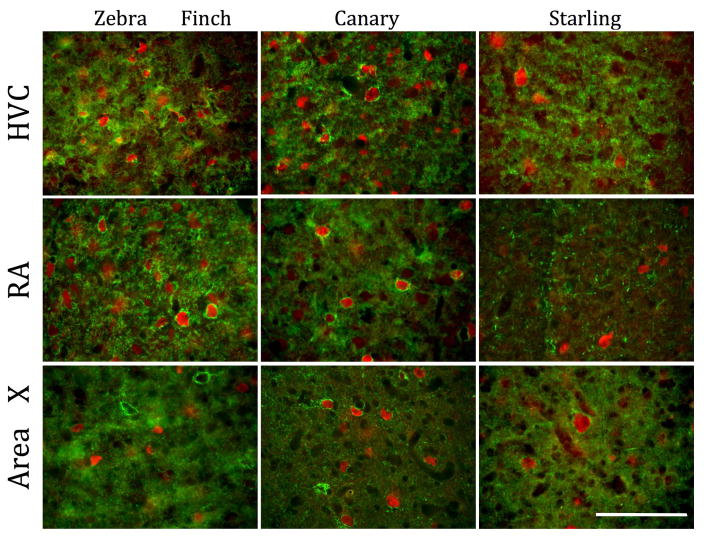
Figure 1.
Representative photomicrographs illustrating the immunostaining for perineuronal nets (PNN; green) and for parvalbumin (red) in two song control nuclei, HVC and Area X and in two auditory brain regions, Field L and NCM of starlings. Arrows point to paravalbumin-immunoreactive cells that were surrounded by PNN. Note the absence of PNN in NCM. Magnification bar = 100 μm.
PNN and PV quantification
Photomicrographs were acquired with a Leica DMRB Fl 100 fluorescence microscope and a Leica DFC-480 color (for zebra finches) or Leica DFC-3000 black and white (for canaries and starlings) camera. Images acquired from the black and white camera were assigned a fictive color similar to the color observed in the microscope (green for PNN: excitation filter BP480/40, dichromatic filter 505, suppression filter BP 527/30; red for PV: excitation filter BP545/30, dichromatic filter 565, suppression filter BP10/75). Photomicrographs were taken of the four primary forebrain nuclei of the song control system (HVC, RA, Area X, and LMAN) that were identified by the overall higher staining intensity compared to the surrounding regions except for LMAN which was located dorsally across the lamina pallio-subpallialis, in the same section where Area X was identified. The magnification used and the number of sample photomicrographs collected for each nucleus differed between species as starling brains are much larger. In this latter species, photomicrographs at 20X (field covered: 481x358μm = 0.172 mm2) were taken to ensure that each photomicrograph contained only the nucleus of interest. Three photomicrographs in Area X, 2 photomicrographs in HVC, and 1 photomicrograph in LMAN and RA, were taken in each hemisphere and in one section from each of the 4 wells, the section where the nucleus of interest was the largest. This number of photomicrographs allowed covering a large part (approximately 50%) of the nuclei area without overlap between photomicrographs. In zebra finches and canaries 40X photomicrographs (field covered: 240 x 179 μm = 0.043 mm2) were acquired in each hemisphere. In Area X, 2 photomicrographs were acquired whereas 1 photomicrograph was acquired in the 3 other nuclei. As these nuclei are approximately 10 times smaller in these species than in starlings, 1 section from each series of 3 wells where the nucleus of interest was the largest was used. The settings (exposure and gain) used to acquire the photomicrographs were similar for all birds from the same species and set specifically for each nucleus.
In starlings, the same procedure was used to quantify PNN and PV cells in 3 auditory telencephalic brain regions: Field L, the primary auditory input region, the caudomedial nidopallium (NCM) and the caudomedial mesopallium. For these regions, two photomicrographs were taken for each region in each hemisphere and in one section from each of the 4 wells, the section where the nucleus of interest was the largest, at a magnification of 20X (field covered: 481x358 μm = 0.172 mm2).
All these photomicrographs were used to count the number of parvalbumin-positive cells, defined as bright red fluorescent cells, and of perineuronal nets defined as green circles or part of circles surrounding a cell body and brighter than the rest of the extracellular milieu. All photomicrographs were adjusted for contrast and brightness following identical settings for all birds from the same species in a specific nucleus. All procedures were carried out with the help of the Image J Software (NIH Research Services Branch) in photomicrographs acquired at the single corresponding wavelength. It was then necessary to merge the PV and PNN staining to count the number of PV cells that were surrounded by PNN (PV+PNN). Photomicrographs of DAPI staining (present in the mounting medium) were merged with PNN images to confirm PNN localization around a cell body, if they were not surrounding a PV-positive cell. PV and PNN were counted visually based on the images of single staining. Photomicrographs acquisition and counting were done blind to the treatment groups.
We also measured the overall staining intensity of the PNN staining on photomicrographs that were not adjusted for contrast and brightness to quantify the chondroitin sulfate expression in the entire extracellular matrix, organized or not in PNN. This measure cannot be reliably compared between species as there are differences in the settings used for acquiring photomicrographs that could influence the measured intensity. Staining intensity was quantified as a measure of average grey values across the entire area obtained by Image J in 8-bit transformed images.
Nuclei volume measurement
In starlings, the volume of song control nuclei was additionally measured in low magnification photomicrographs of the merged PV and PNN staining. This combination allowed us to clearly distinguish the boundaries of HVC, RA, and Area X but not of LMAN that could not be detected by this approach. We took photomicrographs of these nuclei in all sections in the 4 wells of the series with the same microscope and software mentioned above with a 2.5X objective (field covered: 3.85 x 2.87 mm = 11.05 mm2) so that the entire nuclei were covered in each photomicrograph. Using the area measurement function of Image J, we then calculated the surface of each nucleus in each section. The area (mm2) was multiplied by the distance between each section in the series (0.18mm) and these results were summed for all sections of each nucleus in each bird. This procedure was performed separately for each hemisphere and volumes were averaged to obtain a final unilateral volume of the nucleus of interest.
Data analysis
When several photomicrographs of one nucleus were taken within one section, the counts for these images were averaged. The average counts for the left and right hemisphere were then calculated for each section as no lateralization was observed in PV and PNN expression in a previous study of zebra finches (Cornez et al., 2015). The average between all counted sections was then computed. For each bird, we calculated the density of PV, PNN, PV+PNN by dividing the average counts by the counted area expressed in mm2. Finally, the proportion of PV cells surrounded by PNN and the proportion of PNN surrounding PV cells was calculated by dividing their average counts.
We also obtained an estimate of the total number of PNN, PV, and PV+PNN in the entire song control nuclei HVC, RA and Area X by multiplying the average counts obtained in one microscope field by the ratio of the volume of the nucleus divided by the volume of the counted field (Surface x Thickness). These calculations were based on the average volumes measured in each individual starling and canary used in the present experiments and on average volumes of these nuclei measured in 3 of the 6 zebra finches.
Methodological controls
To ensure that differences between species studied in experiment 2 were genuine and did not simply reflect differences in procedures (plane of sectioning, inter-assay variation of staining, camera used for microphotography, …), one series of sections from 3 subjects in each of the 3 species were additionally stained in the same assay and PNN/PV were quantified in parallel in photomicrographs from these sections obtained from the same camera (Leica DFC-3000).
All these sections were alternate sections coming from the brain of the birds that had been used in the initial experiment. These zebra finch and canary sections were also used to measure the volume of the song control nuclei with procedures similar to those described above for starlings. Previous work in our laboratory has shown that volumes of song control nuclei determined in this way based on PNN/PV staining positively correlate with volumes determined in Nissl-stained sections (Cornez G. and Balthazart J., Unpublished data).
In addition, the brain of 2 additional male zebra finches specifically perfused and dissected for this purpose was split in halves along the medial plane and one hemisphere was cut in the sagittal plane while the other was cut in the coronal plane. The left hemisphere was cut in the coronal plane in one bird and in the sagittal plane in the other bird and vice-versa for the right hemisphere in order to balance data for any possible lateralization. Note however that no such lateralization was found in a previous study of male and female zebra finches (Cornez et al., 2015). Two sections from each hemisphere from each bird were stained by immunohistochemistry in the same assay and PNN/PV-positive cells or structures were quantified in HVC, RA and Area X as described above in all the sections. Numbers of positive objects were then compared in the two hemispheres that had been sectioned in orthogonal planes.
