Abstract
The diagnosis of acute myeloid leukemia (AML) can be made based on peripheral blood or bone marrow blasts. In this review, we will discuss the role of bone marrow evaluation and peripheral blood monitoring in the diagnosis, management, and follow up of AML patients. For patients with circulating blasts, it is reasonable to perform the necessary studies needed for diagnosis and risk stratification, including multiparametric flow cytometry, cytogenetics, and molecular analysis, on a peripheral blood specimen. The day 14 marrow is used to document hypocellularity in response to induction chemotherapy, but it is unclear if that assessmentis necessary as it often does not affect immediate management. Currently, response assessments performed at count recovery for evaluation of remission and measurable residual disease rely on bone marrow sampling. For monitoring of relapse, peripheral blood evaluation may be adequate, but the sensitivity of bone marrow testing is in some cases superior. While bone marrow evaluation can certainly be avoided in particular situations, this cumbersome and uncomfortable procedure currently remains the de facto standard for response assessment.
Keywords: Acute myeloid leukemia, Bone marrow evaluation, Flow cytometry, Morphology, Measurable residual disease
1. Use of marrow in initial diagnosis
1.1 Bone marrow evaluation
When the diagnosis of acute myeloid leukemia (AML) is suspected, the treating physician typically recommends a bone marrow evaluation for further morphologic assessment. Indeed, the practice of morphologic assessment of the bone marrow is recommended in the initial diagnostic work-up for suspected AML by the European LeukemiaNet (ELN), the National Comprehensive Cancer Network (NCCN) Guidelines, and the World Health Organization (WHO) 2016 guidelines.1-3 Typically, both bone marrow aspiration and bone marrow trephine biopsy are performed. Some centers perform aspiration alone when possible, with biopsy only in cases of a “dry tap” or diagnostic uncertainty (e.g., distinguishing whether peripheral pancytopenia is related to AML or myelodysplastic syndrome); a biopsy is always indicated if there are no circulating blasts in the peripheral blood and AML is suspected. Analyses typically performed on the bone marrow sample include flow cytometry, metaphase cytogenetics, fluorescence in situ hybridization (FISH), and molecular analyses, which often utilize polymerase chain reaction-based or next-generation sequencing technology to examine specific markers (NPM1, CEBPA, FLT3) or panels of markers with demonstrated importance in myeloid malignancy.4,5 This combined information collected at the time of AML diagnosis is used in prognostication for patients overall,6,7 as well as in recommendations for individual patients regarding whether to proceed to investigational induction chemotherapy and/or allogeneic hematopoietic cell transplant (HCT).8
1.2 Can AML be diagnosed and characterized without bone marrow evaluation?
The diagnosis of AML requires the presence of ≥20% blasts in the peripheral blood or bone marrow; in certain cases, the presence of recurrent cytogenetic abnormalities, such as the characteristic translocation (8;21) define AML even at a lower blast count.3 Frequently the diagnosis of AML can be made without resorting to invasive bone marrow sampling. For patients with high peripheral leukemic blast counts, many of the requisite tests can be performed on peripheral blood. The immunophenotype obtained by flow cytometry has been found to be the same in peripheral blood and bone marrow blasts, though the antibody panel used therein was relatively limited in scope and complexity in this relatively older study9. A small case series confirmed this finding by comparing peripheral blood and bone marrow in patients with acute leukemias, which demonstrated no differences in morphology or immunophenotyping if the peripheral blood blasts were 30% or more.11 Differences have also been found in the cell cycle phase of blasts in these two compartments, though the clinical significance of this finding is yet to be determined.10. In contrast, the particular leukemia-associated immunophenotype (LAIP) for a particular patient may be variable and meaningful, particularly for later monitoring of residual disease.12
In the small case series mentioned previously, the karyotype was insufficient for analysis in 17% of the AML peripheral blood samples (5 out of 29 patients), but in none of the bone marrow samples.11 However, no account was made by the authors of the total blood blast count (i.e., white blood cell count multiplied by percentage of blasts), only the blast percentage in peripheral blood. Since the comparison of karotype between the blood and bone marrow was performed only in patients with a high percentage of peripheral blasts, bone marrow aspiration for karyotype should be performed if few or no circulating blasts are present. Similar findings were demonstrated by the study of Hussein et al, in which patients with high numbers of circulating blasts (at least 0.1 × 109 cells/L) were likely to have successful peripheral blood karyotype (90% success rate or higher).13 In AML patients specifically, peripheral blood karyotyping produced successful metaphases in 32 out of 42 patients (76%).13 Conventional karyotypeing with chromosome banding is the current standard in the diagnosis and work-up of a patient with AML, and several AML entities are defined by in the WHO classification by their recurrent karyotypic abnormalities.1,3 It is possible that next generation sequencing approaches will make conventional chromosomal banding redundant in the future.14
FISH testing, typically performed as part of an AML or myelodysplastic syndrome-specific panel, is less clearly correlated between blood and bone marrow. In a review of 48 cases of AML with paired peripheral blood and bone marrow samples, abnormal peripheral blood FISH results were found in 69% of patients with abnormal bone marrow FISH results (18 of 26), but also in 23% of cases with normal bone marrow FISH results (5 of 22).15 There is uncertainty whether patients with abnormal cytogenetics as assessed by FISH but not standard 20-metaphase karyotype have a prognosis more befitting the FISH results or the standard results; pathologist consultation may help the treating physician in cases of discordance. Molecular analysis has similarly been compared between peripheral blood and bone marrow samples, and peripheral blood has high sensitivity and specificity for detection of FLT3-ITD and NPM1 mutations when the blast count is >2000 cells per microliter.16,17 More recently, the protein expression pattern (so-called proteome) has been shown to be closely correlated between peripheral blood and bone marrow blasts, though this finding is not clinically relevant at the current time.18
Overall, if peripheral blood blasts are high at the time of AML diagnosis (>2000 cells per microliter), we posit that bone marrow examination is an unnecessary adjunct to peripheral blood sampling, which is able to provide morphologic, immunophenotypic, and molecular data; however, discrepancies still remain between the consistency of FISH results in peripheral blood and bone marrow.