Testosterone Enzyme ImmunoAssay(EIA)
Testosterone in starlings samples was quantified by EIA in duplicate during a single assay in the Ball laboratory at Johns Hopkins University (Baltimore). Blood samples were collected in starlings (~200μl) via venipuncture of the brachial vein prior to photoperiodic manipulation (Pre) and trunk blood (~500μl) was collected at the end of the experiment (Post). Plasma testosterone was measured using a competitive enzyme-linked immunoassay (ELISA: Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions with minor modifications. Briefly, the assay was validated for use with starling plasma via a test of parallelism and a recovery of added mass (standard biochemical validations as described in (Gill et al., 2015)). Plasma was extracted by adding 1ml diethyl ether and reconstituted with ELISA buffer. Per the manufacturer’s specifications, the assay has a sensitivity (80% B/B0) of approximately 6 pg/ml and is specific given the cross reactivity to 5a-dihydrotestosterone is 27.4% and estradiol <0.01%. The intraassay coefficient of variation was 3.71%.
For a few samples, we observed poor reproducibility between duplicates and these results were removed from the final analyses together with a few statistical outliers thus leading to a reduced sample size in some experimental groups (T-pre: PSENS n=7, PSTIM n=6, PREF n=6; T-post: PSENS n=7, PSTIM n=7, PREFn=5).
Statistical analysis
Because most data did not meet the normality and/or homoscedasticity criteria, results from the 3 groups of starlings in different photoperiodic conditions were compared for each nucleus and dependent measure by separate one-way non-parametric Kruskal Wallis ANOVA followed when appropriate by post hoc multiple comparison of mean ranks. The Wilcoxon test was used to analyze the difference between pre- and post-treatment testosterone concentration in the blood within each group. The same methods were used to compare data from the three species. Differences were considered significant for p< 0.05. All data are presented by their mean and standard error of the mean (SEM).
RESULTS
EXPERIMENT 1
Photoperiodic effect on reproductive state
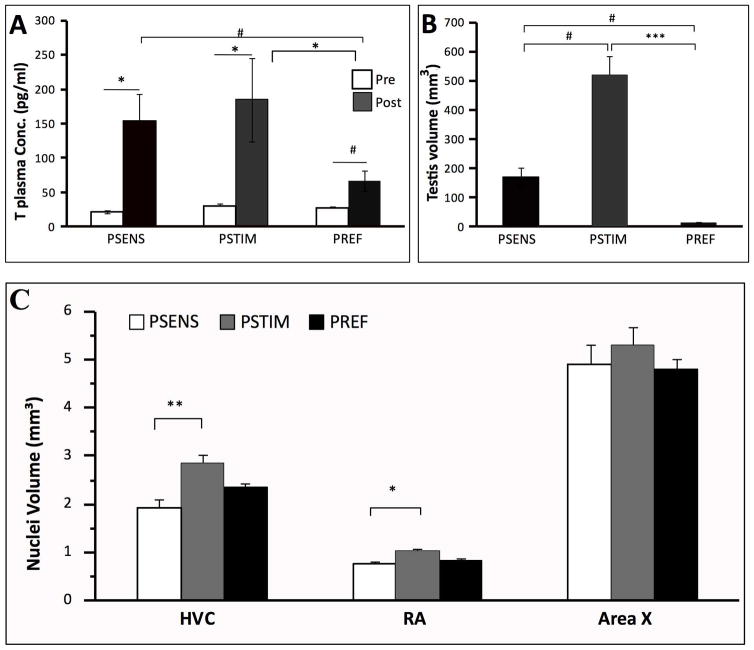
Before treatments, there was no difference in testis volume (H=1.01, p=.605) and plasma testosterone concentrations between groups (H=3.50, p=0.173) whereas these concentrations were significantly different at the end of the experiment (H=7.48, p=0.023). Post hoc tests revealed that the PREF group had significantly lower testosterone concentrations than the PSTIM group (z=2.63, p=0.026) and a tendency to have lower concentrations than the PSENS group (z=2.15, p=0.094). The comparison of pre- and post-treatment concentrations within each group showed that PSTIM (Z=2.02, p=0.043) and PSENS (Z=2.20, p=0.028) birds had a significantly higher testosterone concentration at the end of the experiment whereas there was only a trend for such a difference in the PREF group (Z=1.83, p=0.068) (Fig. 2A). The different photoperiodic regimes also had the expected effects on testis volume and weight that significantly differed between groups at the end of the experiment (volume: H=20.48, p<0.001; weight: H=20.48, p<0.001). Post hoc tests revealed that PSTIM birds had significantly larger (z=4.53, p<0.001) and heavier (z=4.53, p<0.001) testes than PREF birds. There was also a tendency for the other two groups (PSENS and PREF) to be different from each other for both testis volume (z=2.26, p=.0710) and mass (z=2.26, p=.0710) (Fig. 2B).
Figure 2.
Effects of photoperiodic manipulations on plasma testosterone concentrations (A), left testis volumes (B), and volumes of song control nuclei HVC, RA and Area X (C) in male starlings. Testosterone concentrations were measured before (Pre) and at the end (Post) of the experiment. Nuclei volumes are the average left and right nuclei. Data were analyzed by Kruskal-Wallis non parametric ANOVA followed when significant by post-hoc tests. Pre and Post concentrations of testosterone were also compared within each group by Wilcoxon tests. *= p<0.05, ***=p<0.001, #=p<0.10.
Photoperiodic regulation of song control nuclei volume
The volumes of HVC (H=10.51, p=0.005) and RA (H=7.73, p=0.021) nuclei were significantly affected by the photoperiodic treatments. PSTIM birds had significantly larger nuclei than PSENS birds (HVC: z=3.24, p=0.004; RA: z=2.63, p=0.025). The PREF birds had slightly larger volumes of HVC and RA than photosensitive birds but they were not significantly different from any of the two other groups. The volume of Area X was not different among the conditions (H=1.25, p=0.534) (Fig. 2C).
Photoperiodic effect on PV, PNN and chondroitin sulfate in song control nuclei
The density (numbers/mm2) of PV-positive cells, of PNN, of PV cells surrounded by PNN (PV+PNN), their ratio and the signal intensity of chondroitin sulfate was quantified in the 4 song control nuclei of all birds the 3 groups of starlings with the exception that poor staining quality prevented quantification in Area X, RA and LMAN in one subject. Means ± SEM of all these results are presented in Figure 3.
Figure 3.
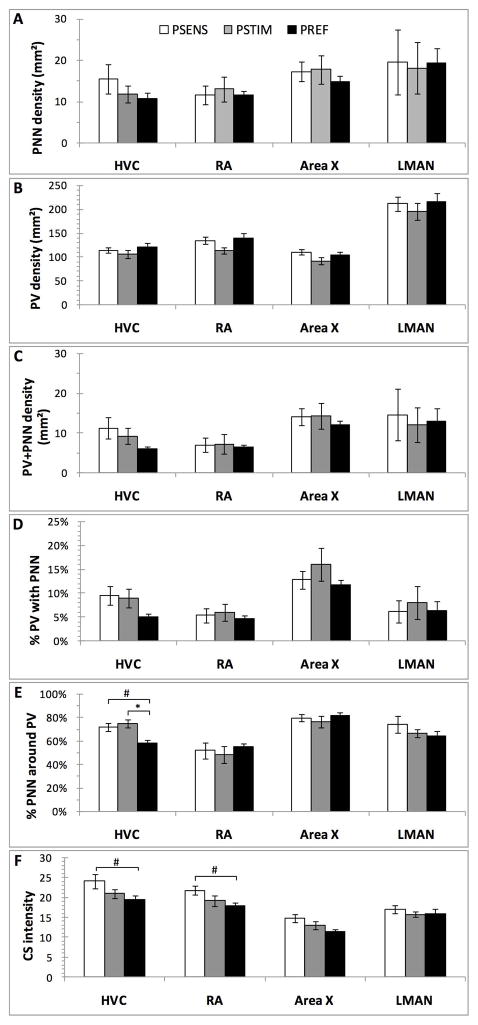
Effects of photoperiodic manipulations on the density (numbers/mm2) of PNN (A), PV-positive cells (B) and PV cells surrounded by PNN (C), on the percentage of PV interneurons surrounded by PNN (D) or of PNN that surrounded PV cells (E), and finally on the chrondroitin sulfate (CS) immunostaining intensity (F)in the4 song control nuclei of male starlings. Data were analyzed by Kruskal Wallis non parametric ANOVA followed when significant by post hoc tests. *= p<0.05, #=p<0.10.
In HVC, the density of PNN (H=1.36, p=0.507; Fig. 3A), of PV cells (H=0.66, p=0.717; Fig. 3B), and of PV+PNN (H=2.14, p=0.342; Fig. 3C) were not affected by the photoperiodic conditions. The percentage of PV interneurons surrounded by PNN was also not significantly affected (H=3.51, p=0.173; Fig. 3D), but the proportion of PNN that surrounded PV cells was significantly different after exposure to the 3 photoperiodic conditions (H=8.22, p=0.016; Fig. 3E). Post hoc tests indicated that PREF birds had a significantly lower proportion of PNN surrounding PV than the PSTIM birds (z=2.58, p=0.029; Fig. 3E) and this percentage also tended to be lower in PREF than PSENS birds (z=2.32, p=0.061; Fig. 3E). Finally, chondroitin sulfate (CS) intensity tended to be affected by the photoperiodic conditions (H=5.18, p=0.075) and this effect was presumably driven by the tendency of PREF to be lower than PSENS birds (multiple comparison test: z=2.21, p=0.081) (Fig. 3F).
In RA, there was similarly no effect of the photoperiodic manipulations on the density of PV cells (H=4.09, p=0.129; Fig. 3A), of PNN (H=0.01, p=0.994; Fig. 3B), of PV+PNN (H=0.27, p=0.874; Fig. 3C) and on the percentages of co-existence (% PV with PNN:H=0.05, p=0.975; % of PNN around PV: H=0.35, p=0.839; Fig. 3D–E). However like in HVC, chondroitin sulfate (CS) intensity tended to be affected by the treatments (H=5.45, p=0.065), presumably because this intensity tended to be higher in PSENS than in PREF birds (multiple comparison test: z=2.24, p=0.076)(Fig. 3F).
No effect of the photoperiodic manipulations was detected in Area X (PV: H=2.34, p=0.310; PNN: H=0.73, p=0.693; PV+PNN: H=0.79,p=0.673; % PV with PNN: H=0.75, p=0.686; % of PNN around PV: H=0.91, p=0.632; CS intensity: H=3.92, p=0.141; Fig. 3A–F). The same global absence of effect was observed in LMAN (PV: =0.30; p=0.860; PNN: H=1.13; p=0.568; PV+PNN: H=1.35; p=0.508; % PV with PNN: H=1.52, p=0.466; % of PNN around PV: H=1.30, p=0.522; CS intensity: H=0.59, p=0.744; Fig. 3A–F).
Since photoperiodic treatments had affected the total volume of HVC and RA (but not Area X), we asked whether the total number of the structures of interest in the whole nuclei could be affected even if their density per unit of surface was unchanged. Total numbers of PV cells, of PNN and of PV cells surrounded by PNN were therefore calculated for the whole volume of these 3 nuclei as described in the methods. This approach did not, however, generate any new significant difference between groups (data not shown). There was only a tendency in HVC for the number of PV-positive cells to be affected by the treatments (H=5.54, p=0.062) resulting from a slightly higher number of PV cells in the PSTIM than in the PSENS birds (z=2.31, p=0.063; data not shown). All other comparisons were not significant although the total number of PNN and the numbers of PV cells surrounded by PNN were numerically higher (10 to 45 %) in PSTIM birds than in birds from the two other groups in both HVC and RA. These differences were especially prominent for the total number of PNN in RA (PSENS: 317±72, PSTIM: 463±120, PREF: 418±31; all means ± SEM) but they were however far from significant (total PNN in HVC: H=0.69, p=0.707; total PV+PNN in HVC: H=1.93, p=0.381; total PNN in RA: H=0.56, p=0.755; total PV+PNN in RA: H=0.24, p=0.889).
Photoperiodic effect on PV, PNN and chondroitin sulfate in auditory brain regions
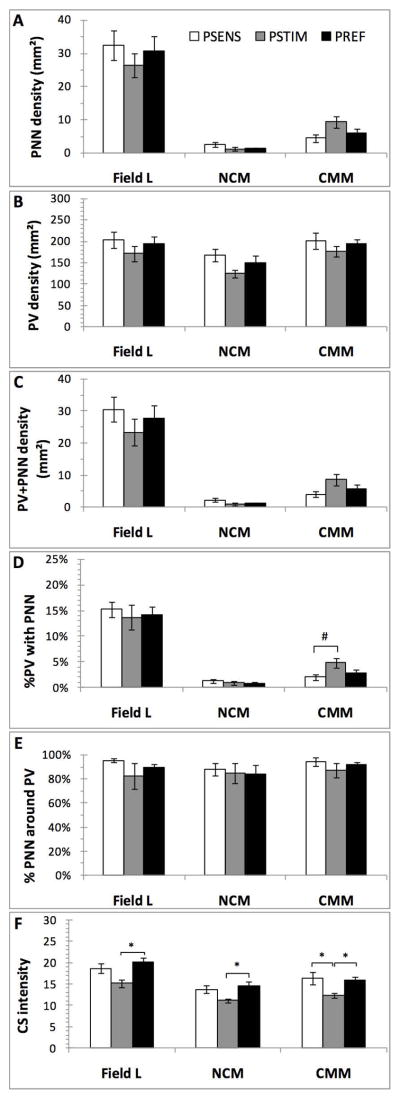
Large numbers of PV-positive cells were also detected in the 3 telencephalic auditory regions, Field L, NCM and CMM but PNN were found in substantial numbers in Field L only; they were essentially absent from NCM and CMM and as a consequence the PV cells in these regions were very rarely surrounded by PNN (Fig. 4). In all 3 regions, the non parametric ANOVA revealed no effect of the photoperiodic conditions on the density of PV cells (Field L: H=0.94; p=0.626; NCM: H=4.10; p=0.129; CMM:H=0.45; p=0.799), of PNN (Field L: H=0.69; p=.707; NCM: H=2.39; p=0.302; CMM: H=4.37; p=0.112) and of PV cells surrounded by PNN (Field L: H=0.95; p=0.622; NCM: H=2.22; p=0.328; CMM: H=3.10; p=0.212). There was also no effect on the percentage of PV cells surrounded by PNN (Field L: H=0.23, p=0.889; NCM: H=0.95, p=0.621; CMM:H=4.60, p=0.100) and on the percentage of PNN surrounding PV cells (Field L: H=3.15, p=0.206; NCM: H=0.03, p=0.986; CMM: H=3.04, p=0.218).
Figure 4.
Effects of photoperiodic manipulations on the density (numbers/mm2) of PNN (A), PV-positive cells (B) and PV cells surrounded by PNN (C), on the percentage of PV interneurons surrounded by PNN (D) or of PNN that surrounded PV cells (E), and finally on the chrondroitin sulfate (CS) immunostaining intensity (F) in telencephalic auditory brain regions of male starlings. Data were analyzed by Kruskal Wallis non parametric ANOVA followed when significant by post hoc tests. *= p<0.05.
In contrast, the chondroitin sulfate overall immunoreactivity (intensity) was significantly affected in all 3 regions (Field L: H=7.64, p=0.021; NCM: H=6.71, p=0.034; CMM: H=7.87, p=0.019), with lower values being observed in the PSTIM birds than in the PREF group in Field L and NCM (z=2.73, p=0.019 and z=2.54, p=0.033 respectively) or even than in both other groups in CMM (z=2.46, p=0.0420 in both cases: see Fig. 4F). Since PNN were either not affected (Field L) or present in extremely low number (NCM and CMM) this overall change in intensity of the chondroitin sulfate must obviously relate to an overall change in the immunoreactive material.
EXPERIMENT 2
Species differences in PV & PNN expression
We next considered whether the relative absence of seasonal variations of PNN in starlings as compared to the marked changes had been detected during the ontogeny of zebra finches (Balmer et al., 2009) reflected a differential regulation or a more fundamental difference in the expression of these compounds. Therefore we compared the density of PNN and PV-positive cells in photostimulated starlings (n=7) with data previously obtained in zebra finches (n=6; data from Cornez et al., 2015) as well as in testosterone-treated canaries (n=3; present study).