1.3 Is morphologic assessment at diagnosis necessary?
Morphologic assessment of the bone marrow for the evaluation of acute leukemias was initially standardized through pathologic review by the French-American-British (FAB) cooperative group in 1976, and revised a decade later.19,20 While the small study mentioned previously showed good correlation between morphology in the peripheral blood and bone marrow,11 the question remains whether morphology is necessary at all for the diagnosis of AML. In fact, at times, the bone marrow may be inaspirable, making morphology moot. In limited subtypes, including acute megakaryoblastic leukemia, acute panmyelosis with myelofibrosis, and acute myeloid leukemia with myelodysplasia-related changes, some have argued that immunohistochemistry performed on a bone marrow biopsy is a crucial adjunct to peripheral blood analysis in order to make the final diagnosis.21 It should be noted that one way for the diagnosis of AML with myelodysplasia-related changes to be made in the 2016 WHO classification is with multilineage dysplasia (defined as >50% of cells with dysplasia in at least two cell lines), which can only be assessed on bone marrow sampling.3 It has recently been suggested that the presence of a mutation in any of SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 in AML is greater than 95% specific for the diagnosis of secondary AML22 suggesting the possibility that future revisions of the WHO classification will continue the trend to move away from morphological descriptors as surrogates of underlying etiology.
However, shortly after the FAB classification was released, flow cytometry was developed and rapidly incorporated into clinical diagnosis, allowing for precise surface marker characterization of acute leukemias at diagnosis and beyond.9,23 Furthermore, it is increasingly becoming evident that morphological and even immunophenotypic assessments cannot accurately reflect the diverse genetic etiology of this class of diseases,24,25 including even clonal heterogeneity within a single patient.26-31 Increasingly sophisticated molecular tools are able to better refine an AML diagnosis based on genetic abnormality,4,14,32 with the important caveats that 1) somatic mutations are not typically in themselves disease defining and can be seen in healthy older adults33-35 or patients in prolonged remissions after chemotherapy36 and that 2) such genomic profiling pre-treatment is currently suboptimal in predicting resistance to induction therapy.37
2. Use of marrow in response assessment
2.1 Utility of the day 14 marrow
Soon after initial diagnosis with AML, fit patients are treated with intensive induction chemotherapy. The NCCN guidelines for AML recommend performing bone marrow evaluation 7-10 days after completion of induction chemotherapy, which is around day 14 if traditional 7+3 chemotherapy (combining continuous low-dose cytarabine with three days of an anthracycline) is used.2 If persistent or residual disease is identified (generally >5-10% blasts, though the background marrow cellularity may also be important), the recommendation is to administer more chemotherapy. A study of clinical flow cytometry examined a novel method to calculate the degree of cytoreduction with induction chemotherapy;38 when combined with knowledge that time to complete remission (CR) is an important prognostic factor,39,40 it seems intuitive that a day 14 marrow would provide important clinical information. Indeed, Liso et al. examined the prognostic value of a day 14 marrow in 198 de novo AML patients in an attempt to derive a predictive tool based on blast percentage.41 Similarly, a German study evaluated outcomes in 449 patients enrolled in the German AML Cooperative Group 1992 trial, and found that day 16 blasts as a continuous variable were significantly related to rates of CR and persistent disease, as well as to overall and relapse-free survival.42 It should be noted that patients in this study and similar studies from European groups received a double induction regardless of early marrow status and achievement of CR after first induction course.42-44 Additionally, there is wide variation when assessing blast clearance in the aforementioned and other studies, having a nadir bone marrow blast cut-off ranging from ‘too few to count’ to <22%. Patients who had blast cut-offs below these ranges had higher rates of CR and improved outcomes. However, intra-observer variability remains an important factor when evaluating hypoplastic marrows.45 More recent studies have questioned the utility of the day 14 marrow, as the marrow results are not always correlated with level of disease and may not reliably predict achievement of CR.
In a retrospective analysis of 194 untreated AML patients, Hussein et al. found that day 14 marrow was highly sensitive in predicting CR (90% sensitivity), but did not predict overall survival.46 In fact, some patients with a high BM blast percentage at day 14 were still able to achieve CR at day 2147 or day 28 without re-induction chemotherapy, a finding also seen in other retrospective analyses using morphology and even flow cytometry.48-50 The method of assessment of residual disease at day 14 or later by flow cytometry may also be important, evaluating a leukemia-associated immunophenotype (so-called LAIP) or a “different-fron-normal” phenotype.12 Further, the treatment algorithm for patients with evidence of disease on a day 14 marrow is not standardized even by practitioners at a single institution,51 as summarized in a recent review.52
2.2 Marrow sampling after induction
It is difficult to debate the necessity of an end of treatment bone marrow performed at count recovery after the first cycle of induction chemotherapy. Remission status is formally assessed around day 28-35, as the peripheral blood counts recover from induction chemotherapy. The definition of CR requires peripheral blood count recovery, generally defined as neutrophils > 1000/microliter, platelets > 100,000/microliter, and independence from red blood cell transfusion, along with a concomitant decrease in marrow blasts to <5%. Such criteria were first proposed in 195653 and were updated in 200354 and 2010,1 and are expected to be updated again within the next year in the 2017 ELN guidelines.55 However, despite patients being in CR by morphology, patients often have measurable residual disease (MRD) detectable by more sensitive flow or polymerase chain reaction (PCR)-based assays. Patients with MRD at the end of treatment or prior to transplant have similar outcomes as patients who have bone marrow blasts > 5%.56-59 Prospective randomized studies need to be peformed evaluating patients in CR but with MRD to determine if further treatment improves outcomes (see section 3.2 Measurable Residual Disease below).
2.3 Use of marrow in patients receiving less intensive therapy
Increasingly, less intensive therapies are being used in the management of patients with AML who are considered not to be candidates for induction chemotherapy; these are typically “less fit” newly-diagnosed patients or relapsed/refractory patients who have not responded to conventional chemotherapy. While the ELN response guidelines do not specify required intensity of treatment, remission status is generally determined after one or two cycles of induction chemotherapy. Less intensive therapies, including hypomethylating agents such as azacitidine and decitabine, may take months in order to achieve CR.60-63 The frequency at which bone marrow evaluation should be performed is not clear; the AZA-AML-001 study, in which patients were randomized to azacitidine or a conventional care regimen, specified that peripheral blood and bone marrow aspirate/biopsy would be collected every second cycle beginning at cycle 3, but the authors did not comment on time to best response.62 A retrospective analysis of patients treated with three cycles of decitabine at MD Anderson suggested patients were more likely to achieve CR if they had a significant (p < 0.05) reduction in the ratio of number of blasts to number of non-blasts in the bone marrow (Estey, unpublished data).
Targeted therapies, whether used alone or combined with intensive chemotherapy, are growing in importance, as investigators seek to exploit the molecular heterogeneity of the disease.64 FLT3 inhibitors have been under investigation for over a decade, but the multikinase inhibitor midostaurin is the first to show an overall survival benefit when studied in a randomized controlled fashion in combination with 7+3 chemotherapy in newly-diagnosed FLT3-mutated patients.65 While midostaurin has primarily been studied in combination with other drugs, the IDH inhibitors used in patients with IDH1 and IDH2 mutations have been used as single agents to date.66 Anecdotally, these drugs have led to a differentiation syndrome with high numbers of blasts seen both in the circulation and in the marrow after multiple weeks of therapy, a finding which seems to correspond to clinical response.67 Such phenomena are provocative, and require a reassessment of the standard timing of blood and marrow response assessments for patients receiving these novel targeted drugs. Simultaneously, some might argue that bone marrow assessment may be advantageous in these patients to avoid prolonged, expensive treatment without demonstrable clinical benefit.