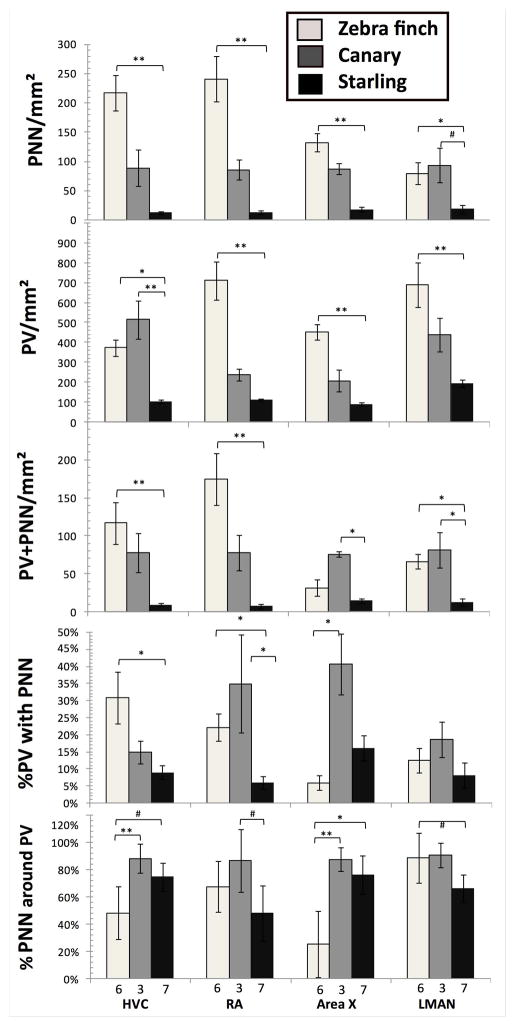
Substantial differences between species were detected in the density (numbers per mm2) of PNN, PV cells and PV cells surrounded by PNN in the 4 song control nuclei (Fig. 5 for representative images, Fig 6 for quantitative data). All measures were higher in zebra finches than in starlings with canaries having usually an intermediate density, except for the PNN in LMAN, PV cells in HVC and PV cells surrounded by PNN in Area X and LMAN where values in canaries where higher than in starlings. Kruskal Wallis ANOVAs confirmed the statistical significance of these differences between the 3 species (see Table 1) and post hoc tests demonstrated in every single case a significant difference between zebra finches and starlings (PNN -in HVC: z=3.52, p=0.001; -in RA: z=3.59, p=0.001; -in Area X: z=3.46, p=0.002; -in LMAN: z=2.49, p=0.038; PV –in HVC: z=2.67, p=0.022; -in RA: z=3.59, p=0.001; -in Area X: z=3.52, p=0.001; -in LMAN: z=3.22, p=0.004; PV+PNN –in HVC: z=3.27, p=0.003; -in RA: z=3.46, p=0.002; -in LMAN: z=2.53, p=0.034), except of PV cells surrounded by PNN in Area X (z=0.82, p=1.0). There was in addition in a few cases a significant difference between canaries and starlings (PNN in LMAN: z=2.36, p=0.054; PV in HVC: z=2.99, p=0.008; PV+PNN in Area X: z=2.74, p=0.018; and PV+PNN in LMAN: z= 2.75, p=0.018; see detail in Fig. 5).
Figure 5.
Representative photomicrographs illustrating the species differences in PNN and PV-positive cells density between zebra finches, canaries and starlings. Magnification bar = 100 μm.
Figure 6.
Comparison of the density (numbers/mm2) of PNN (A), PV-positive cells (B) and PV cells surrounded by PNN (C), of the percentage of PV interneurons surrounded by PNN (D) or of PNN that surrounded PV cells (E) in 3 songbird species. Data were analyzed by Kruskal Wallis non parametric ANOVA followed when significant by post hoc tests. *= p<0.05, **=p<0.01, #=p<0.10.
Table 1.
Statistical analysis by Kruskal Wallis non parametric ANOVA of the species differences in density of PNN, PV cells, double labeled structures and on the relevant percentages. The table shows the H statistics and the associated probabilities.
| HVC | RA | Area X | LMAN | |
|---|---|---|---|---|
| PNN/mm2 | H=12.57 p<0.001 |
H=12.90 p=0.001 |
H=12.23 p=0.002 |
H=8.64 p=0.013 |
| PV/mm2 | H=11.80 p=0.002 |
H=12.90 p=0.001 |
H=12.53 p=0.001 |
H=10.90 p=0.004 |
| PNN+PV/mm2 | H=11.56 p=0.003 |
H=12.22 p=0.002 |
H=7.58 p=0.022 |
H=10.26 p=0.005 |
| % PV with PNN | H=7.07 p=0.029 |
H=9.19 p=0.010 |
H=8.00 p=0.018 |
H=3.32 p=0.190 |
| % PNN with PV | H=10.07 p=0.006 |
H=5.68 p=0.058 |
H=10.88 p=0.004 |
H=6.10 p=0.047 |
Similarly, the percentage of PV cells surrounded by PNN differed between the 3 species in HVC, RA and Area X but not in LMAN (see Table 1; Fig. 5). In HVC and RA, this percentage was significantly higher in zebra finches than in starlings (HVC: z=2.65, p=0.024; RA: z=2.61, p=0.027). In HVC canaries were intermediate while they displayed a higher but not statistically significant percentage than zebra finches (z=0.30, p=1.0) and were statistically higher than starlings (z=2.41, p=0.048) in RA. In Area X, post-hoc tests showed a single significant difference with canaries showing a higher percentage of co-expression than zebra finches (z=2.82, p=0.014).
Interestingly a very large percentage of the PNN were located in most species and nuclei around PV cells. However these percentages also differed significantly among the three species, except in RA and LMAN where only statistical tendencies (p<0.10) were observed, but the pattern of differences was here quite different with the zebra finches showing the smallest percentage of co-expression, except in LMAN (see Fig. 5).
It should be noted that the treatment with testosterone of male canaries made them, if anything, more similar to zebra finches and more different from starlings. Comparison of the 3 testosterone-treated males with the 2 untreated controls indeed suggested that testosterone increases the density of PNN (e.g., in HVC: 3.83±1.33 vs. 0.75±0.75, in RA: 3.67±0.73 vs. 2.75±0.75), of PV cells (e.g., in HVC: 22.17±4.09 vs. 19.00±4.50, in RA: 10.33±1.30 vs. 8.00±0.00) and of PV cells surrounded by PNN (e.g., in HVC: 3.33±1.09 vs. 0.75±0.75, in RA: 3.33±1.01 vs. 2.50±1.00; all numbers are means ± SEM of PNN or PV/0.043 mm2 counting field). These differences are not significant but are confirmed by experiments based on larger sample size currently ongoing in our laboratory.
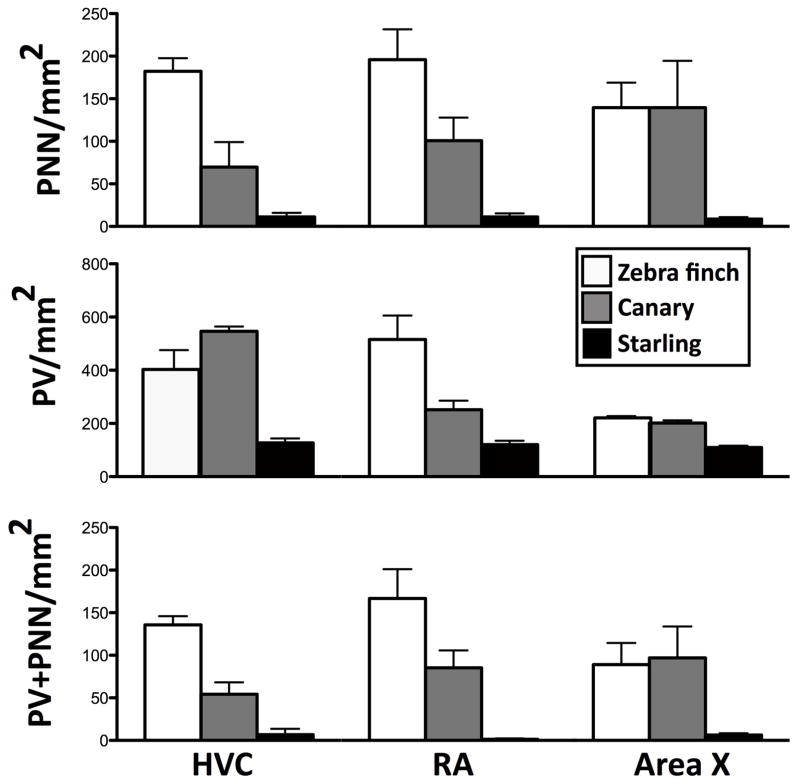
Starling brains are obviously much larger than the brains of zebra finches or canaries (estimated body mass= 75 g in starlings, 12 g in zebra finches and 8–24 g in canaries depending on the strain, about 20 g in our birds). One could thus speculate that the lower densities of PNN or PV-positive cells only reflect the distribution of similar total numbers in a larger brain volume. To test this idea, we calculated the total numbers of PNN or PV cells in the entire nuclei based on their average volume measured in 3 zebra finches, 3 canaries and 7 starlings used in the present experiments based on the sections double stained for PNN and PV cells (see Table 2).