3. Peripheral Blood Monitoring
3.1 Prognostic value of clearing peripheral blood blasts
One non-invasive marker to consider using in place of an invasive day 14 marrow would be kinetics of peripheral blood blast clearance in applicable patients. Clearance of leukemic blasts in the periphery has been correlated with day 14 marrow when analyzed by prospective daily flow cytometry in a small group of 30 patients; in 17 of 19 patients who had a decrease in peripheral blasts of > 2 logs by day 6 of therapy with induction chemotherapy, CR was achieved.68,69 In another study, time to blast clearance monitored by manual differentials in 162 AML patients receiving induction chemotherapy showed that early blast clearance (prior to day 6 of treatment) was able to predict for early marrow blast clearance, CR, relapse free survival, and overall survival.70 Similarly, a retrospective analysis by Elliott et al stratified relapse-free survival in 73 patients with de novo AML who ultimately achieved CR; time of peripheral blast clearance (at or before day 3, on days 4 or 5, and on day 6 or later) was highly significant, with early clearance associated with a relapse rate of 12.5% and late clearance with a much higher relapse rate of 78%.71 These findings were confirmed by an analysis examining peripheral blood blast clearance by more sensitive multiparametric flow cytometry in 130 AML patients.72 Mathematical modeling of the peripheral blood blast clearance has been performed by at least two groups to evaluate kinetics, and rapid blast clearance is strong and independent predictor of CR.72,73 Lacombe et al. evaluated the slope of blast cell decrease in each individual patient over the first four days of treatment, and the slope was strongly correlated with the achievement of CR and risk of relapse.72 Vainstein V et al. examined peripheral blood blast dynamics by modeling an exponential decay curve for 106 patients, and using this methodology calculated an area under the ROC curve of 0.79.73
A major limitation of examining peripheral blood blasts is that not all patients have circulating blasts at diagnosis. Changes in treatment course in response to rate of peripheral blood blast clearance have not been studied in a prospective fashion. An association between mutation clearance (as measured by next-generation sequencing of bone marrow) and clinical outcome has been reported in a retrospective cohort.74 It is possible that kinetics of changes measured with high sensitivity tools, such as used for MRD, on peripheral blood samples during induction may provide early information regarding clinical response; a clinical trial is currently underway at the NIH to test this hypothesis (NCT02527447).
3.2 Measurable Residual Disease (MRD)
As discussed above, CR requires peripheral blood count recovery in addition to morphologic remission in the marrow with blasts <5%. Subtypes of CR include CR with incomplete platelet recovery (CRp) and CR with incomplete neutrophil recovery (CRi), and these subtypes have a significantly worse overall prognosis in terms of both response to chemotherapy and survival.55,75-77
It is possible to further risk stratify patients in a CR by using high sensitivity techniques to detect biomarkers associated with increased relapse risk. These can include flow cytometry, PCR for gene expression, PCR for abnormal gene sequence, and increasingly next generation sequencing.12,78-82 The 2017 ELN guidelines for the diagnosis and treatment of AML that are currently under development will move toward such MRD-based response criteria.55 That is, the most stringent definition of CR will require no evidence of MRD, as detected by multiparametric flow cytometry (MFC)83 or molecular techniques where appropriate for individual patients in the bone marrow. This change is made in response to the fact that post-induction factors, particularly MRD status, have a very strong correlation with outcomes after either further chemotherapy or after allogeneic HCT. MRD provides prognostic information independent of type of response to induction chemotherapy, which can be important in future treatment planning for younger and older patients.77,84-86 Additionally, presence of MRD is a critical factor in determining outcomes for AML patients following allogeneic HCT, to such a degree that patients with MRD, but morphologic remission, behave similarly poorly to those with active disease at the time of allogeneic HCT.58,87-90 These observations have led investigators to suggest that “minimal” in the traditional definition of MRD should be replaced with “measurable,” since any detectable evidence of disease leads to a worse prognosis. The sensitivity of any particular MRD technique used is likely of lesser importance than issues of amount, type, and frequency of sampling; clonal heterogeneity and antigen drift; technical reproducibility; and interpretation and integration of such measurements into clinical care.79,91 The sensitivities of multiple targets assessed by MFC or PCR, which range from 1:100 to 1:200,000, have been comprehensively summarized by Hokland et al.82
Persistence of cytogenetic abnormalities for those patients in remission after therapy is known to be associated with worse outcomes,92,93 though this technique is not sensitive for the presence of residual disease. A number of more sensitive tools exist to detect MRD,81 many of which may be used on peripheral blood, lessening the need for invasive bone marrow sampling. No clear superiority of one MRD technology over another in AML has been proven, with typically at least one hundred fold improvement in sensitivity compared with morphology alone, however flow cytometry methods may suffer from greater variability between centers than molecular approaches.81 PCR-based monitoring of disease in the peripheral blood has been used successfully to monitor for patients with favorable risk AML for translocation(15;17), inversion(16) and translocation(8;21) AML 94-97 and more recently somatic mutations such as in NPM1.98 The ELN performed extensive testing on expression based MRD using WT1.99 Despite being expressed in approximately 90% cases of AML, it was overexpressed to a level useful for MRD monitoring in only around 50% of cases. This limitation may be mitigated, in part, by using a multiple gene approach as studied in patients receiving allogeneic HCT 59,100 and autologous HCT.101 Though sampling of peripheral blood every three months is typically used for monitoring, different molecular aberrations may require more frequent testing or even bone marrow sampling, due to distinct differences in the doubling time of abnormal clones.102,103
MRD assessment using flow cytometric techniques has traditionally been done on marrow samples, but there is increasing evidence that peripheral blood can be used to monitor for MRD. A recent study examined the cumulative incidence of relapse and 3-year overall survival for patients with MRD detected by immunophenotyping of the peripheral blood, and found that both differences were significant. Specifically, the cumulative incidence of relapse at 1 year for patients with peripheral blood MRD positivity was 89% vs. 29% (p<0.001).104 Caveats include that the study included only 114 AML patients with paired bone marrow and peripheral blood samples, primarily at a single center.
Importantly, however, though each of these methods has shown that detection of disease is associated with a worse prognosis, early intervention for MRD-positive patients has not been studied in a systematic manner to demonstrate improvement in outcome when MRD is detected. There is provocative evidence in childhood AML that a risk-stratified approach based on genetic classification and MRD may improve outcomes.105
In the future, there may also be a role for whole-genome or whole-exome sequencing to follow patients for MRD. In an analysis of comprehensive sequencing data for 50 patients with paired samples from diagnosis and remission, the 24 patients who had persistent leukemia-associated mutations had significantly worse survival.74 Though the cost of large-scale sequencing has decreased considerably in recent years, concerns still remain about the interpretation and utility of the large amount of data generated for each individual AML patient. Additionally, the sensitivity and specificity of MRD on outcomes, however the MRD is detected, are such that it can be difficult to counsel individual patients about treatment planning; indeed, though a patient with MRD after induction chemotherapy may be more likely to relapse after allogeneic HCT than one without MRD, that same patient may be more likely to benefit from a graft-versus-leukemia effect than from more cytotoxic chemotherapy.
The optimal frequency of monitoring for the development of MRD is unknown at this time, and likely depends on the specific type of mutations that are identified, as discussed above, because of differences in both test sensitivity and leukemic clone doubling time.98,102,103,106 Whether early intervention will be beneficial for relapsed disease is also unknown; for example, an older study suggested that routine bone marrow examination was not beneficial during first CR,107 and it remains to be proven that early detection of MRD leads to improved survival outcomes. Given the current limitations of the technology, our practice is not to make clinical decisions on the basis of a single MRD result; our viewpoints regarding necessary times for bone marrow evaluation are summarized in Table 1.
Table 1.
The authors' viewpoint on necessity of bone marrow evaluation at standard times during the course of AML diagnosis therapy.
| Time point | Is bone marrow evaluation necessary? | Why or why not? |
|---|---|---|
| Diagnosis | Sometimes | If absolute peripheral blast count >2,000/μl, peripheral blood is sufficient for diagnosis and characterization of AML (morphology, karyotype, and molecular) |
| Day 14 after induction | No | Enough discordance exists in the AML community about how to utilize this information in terms of further chemotherapy that we do not advocate performing a marrow until at least day 21 or at the time of count recovery |
| Count recovery after induction | Yes | MRD is extremely important from a prognostic standpoint, and sensitivity of peripheral blood studies is as yet insufficient to detect low levels of MRD |
| Monitoring for MRD | Possibly | The benefit of early intervention for relapse has not yet been established in AML, meaning that we do not typically monitor disease with routine bone marrow evaluation after the completion of planned treatment. Additionally, for gene expression PCR-based MRD monitoring, peripheral blood may be superior to bone marrow due to high background rates in the bone marrow |
Abbreviations: AML (acute myeloid leukemia); MRD (measureable residual disease).
4. Novel approaches to track disease burden
Bone marrow biopsies at diagnosis and count recovery time-points are currently still the “gold standard.” With the improvement of peripheral blood monitoring techniques, in combination with better imaging modalities, it may be possible to create a new standard for evaluating response to treatment. There are limited studies exploring imaging as a prognostic and predictive indicator of response and survival, likely related to cost and time needed to complete these studies. While provocative, these radiologic studies are not yet ready for incorporation into routine clinical practice.
4.1 FDG PET
Positron emission tomography (PET) is a functional imaging technique used to evaluate metabolic processes. In fludeoxyglucose (FDG) PET, a biologically active analogue of glucose is used as a tracer and is very sensitive at measuring glucose uptake as a function of metabolic activity. However, FDG PET is not specific for distinguishing inflammation secondary to tumor versus infection in the majority of cases. Interestingly, FDG PET has shown efficacy in visualizing extramedullary disease (EMD). In a small study of 10 patients, FDG PET was able to detect known EMD in 90% of patients and additional EMD in 60% with an SUV max range 2.1-8.1.108 Cribe et al. evaluated 26 patients with newly diagnosed AML in which FDG PET found 65% of patients to have EMD compared to 31% by clinical exam. There was a high degree of concordance with bone marrow response and FDG PET response at the end of treatment, with 4 of 6 patients achieving a PR on FDG PET but CR on bone marrow biopsy experiencing an early relapse.109 The utility of FDG PET is unknown, but given the sensitivity of the imaging, FDG PET may be useful as an adjunct at diagnosis for patients with EMD AML to determine extent of disease, and at the end of treatment to document response.110
4.2 18F-FLT PET
18F-FLT PET may be more suitable for the evaluation of AML patients given that 3′-deoxy-3′-18F-fluorothymidine (FLT) is a thymidine analog that is resistant to in vivo degradation and accumulates in proliferating tissues, including rapidly dividing hematopoietic stem cells in the bone marrow.111 The first demonstration of FLT PET in AML patients showed higher rates of biodistribution in the bone marrow, spleen and EMD compared to normal healthy controls.112 In a pilot study of eight patients, 18F-FLT PET was used as an early assessment of treatment response. Eight newly diagnosed AML patients were treated with induction chemotherapy and completed 18F-FLT PET during therapy (range from 2-6 days from start of treatment). Patients with a CR showed SUV uptake < 2 while patients with resistant disease (RD) displayed SUV > 2. SUVmean and SUVmax were also significantly lower in patients with CR compared to RD and normal controls had SUVs similar to patients in CR.113 While the numbers in this study are too small to generalize to larger populations, it addresses an interesting question of using imaging as an early assessment tool of response. For patients who are not responding, it may be worth changing therapy early to avoid unnecessary toxicity from an unsuccessful regimen. Taken in combination with peripheral blood monitoring, there is potential to predict response minimizing the need for an invasive testing. Currently, ECOG-ACRIN Cancer Research Group is conducting a phase 2 study of FLT PET/CT at the time of the nadir bone marrow (days 10-17) in newly diagnosed AML patients being treated with standard induction chemotherapy (NCT02392429).