Table 2.
Estimations of the total numbers (±SEM) of PNN, of PV-positive cells and of PV cells surrounded by PNN in the HVC, RA and Area X of zebra finches, canaries and starlings based on average volumes of these nuclei measured in 3 zebra finches, 3 canaries and 7 starlings used in the present experiments. Species differences were analyzed using 1-Way Kruskal-Wallis ANOVA. astatistically different from b(p<0.05) by post hoc multiple comparison.
| HVC | K-W ANOVA | Zebra Finches | Canaries | Starlings |
|---|---|---|---|---|
| Volume (mean) | 0.35mm3(±0.03) | 0.41mm3(±0.08) | 2.87mm3(±0.17) | |
| Total PNN | H=8.87, p=0.011 | 2540 (±351) a | 1215 (±423) | 1133 (±217) b |
| Total PV | H=10.51, p=0.005 | 4378 (±495)a | 7024 (±1295) | 10165 (±864)b |
| Total PV+PNN | H=1.31, p=0.519 | 1361 (±318) | 1056 (±346) | 890 (±199) |
| RA | K-W ANOVA | Zebra Finches | Canaries | Starlings |
|---|---|---|---|---|
| Volume (mean) | 0.23mm3(±0.01) | 0.23mm3(±0.02) | 1.02mm3(±0.05) | |
| Total PNN | H=10.46, p=0.005 | 1811 (±294)a | 646 (±128) | 446 (±108)b |
| Total PV | H=9.19, p=0.010 | 5360 (±722)a | 1821 (±229)b | 3888 (±224) |
| Total PV+PNN | H=9.80, p=0.007 | 1314 (±255)a | 587 (±179) | 248 (±90)b |
| Area X | K-W ANOVA | Zebra Finches | Canaries | Starlings |
|---|---|---|---|---|
| Volume (mean) | 1.41mm3(±0.08) | 0.88mm3(±0.03) | 5.30mm3(±0.40) | |
| Total PNN | H=7.54, p=0.023 | 6182 (±741) | 2556 (±260) | 3142 (±656) |
| Total PV | H=9.56, p=0.008 | 21274 (±1804) a | 6134 (±1655) b | 16335 (±1524) |
| Total PV+PNN | H=1.79, p=0.407 | 1455 (±501) | 2215 (±98) | 2529 (±593) |
These total numbers were slightly more similar between species but some important species differences were still present. Starlings and canaries had similar total numbers of PNN whereas zebra finches had more PNN than the two other species in HVC, RA and Area X (see Table 2). This difference between species reached statistical significance in all three nuclei (see Table 2) and post hoc showed a difference between zebra finches and starlings in HVC (z=2.91; p=0.011) and RA (z=3.20; p=0.004). The same statistical differences were observed for PV+PNN in RA that were 4 fold more numerous in zebra finches than in starlings (z=3.13; p=0.005). Interestingly zebra finches had smaller total number of PV+PNN in Area X than the two other species but this difference was not statistically significant (see Table 2).
Total numbers of PV cells were also present in different numbers in the song control nuclei of the three species and overall these differences were statistically significant (p≤0.010 in Kruskal Wallis ANOVA). Post hoc tests showed that these cells were significantly more numerous in the HVC of starlings than in zebra finches (z=3.24; p=0.004). Interestingly, canaries had a significantly smaller total number of PV in RA (z=3.02; p=0.008) and Area X (z=3.07; p=0.006) compared to zebra finches.
Methodological validations
The staining of sections from the 3 species within a single assay confirmed the species differences discussed in the preceding paragraphs (Figure 7). The pattern of species differences identified in this methodological control was essentially identical to the pattern described in the main experiment (compare with Figure 6). In particular the much higher density of PNN and of PNN surrounding PV cells in the HVC and RA of zebra finches as compared to starlings was clearly present in this set of directly comparable data and the canaries were as in the main set of data occupying an intermediate position.
Figure 7.
Comparison of the density (numbers/mm2) of PNN (top), PV-positive cells (middle) and PV cells surrounded by PNN (bottom) in the HVC, RA and Area X of the three species based on brain sections from 3 subjects in each species stained in the same assay. The pattern of species differences is very similar to the pattern observed with data coming from separate staining batches.
In addition, comparison of the numbers of PNN, PV cells and PV cells surrounded by PNN in the two hemispheres of two additional zebra finches that had been cut coronally or sagittally found no evidence for a difference that could relate to the plane of sectioning in HVC, RA or Area X (see Table 3).
Table 3.
Comparison of the numbers of PNN, PV positive cells, and PV cells surrounded by PNN in zebra finches brain sections that had been cut in the coronal or sagittal plane. All numbers are means ± SEM of numbers of positive structures per mm2 in 2 birds in which in two sections were counted per bird. No systematic difference related to the plane of section can be detected.
| PNN | PV | PNN+PV | ||||
|---|---|---|---|---|---|---|
| Coronal | Sagittal | Coronal | Sagittal | Coronal | Sagittal | |
| HVC | 168.6±5.8 | 151.2±58.1 | 331,4±17.4 | 255.8±0 | 127.9±11.6 | 127.9±34.9 |
| RA | 133.7±17.4 | 133.7±5.8 | 622.1±122.1 | 598.8±98.8 | 127.9±11.6 | 116.3±11.6 |
| Area X | 98.8±5.8 | 58.1±23.2 | 186.0±23.2 | 156.9±17.4 | 52.3±5.8 | 40.7±40.7 |
DISCUSSION
The two studies described here demonstrate that there is only a limited variation in the density of perineuronal nets (PNN) observed in the song control nuclei and auditory areas of European starlings when one compares birds in the three photoperiodic states that mimic the different physiological conditions observed across the yearly cycle. This is in contrast to what had been previously observed over the course zebra finch ontogeny (Balmer et al., 2009) where marked changes in PNN density were reported. A direct comparison of PNN in zebra finches, canaries and starlings interestingly revealed that PNN densities are significantly higher in the zebra finch, a closed-ended learner than in the other two species that are able to modify their song during adulthood. Taken together, these data are consistent with the notion that PNN play a critical role in the control of neural plasticity associated with song learning in ontogeny (Balmer et al., 2009) and possibly seasonal song plasticity as previously reported for brain plasticity during development and in adulthood in mammalian models such as in the ocular dominance model (Pizzorusso et al., 2002; Pizzorusso et al., 2006), in fear conditioning (Gogolla et al., 2009)) or in neural plasticity associated with various pathologies such as epilepsy (Galtrey & Fawcett, 2007), stroke (Bidmon et al., 1998) or Alzheimer disease (Bruckner et al., 1999; Baig et al., 2005; Galtrey & Fawcett, 2007). Several aspects of these results deserve further clarification and discussion.
Seasonal plasticity and PNN/parvalbumin expression in song control nuclei
In seasonally breeding songbirds that modify their song repertoire from one season to the next, there is essentially a recapitulation of the developmental processes leading to the final adult song (see for example in canaries (Nottebohm et al., 1986)). During the fall and winter, a new sensorimotor and possibly in some species a new sensory phase takes place that permits the acquisition of new syllables and motifs that will be later rehearsed during a phase of sub-song and plastic song leading in the spring to the fixed crystallized song that will be sung during the reproductive period (Brainard & Doupe, 2002; Brenowitz, 2008). One could thus anticipate that brain plasticity should be more pronounced in periods of vocal learning and development than in periods when a crystallized song is produced.
Similarly, PV-positive cells are present within the song system (dense expression in nucleus RA (Wild et al., 2001) and in HVC (Wild et al., 2005)) and have been associated with the vocal learning capacities in a variety of animal models (Hara et al., 2012). Periods of brain plasticity exhibit a neurophysiological signature characteristic of immature neurons. The appearance of PV cells corresponds to the onset of experience-dependent plasticity and the development of PNN around them marks the end of this plastic period (Balmer et al., 2009). More specifically the critical period for ocular dominance column formation is extended when PNN around GABAergic interneurons are inactivated in the visual cortex (Pizzorusso et al., 2002; Hensch, 2005a) and the number of PV cells surrounded by PNN increases when song crystallizes during maturation of zebra finches (Balmer et al., 2009). Neural changes of this sort might also occur across photoperiodic conditions that mimic the different physiological states that characterize the annual cycle of adult starlings in correlation with seasonal changes in song behavior and the associated neuroplasticity.