4.3 DCE-MRI
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) provides global and functional imaging of bone marrow angiogenesis as compared to traditional MRI which uses radio waves in a magnetic field to identify anatomy. In the studies conducted, DCE-MRI has been examined as a predictor for overall survival using a calculated peak enhancement ratio (Peak) and quantification of vascularity (Amp). In 78 de novo AML patients, those with a low Peak and Amp at diagnosis had an improved disease free survival and overall survival compared to patients with a high Peak and Amp.114 Another study by the same group looked at DCE-MRI at day 0 and day 7 of chemotherapy and found that patients with a decrease in Peak values (decrease in angiogenesis compared to baseline) had a higher chance of achieving CR and longer disease free survival compared to patients that had an increase in Peak values (increase in angiogenesis compared to baseline).115 Similar to the results seen with FLT PET, imaging modalities have the potential to strengthen our current testing methods for response assessment.
Future imaging studies have the potential to answer some important outstanding questions before imaging technology can be incorporated into standard assessments for AML monitoring: 1) Which imaging technique most accurately reflects the total burden of disease? 2) When is the ideal time, during or after treatment, for imaging to take place? 3) Can imaging accurately predict which patients will go into a complete remission and measure depth of response? and 4) What is the role of imaging in surveillance?
5. Future directions
Bone marrow evaluation, therefore, remains an important adjunct to peripheral blood analysis in patients with AML, and perhaps always will since some patients do not have circulating peripheral blood blasts. However, we feel that some “standard” tests such as the day 14 marrow are of questionable importance in the management of AML patients and should not be incorporated into routine clinical practice. The recently published WHO guidelines for the diagnosis of AML still espouse morphology as the most important characteristic for the diagnosis and management of myeloid neoplasms,3 though more modern techniques may threaten the hegemony of morphology, as the biology underlying this diverse set of malignancies is better elucidated. While it is likely that highly sensitive tools will be increasingly used on peripheral blood for response assessment and to monitor for clinical relapse, at present, sampling of the bone marrow compartment remains an important component of initial AML diagnosis and at the end of induction treatment in the majority of patients. Figure 1 summarizes the current recommendations for bone marrow evaluation, as well as areas for possible future modifications to the algorithm as methods for diagnosis and monitoring of AML are refined.
Figure 1.
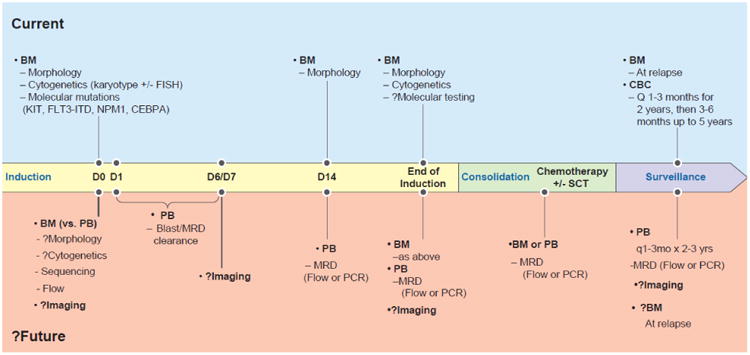
The possible future landscape for diagnosis and monitoring of acute myeloid leukemia at various time points during treatment and subsequent surveillance. The current schema follows the guidelines of the National Comprehensive Cancer Network and others. In the future, we posit that advances in flow cytometry and sequencing (and possibly imaging) may circumvent our current reliance on morphology and cytogenetics. Though the current sensitivity of bone marrow testing is generally 10-fold higher than in the peripheral blood, many tests may be done on peripheral blood only in the future. Timing of surveillance monitoring for measurable residual disease on the peripheral blood will likely depend on the abnormalities being followed for a particular patient.