One can speculate that this plasticity in song behavior and the brain takes place outside the breeding season, most likely in the late summer and fall when birds are photorefractory and transitioning to photosensitivity. A few elements suggesting increased brain plasticity at that time were in fact detected here. Three statistical tendencies toward a decrease were indeed observed in PREF birds: they concerned the percentage of PNN located around PV in HVC and the CS intensity in HVC and RA. Numerical differences suggesting a decreased plasticity during the PSTIM phase were also present in different cases (e.g. % PV with PNN in Area X and LMAN) but these differences were not statistically significant.
Additionally we compared the densities of immunoreactive structures (numbers per mm2) to their numbers in the entire nuclei of interest. Because the volume of HVC and of RA was larger in PSTIM than in PSENS birds, we tested whether the photoperiodic condition had affected total numbers of PNN, PV cells and their co-existence. The global numbers of target structures in the entire song control nuclei were very similar in the three photoperiodic conditions as observed for densities per unit surface. The absence of effects on densities is therefore not the result of a dilution of global increases in numbers into larger volumes. Overall, contrary to the predictions discussed above, very few changes were thus detected as a function of the photoperiodic stage in PNN, PV cells or their co-existence.
Why does the PNN expression change so little in adult starlings?
Several possibilities could explain this absence of changes in PNN in brains collected in three photoperiodic conditions representing key stages in the annual cycle. One could first propose that this study lacked statistical power but this possibility seems unlikely given that significant effects on song control nuclei volumes were detected and the effect size of these differences was moderate to large (HVC: d=0.93 for the comparison of the 3 groups, d=2.02 for the difference between PSENS and PSTIM; RA: d=0.70 or the comparison of the 3 groups, d=1.63 for the difference between PSENS and PSTIM). Therefore if changes in photoperiod affected the PNN and PV cells to the same extent as the volume of nuclei, this study had enough power to detect the effect. There is however no available information allowing us to decide whether or not changes in PNN and PV cell densities do or do not have the same magnitude as the changes in song control nuclei volumes. An alternative second option is that the changes in photoperiod did not have the expected physiological effects and failed to put the 3 groups of birds in the expected “seasonal” stage. This was however clearly not the case since these birds displayed the expected changes in testes size, plasma testosterone concentrations and even exhibited the expected variations in the volumes of the song control nuclei HVC and RA. A third possibility is that changing the photoperiodic state of the birds was not sufficient to modify accordingly their singing behavior and its neural substrate. The vocal behavior of these birds was indeed not recorded and one might imagine that the transition from subsong to plastic song and to crystallized song is not only a matter of being in the proper endocrine and photoperiodic condition but that additional factors are also implicated such as an underlying endogenous rhythm or the influence of social factors (other congeners). The specific time point when brains were collected could also not be optimal and more important changes could have taken place before or after. A fourth option would be to relate the absence of differences to an effect of the age of the birds that was not precisely controlled here. Experimental subjects had indeed been wild caught and although they were all over one year old, we could not determine their exact age. It has been shown that the starling repertoire size and song bout length increase with age and this increase is paralleled by an increase in the volume of song control nuclei (Eens et al., 1992; Bernard et al., 1996; Eens, 1997). If these age-related changes also affect the expression of PNN and PV cells in the song control nuclei, age differences could have increased the within group variance and have a confounding effect preventing detection of the photoperiod effects.
These possibilities are clearly testable but relatively unlikely. A more likely explanation relates however to the ability of starlings to increase their repertoire via the incorporation of new elements throughout their life (Eens et al., 1992; Chaiken et al., 1994; Bernard et al., 1996) and to learn and add new song elements in their repertoire independently of the season and plasma concentrations of androgens (Böhner et al., 1990). Other aspects of the singing behavior vary however seasonally in this species such as the singing rate (Feare, 1984; Eens et al. 1997) and social context in which song is produced (Eens, 1997; Riters et al., 2000) (see introduction). The fact that these phenotypic changes are not associated with changes in PNN expression, thus reinforce the idea that PNN do not relate in a general manner to song production but more specifically to the song learning abilities that do not seem to vary much across the year in starlings.
The comparison with canaries, who produce new syllables each year but may not learn them (entirely) in adulthood (Leitner et al., 2001b), could represent in the future a useful approach to determine the specific relationships between changes in brain structure or function (e.g., expression of PNN and PV) and specific aspects of vocal plasticity (learning vs. practicing, i.e. presence of a sensory vs. sensorimotor period) in seasonally breeding birds.
Seasonal changes in PNN are also limited in telencephalic auditory areas
PNN and PV cells were also present in high density in the primary auditory telencephalic area, Field L, while CMM and NCM only had very low density of PNN. These few PNN were however located almost exclusively around the abundant PV-positive cells. As was observed in song control nuclei, these PNN were only marginally affected by the photoperiodic state of the birds. This could also be considered unexpected since the auditory telencephalon is considered as a site implicated in tutor song memorization (London & Clayton, 2008) and some seasonal changes in this process might be anticipated (but see discussion in the previous section concerning song learning of starlings across the year).
The only significant differences observed here concerned again the overall CS overall density but quite interestingly the pattern of differences was not the same as in song control nuclei. In both HVC and RA, CS density was lowest in the PREF group while in auditory areas the highest densities were precisely in this PREF group with lowest values being observed in the PSTIM birds.
It must be noted that changes in overall CS intensity are difficult to interpret. They indeed reflect modifications of the overall amount of immunoreactive chondroitin sulfate present in the brain area of interest. Therefore this measure integrates changes in the number of PNN, in the density of the immunoreactive material contained in these PNN, and in the immunoreactive material that is not included in PNN and is more or less evenly distributed in the extracellular matrix. Since most of this latter material is amorphous, it is difficult to exclude that this only represents a background staining in the sections. Nevertheless the density of chondroitin sulfate in the extracellular matrix is clearly higher in regions such as HVC or RA and it allows delineating the boundaries of these nuclei.
Why PNN were essentially lacking in CMM and NCM is unclear at present. CMM and NCM may remain highly plastic in starlings to permit year-round vocal learning but why this plasticity would even increase (decrease in CS intensity) in PSTIM birds is not clear. One may hypothesize that during the breeding season, starlings have to interpret a greater variety of acoustic signals (e.g., conspecific vocalizations, predator noises…) to ensure successful reproduction and this requires more flexibility in the secondary auditory areas. Experiments relating brain changes to song learning, song production and auditory discrimination would be needed to test these ideas.
Species differences in PNN and PV expression
The comparison of zebra finches, canaries and starlings revealed striking patterns of differences. In the four song control nuclei, starlings had a significantly lower density of PNN, PV+ interneurons, and PV+ interneurons with PNN than zebra finches. Canaries had in most cases intermediate densities between the two other species (see Fig. 5–7). These interspecific differences persisted to a large extent when numbers of positive structures were extrapolated to the entire nuclei (Table 2) although they were to some extent dampened by the much larger volume of the brain and of the song control nuclei in starlings than in the other two species (5 to almost 10 fold). Total PNN numbers were still larger in zebra finches than in starlings (significant in HVC and RA) and the single major exception to this rule concerned the numbers of PV+ cells in HVC that were more numerous in starlings than in the other two species.
It is quite striking that in HVC and RA, the densities and numbers of PNN are larger in zebra finches than in starlings even though the volume of these nuclei are approximately 10 (HVC) and 5 (RA) times larger in starlings. This demonstrates that the lower density of PNN measured in starlings does not simply reflect a dilution of a fairly constant number of PNN in a larger volume. In contrast however, the total number of PV+ cells was larger in the HVC of starlings compared to zebra finches whereas the density demonstrated a reverse pattern. The low density of PV+ neurons in starlings thus reflects here in part a dilution in a larger volume of a larger total number of inhibitory interneurons. It would be interesting to compare the role of these neurons in the two species. The slightly different patterns of results when densities and total numbers are considered raise the question of which of these variables are more directly relevant to the control of behavior, a question that remains largely unanswered.
The percentage of PV+ cells that were surrounded by PNN displayed a similar pattern as the density of PNN with percentages being much lower in starlings than in zebra finches. However in contrast, the percentage of PNN that were located around PV+ cells was much lower in zebra finches than in the other two species in HVC, RA and Area X. Whereas 80–90% of PNN were located around PV+ cells in canaries and starlings, this percentage was in zebra finches below 50% in HVC and even lower (25.6%) in Area X. Thus in this species, PNN mostly develop around other cell types, possibly interneurons expressing other calcium-binding proteins such as calretinin or calbindin (Wild et al., 2005) or even other types of possibly GABAergic neurons.
It should also be noted that although the main species comparisons were based here on data collected in different experiments, but by the same person in the same laboratory and with nearly identical methods, methodological controls indicated that these differences are reliable and do not simply represent methodological artifacts. The same differences were detected in a set of sections stained in the same assay and the plane of brain sectioning had no impact on the final densities of positive structures.