In an aging population experiencing an increased incidence in AML (de novo, progression from MDS, and treatment related), it is imperative that we consider the patient's ability, willingness, and pain threshold in continuing to do bone marrows. Table 2 summarizes the pros and cons of peripheral blood versus bone marrow sampling for diagnosis and monitoring of AML. Notably, clinical trials often include patients with the best performance status, and the findings generated by such patients may not hold true for the general population.116,117 Work on alternatives to bone marrow examination in AML will continue, primarily using sensitive assays on the peripheral blood and newer imaging technologies, but for now bone marrow evaluation remains an important diagnostic tool in the care of AML patients.
Table 2.
Pros and cons of sampling from the peripheral blood and bone marrow in AML.
| Peripheral blood | Bone marrow |
|---|---|
| Pros | |
|
|
|
|
| Cons | |
|
|
|
|
|
|
|
|
Abbreviations: AML (acute myeloid leukemia); MRD (measurable residual disease)
6. Practice points
Morphologic diagnosis of AML, immunophenotyping, and karyotype can all be performed on a peripheral blood sample if the absolute blast count is > 2,000/μl.
The day 14 marrow is of questionable clinical utility, since it is predictive of CR rates, but not of OS.
Marrow sampling at count recovery after induction chemotherapy is critical for response assessment; patients with a morphologic CR but evidence of MRD have worse outcomes
7. Research agenda
The 2017 ELN guidelines for AML will include a response category of so-called “stringent” CR (i.e., CR without MRD); meanwhile, new techniques are being developed for monitoring MRD, including next-generation sequencing and array-based approaches
The recommended frequency of marrow sampling for less intensive therapies, such as with targeted inhibitors, is unclear
Novel imaging technology, using both PET and MRI, may have a role in future monitoring of AML patients, though many questions still remain about the predictive ability, utility, and cost
Acknowledgments
Funding: This work was supported in part by the Intramural Research Program of the National Heart, Lung, and Blood Institute of the National Institutes of Health.
Footnotes
Conflict of interest: C.S.H. receives research funding from SELLAS Life Sciences Group AG.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–74. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 2.O'Donnell MR, Tallman MS, Abboud CN, et al. Acute myeloid leukemia, version 2. 2013 J Natl Compr Canc Netw. 2013;11:1047–55. doi: 10.6004/jnccn.2013.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 4.Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18. doi: 10.1056/NEJMoa074306. [DOI] [PubMed] [Google Scholar]
- 6.Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–23. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rollig C, Bornhauser M, Thiede C, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29:2758–65. doi: 10.1200/JCO.2010.32.8500. [DOI] [PubMed] [Google Scholar]
- 8.Appelbaum FR. Indications for allogeneic hematopoietic cell transplantation for acute myeloid leukemia in the genomic era. Am Soc Clin Oncol Educ Book. 2014:e327–33. doi: 10.14694/EdBook_AM.2014.34.e327. [DOI] [PubMed] [Google Scholar]
- 9.Griffin JD, Davis R, Nelson DA, et al. Use of surface marker analysis to predict outcome of adult acute myeloblastic leukemia. Blood. 1986;68:1232–41. [PubMed] [Google Scholar]
- 10.Sellar RS, Fraser L, Khwaja A, et al. Cell cycle status in AML blast cells from peripheral blood, bone marrow aspirates and trephines and implications for biological studies and treatment. Br J Haematol. 2016;174:275–9. doi: 10.1111/bjh.14055. [DOI] [PubMed] [Google Scholar]
- 11.Weinkauff R, Estey EH, Starostik P, et al. Use of peripheral blood blasts vs bone marrow blasts for diagnosis of acute leukemia. Am J Clin Pathol. 1999;111:733–40. doi: 10.1093/ajcp/111.6.733. [DOI] [PubMed] [Google Scholar]
- 12.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? Blood. 2014;124:3345–55. doi: 10.1182/blood-2014-05-577593. [DOI] [PubMed] [Google Scholar]
- 13.Hussein K, Ketterling RP, Hulshizer RL, et al. Peripheral blood cytogenetic studies in hematological neoplasms: predictors of obtaining metaphases for analysis. Eur J Haematol. 2008;80:318–21. doi: 10.1111/j.1600-0609.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- 14.McKerrell T, Moreno T, Ponstingl H, et al. Development and validation of a comprehensive genomic diagnostic tool for myeloid malignancies. Blood. 2016;128:e1–9. doi: 10.1182/blood-2015-11-683334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman JF, Theil KS, Tubbs RR, Cook JR. Diagnostic yield of bone marrow and peripheral blood FISH panel testing in clinically suspected myelodysplastic syndromes and/or acute myeloid leukemia: a prospective analysis of 433 cases. Am J Clin Pathol. 2011;135:915–20. doi: 10.1309/AJCPW10YBRMWSWYE. [DOI] [PubMed] [Google Scholar]