A molecular basis for the song learning strategies of songbirds? Closed- vs. open-ended learners
Songbirds are often divided in two categories based on the plasticity of their vocalizations and their aptitude to modify their song in adulthood, the closed-ended or age-limited learners and the open-ended or age-independent learners. Variations in learning abilities have been identified between different open-ended learners (Beecher & Brenowitz, 2005; Brenowitz, 2008). Some species memorize all aspects of their song during ontogeny or their first reproductive season but only express various aspects of what they have memorized after one, two or three years. This is namely the case for song sparrows (Melospiza melodia), indigo buntings (Passerina cyanea) and brown-headed cowbirds (Molothrus ater) (see (Brenowitz, 2008)) This might also be the case for canaries. No study has tested in this species whether males are able to memorize new syllables during adulthood so that the changes observed from year to year in their song production might well reflect the production of different sets of syllables that were memorized during development (Leitner et al., 2001b). Other species in contrast are truly able to learn and produce new syllables during adulthood. This is the case of the nightingales (Luscinia megarhynchos) but also of European starlings that learn new songs throughout the annual cycle (Böhner et al., 1990; Chaiken et al., 1994; Geberzahn & Hultsch, 2003) and consequently increase their repertoire with age (Eens et al., 1992; Bernard et al., 1996).
There are thus various degrees of plasticity in the learning flexibility in different songbird species: zebra finches and starlings that were studied here probably represent the two extremes of the range while canaries presumably represent an intermediate case. Interestingly the density and total numbers of PNN and the percentage of PV+ neurons surrounded by PNN essentially followed a similar pattern of interspecies differences (zebra finches>canaries>starlings).
Since PNN are mostly located around GABAergic interneurons and restrict synaptic plasticity in various animal models (see introduction), it is tempting to postulate that these structures represent (one of) the mechanism(s) that limit(s) the morphological plasticity of the song control nuclei and their aptitude to support new learning.
The specific cellular mechanisms by which these PNN could control song learning abilities remain however to be identified. More work should also investigate whether the species differences in PNN expression specifically relate to the song learning strategies rather than to other behavioral differences between these species. Starlings are indeed singing all year round even if at somewhat different rates (Eens, 1997; Riters et al., 2000) while in other species song seems to be more closely limited to periods of reproduction. One could thus imagine that the lower level of PNN expression in starlings relates to their extensive singing activity. This idea is however negated by our unpublished observations showing that testosterone increases in parallel with singing rate and PNN expression in the canary song control nuclei (Cornez et al., 2016). More work would be needed to test this idea.
Together these data suggests that PNN expression, and their localization around PV+ interneurons and/or other types of neurons could have diverged during evolution to control the different learning capacities in songbird species. The differential expression of PNN in the song control nuclei of different songbird species could represent a key cellular mechanism controlling the behavioral differences. More detailed studies would however be needed to test the general character of the correlations identified here. For example, ideally one would want to compare species in the same subfamily or genus that exhibit variation in the song learning process. It would also be important to assess in a causal manner the role of PNN is song plasticity.
Acknowledgments
This work was supported by Grant SSTC PAI P7/17 from the Belgian Science Policy to J.B., A.vdL., C.A.C and G.F.B. and by a grant from the NIH (NS035467) to G.F.B, J.B. and C.A.C. C.A.C. is F.R.S.-FNRS Research Associate.
References
- Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22:8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Alward BA, Balthazart J, Ball GF. Differential effects of global versus local testosterone on singing behavior and its underlying neural substrate. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:19573–19578. doi: 10.1073/pnas.1311371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alward BA, Madison FN, Parker SE, Balthazart J, Ball GF. Pleiotropic Control by Testosterone of a Learned Vocal Behavior and Its Underlying Neuroplasticity(1,2,3) eNeuro. 2016:3. doi: 10.1523/ENEURO.0145-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig S, Wilcock GK, Love S. Loss of perineuronal net N-acetylgalactosamine in Alzheimer’s disease. Acta neuropathologica. 2005;110:393–401. doi: 10.1007/s00401-005-1060-2. [DOI] [PubMed] [Google Scholar]
- Balmer TS, Carels VM, Frisch JL, Nick TA. Modulation of perineuronal nets and parvalbumin with developmental song learning. J Neurosci. 2009;29:12878–12885. doi: 10.1523/JNEUROSCI.2974-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Endocrine and social regulation of adult neurogenesis in songbirds. Frontiers in neuroendocrinology. 2016 doi: 10.1016/j.yfrne.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Boseret G, Konkle ATM, Hurley LL, Ball GF. Doublecortin as a marker of adult neuroplasticity in the canary song control nucleus HVC. European Journal of Neuroscience. 2008;27:801–817. doi: 10.1111/j.1460-9568.2008.06059.x. [DOI] [PubMed] [Google Scholar]
- Beecher MD, Brenowitz EA. Functional aspects of song learning in songbirds. Trends in ecology & evolution. 2005;20:143–149. doi: 10.1016/j.tree.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Ball GF. Two histological markers reveal a similar photoperiodic difference in the volume of the high vocal center in male European starlings. J Comp Neurol. 1995;360:726–734. doi: 10.1002/cne.903600415. [DOI] [PubMed] [Google Scholar]
- Bernard DJ, Eens M, Ball GF. Age- and behavior-related variation in volumes of song control nuclei in male European starlings. J Neurobiol. 1996;30:329–339. doi: 10.1002/(SICI)1097-4695(199607)30:3<329::AID-NEU2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Best BJ, Day NF, Larson GT, Carels VM, Nick TA. Vocal effects of perineuronal net destruction in adult zebra finc. Abst Soc for Neurosc nbr 30318 2012 [Google Scholar]
- Bidmon HJ, Jancsik V, Schleicher A, Hagemann G, Witte OW, Woodhams P, Zilles K. Structural alterations and changes in cytoskeletal proteins and proteoglycans after focal cortical ischemia. Neuroscience. 1998;82:397–420. doi: 10.1016/s0306-4522(97)00289-3. [DOI] [PubMed] [Google Scholar]
- Böhner J. Early acquisition of song in the zebra finch, Taeniopygia guttata. Anim Behav. 1990;39:369–374. [Google Scholar]
- Böhner J, Chaiken M, Ball GF, Marler P. Song acquisition in photosensitive and photorefractory male European starlings. Horm Behav. 1990;24:582–594. doi: 10.1016/0018-506x(90)90043-w. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) Journal of neurobiology. 2006;66:1044–1060. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature : Insight reviews. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;1016:560–585. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA. Plasticity of the song control system in adult birds. In: Zeigler HP, Marler P, editors. Neuroscience of birdsong. Cambridge University Press; Cambridge: 2008. pp. 332–349. [Google Scholar]
- Brenowitz EA, Beecher MD. Song learning in birds: diversity and plasticity, opportunities and challenges. Trends Neurosci. 2005;28:127–132. doi: 10.1016/j.tins.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Larson TA. Neurogenesis in the adult avian song-control system. Cold Spring Harbor perspectives in biology. 2015:7. doi: 10.1101/cshperspect.a019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner G, Hausen D, Hartig W, Drlicek M, Arendt T, Brauer K. Cortical areas abundant in extracellular matrix chondroitin sulphate proteoglycans are less affected by cytoskeletal changes in Alzheimer’s disease. Neuroscience. 1999;92:791–805. doi: 10.1016/s0306-4522(99)00071-8. [DOI] [PubMed] [Google Scholar]
- Chaiken M, Bohner J, Marler P. Repertoire turnover and the timing of song acquisition in European starlings. Behaviour. 1994;128:25–39. [Google Scholar]
- Cornez G, Shevchouk O, Madison FN, Ball GF, Van Der Linden A, Cornil CA, Balthazart J. Seasonal changes and steroid control of perineuronal nets in the song control system. Abstract of the International Symposium on Avian Endocirnology; Niagara Falls Canada. October 2016.2016. [Google Scholar]
- Cornez G, ter Haar SM, Cornil CA, Balthazart J. Anatomically discrete sex differences in neuroplasticity in zebra finches as reflected by perineuronal nets. PloS one. 2015;10:e0123199. doi: 10.1371/journal.pone.0123199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. Journal of biological rhythms. 2001;16:365–380. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- De Groof G, Verhoye M, Van Meir V, Balthazart J, Van Der Linden A. Seasonal rewiring of the songbird brain: An in vivo MRI study. European Journal of Neuroscience. 2008;28:2475–2485. doi: 10.1111/j.1460-9568.2008.06545.x. [DOI] [PubMed] [Google Scholar]
- Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. The Journal of biological chemistry. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- Eens M. Understanding the complex song of the European starling: an integrated ethological approach. Adv Study Anim Behav. 1997;26:355–434. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. No overlap in song repertoire size between yearling and older starlings Sturnus vulgaris. Ibis. 1992;134:72–76. [Google Scholar]
- Feare CJ. The starling. Oxford Press; Oxford: 1984. [Google Scholar]
- Galtrey CM, Fawcett JW. The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain research reviews. 2007;54:1–18. doi: 10.1016/j.brainresrev.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Geberzahn N, Hultsch H. Long-time storage of song types in birds: evidence from interactive playbacks. Proceedings Biological sciences / The Royal Society. 2003;270:1085–1090. doi: 10.1098/rspb.2003.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill KE, Madison FN, Akins CK. Cocaine-induced sensitization correlates with testosterone in male Japanese quail but not with estradiol in female Japanese quail. Horm Behav. 2015;67:21–27. doi: 10.1016/j.yhbeh.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–1261. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Hara E, Rivas MV, Ward JM, Okanoya K, Jarvis ED. Convergent differential regulation of parvalbumin in the brains of vocal learners. PloS one. 2012;7:e29457. doi: 10.1371/journal.pone.0029457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartig W, Bruckner G, Brauer K, Schmidt C, Bigl V. Allocation of perineuronal nets and parvalbumin-, calbindin-D28k- and glutamic acid decarboxylase-immunoreactivity in the amygdala of the rhesus monkey. Brain research. 1995;698:265–269. doi: 10.1016/0006-8993(95)01016-o. [DOI] [PubMed] [Google Scholar]
- Hartig W, Derouiche A, Welt K, Brauer K, Grosche J, Mader M, Reichenbach A, Bruckner G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain research. 1999;842:15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period mechanisms in developing visual cortex. Current topics in developmental biology. 2005a;69:215–237. doi: 10.1016/S0070-2153(05)69008-4. [DOI] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nature reviews. Neuroscience. 2005b;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hough GE, 2nd, Nelson DA, Volman SF. Re-expression of songs deleted during vocal development in white-crowned sparrows, Zonotrichia leucophrys. Animal behaviour. 2000;60:279–287. doi: 10.1006/anbe.2000.1498. [DOI] [PubMed] [Google Scholar]
- Hurley LL, Wallace AM, Sartor JJ, Ball GF. Photoperiodic induced changes in reproductive state of border canaries (Serinus canaria) are associated with marked variation in hypothalamic gonadotropin-releasing hormone immunoreactivity and the volume of song control regions. General and comparative endocrinology. 2008;158:10–19. doi: 10.1016/j.ygcen.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann N Y Acad Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Karetko M, Skangiel-Kramska J. Diverse functions of perineuronal nets. Acta neurobiologiae experimentalis. 2009;69:564–577. doi: 10.55782/ane-2009-1766. [DOI] [PubMed] [Google Scholar]
- Kirn JR, Nottebohm F. Direct evidence for loss and replacement of projection neurons in adult canary brain. JNeurosci. 1993;13:1654–1663. doi: 10.1523/JNEUROSCI.13-04-01654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Gahr M. Seasonal changes in the song pattern of the non-domesticated canary (Serinus canaria), a field study. Behaviour. 2001a;138:885–904. [Google Scholar]
- Leitner S, Voigt C, Garcia-Segura LM, Van’t Hof T, Gahr M. Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Horm Behav. 2001b;40:160–168. doi: 10.1006/hbeh.2001.1700. [DOI] [PubMed] [Google Scholar]
- Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. PNAS. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nature neuroscience. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison FN, Rouse ML, Jr, Balthazart J, Ball GF. Reversing song behavior phenotype: Testosterone driven induction of singing and measures of song quality in adult male and female canaries (Serinus canaria) General and comparative endocrinology. 2015;215:61–75. doi: 10.1016/j.ygcen.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marler P. Sensitive period and the role of specific and general sensory stimulation in birdsong learning. In: Rauschecker JP, Marler P, editors. Imprinting and cortical plasticity. John Wiley & Sons; New York: 1987a. pp. 99–135. [Google Scholar]
- Marler P. Sensitive periods and the roles of specific and general sensory stimulation in birdsong learning. In: Rauschecker JP, Marler P, editors. Imprinting and cortical plasticity. John Wiley and Sons; New York: 1987b. pp. 99–135. [Google Scholar]
- Marler P. Song learning: the interface between behaviour and neuroethology. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1990;329:109–114. doi: 10.1098/rstb.1990.0155. [DOI] [PubMed] [Google Scholar]
- Meyer CA, Boroda E, Nick TA. Sexually dimorphic perineuronal net expression in the songbird. Basal Ganglia. 2014;3:229–237. [Google Scholar]
- Morris NP, Henderson Z. Perineuronal nets ensheath fast spiking, parvalbumin-immunoreactive neurons in the medial septum/diagonal band complex. The European journal of neuroscience. 2000;12:828–838. doi: 10.1046/j.1460-9568.2000.00970.x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: Cyclical anatomical changes in song-control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci. 2004;1016:628–658. doi: 10.1196/annals.1298.027. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The neural basis of birdsong. PLoS Biol. 2005;3:e164. doi: 10.1371/journal.pbio.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane L. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane LA, Wingfield JC. Seasonal changes in gonadal hormone levels of adult male canaries and their relation to song. Behav Neural Biol. 1987;47:197–211. doi: 10.1016/s0163-1047(87)90327-x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Andalman AS, Fee MS. Vocal experimentation in the juvenile songbird requires a basal ganglia circuit. PLoS Biol. 2005;3:e153. doi: 10.1371/journal.pbio.0030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillmore LS, Hoshooley JS, Sherry DF, MacDougall-Shackleton SA. Annual cycle of the black-capped chickadee: seasonality of singing rates and vocal-control brain regions. Journal of neurobiology. 2006;66:1002–1010. doi: 10.1002/neu.20282. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlngs (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha2-noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria) Hormones and Behavior. 2005;47:467–476. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker M, Churchill L, Todd RP, Blacktop JM, Zuloaga DG, Raber J, Darling RA, Brown TE, Sorg BA. Removal of perineuronal nets in the medial prefrontal cortex impairs the acquisition and reconsolidation of a cocaine-induced conditioned place preference memory. J Neurosci. 2015;35:4190–4202. doi: 10.1523/JNEUROSCI.3592-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. TINS. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Van Hout AJ, Pinxten R, Darras VM, Eens M. Testosterone increases repertoire size in an open-ended learner: an experimental study using adult male European starlings (Sturnus vulgaris) Horm Behav. 2012;62:563–568. doi: 10.1016/j.yhbeh.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Van Hout AJM, Eens M, Balthazart J, Pinxten R. Complex modulation of singing behavior by testosterone in an open-ended learner, the European Starling. Hormones and Behavior. 2009;56:564–573. doi: 10.1016/j.yhbeh.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Voigt C, Leitner S. Seasonality in song behaviour revisited: seasonal and annual variants and invariants in the song of the domesticated canary (Serinus canaria) Horm Behav. 2008;54:373–378. doi: 10.1016/j.yhbeh.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Wang D, Fawcett J. The perineuronal net and the control of CNS plasticity. Cell and tissue research. 2012;349:147–160. doi: 10.1007/s00441-012-1375-y. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Howie GJ, Mooney R. Calcium-binding proteins define Interneurons in HVC of the zebra finch (Taeniopygia guttata) J Comp Neurol. 2005;483:76–90. doi: 10.1002/cne.20403. [DOI] [PubMed] [Google Scholar]
- Wild JM, Williams MN, Suthers RA. Parvalbumin-positive projection neurons characterise the vocal premotor pathway in male, but not female, zebra finches. Brain research. 2001;917:235–252. doi: 10.1016/s0006-8993(01)02938-9. [DOI] [PubMed] [Google Scholar]
- Williams H. Birdsong and singing behavior. Ann N Y Acad Sci. 2004;1016:1–30. doi: 10.1196/annals.1298.029. [DOI] [PubMed] [Google Scholar]
- Xu JC, Xiao MF, Jakovcevski I, Sivukhina E, Hargus G, Cui YF, Irintchev A, Schachner M, Bernreuther C. The extracellular matrix glycoprotein tenascin-R regulates neurogenesis during development and in the adult dentate gyrus of mice. Journal of cell science. 2014;127:641–652. doi: 10.1242/jcs.137612. [DOI] [PubMed] [Google Scholar]