- 16.Tong WG, Sandhu VK, Wood BL, et al. Correlation between peripheral blood and bone marrow regarding FLT3-ITD and NPM1 mutational status in patients with acute myeloid leukemia. Haematologica. 2015;100:e97–8. doi: 10.3324/haematol.2014.118422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jilani I, Estey E, Manshuri T, et al. Better detection of FLT3 internal tandem duplication using peripheral blood plasma DNA. Leukemia. 2003;17:114–9. doi: 10.1038/sj.leu.2402743. [DOI] [PubMed] [Google Scholar]
- 18.Hutter G, Letsch A, Nowak D, et al. High correlation of the proteome patterns in bone marrow and peripheral blood blast cells in patients with acute myeloid leukemia. J Transl Med. 2009;7:7. doi: 10.1186/1479-5876-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33:451–8. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 20.Bennett JM, Catovsky D, Daniel MT, et al. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985;103:620–5. doi: 10.7326/0003-4819-103-4-620. [DOI] [PubMed] [Google Scholar]
- 21.Orazi A. Histopathology in the diagnosis and classification of acute myeloid leukemia, myelodysplastic syndromes, and myelodysplastic/myeloproliferative diseases. Pathobiology. 2007;74:97–114. doi: 10.1159/000101709. [DOI] [PubMed] [Google Scholar]
- 22.Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125:1367–76. doi: 10.1182/blood-2014-11-610543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin JD, Mayer RJ, Weinstein HJ, et al. Surface marker analysis of acute myeloblastic leukemia: identification of differentiation-associated phenotypes. Blood. 1983;62:557–63. [PubMed] [Google Scholar]
- 24.Cancer Genome Atlas Research N. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med. 2016;374:2209–21. doi: 10.1056/NEJMoa1516192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding L, Ley TJ, Larson DE, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–10. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klco JM, Spencer DH, Miller CA, et al. Functional heterogeneity of genetically defined subclones in acute myeloid leukemia. Cancer Cell. 2014;25:379–92. doi: 10.1016/j.ccr.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li S, Garrett-Bakelman FE, Chung SS, et al. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat Med. 2016;22:792–9. doi: 10.1038/nm.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bochtler T, Stolzel F, Heilig CE, et al. Clonal heterogeneity as detected by metaphase karyotyping is an indicator of poor prognosis in acute myeloid leukemia. J Clin Oncol. 2013;31:3898–905. doi: 10.1200/JCO.2013.50.7921. [DOI] [PubMed] [Google Scholar]
- 30.Griffith M, Miller CA, Griffith OL, et al. Optimizing cancer genome sequencing and analysis. Cell Syst. 2015;1:210–23. doi: 10.1016/j.cels.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parkin B, Ouillette P, Li Y, et al. Clonal evolution and devolution after chemotherapy in adult acute myelogenous leukemia. Blood. 2013;121:369–77. doi: 10.1182/blood-2012-04-427039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He J, Abdel-Wahab O, Nahas MK, et al. Integrated genomic DNA/RNA profiling of hematologic malignancies in the clinical setting. Blood. 2016;127:3004–14. doi: 10.1182/blood-2015-08-664649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie M, Lu C, Wang J, et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med. 2014;20:1472–8. doi: 10.1038/nm.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med. 2014;371:2477–87. doi: 10.1056/NEJMoa1409405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med. 2014;371:2488–98. doi: 10.1056/NEJMoa1408617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong TN, Miller CA, Klco JM, et al. Rapid expansion of preexisting nonleukemic hematopoietic clones frequently follows induction therapy for de novo AML. Blood. 2016;127:893–7. doi: 10.1182/blood-2015-10-677021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter RB, Othus M, Paietta EM, et al. Effect of genetic profiling on prediction of therapeutic resistance and survival in adult acute myeloid leukemia. Leukemia. 2015;29:2104–7. doi: 10.1038/leu.2015.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiddemann W, Clarkson BD, Buchner T, Melamed MR, Andreeff M. Bone marrow cell count per cubic millimeter bone marrow: a new parameter for quantitating therapy-induced cytoreduction in acute leukemia. Blood. 1982;59:216–25. [PubMed] [Google Scholar]
- 39.Estey EH, Shen Y, Thall PF. Effect of time to complete remission on subsequent survival and disease-free survival time in AML, RAEB-t, and RAEB. Blood. 2000;95:72–7. [PubMed] [Google Scholar]
- 40.Estey EH. Early blast clearance by remission induction as a prognostic factor in acute myeloid leukemia. Curr Oncol Rep. 2003;5:389. doi: 10.1007/s11912-003-0023-9. [DOI] [PubMed] [Google Scholar]
- 41.Liso V, Albano F, Pastore D, et al. Bone marrow aspirate on the 14th day of induction treatment as a prognostic tool in de novo adult acute myeloid leukemia. Haematologica. 2000;85:1285–90. [PubMed] [Google Scholar]
- 42.Kern W, Haferlach T, Schoch C, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003;101:64–70. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- 43.Wheatley K, Burnett AK, Goldstone AH, et al. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999;107:69–79. doi: 10.1046/j.1365-2141.1999.01684.x. [DOI] [PubMed] [Google Scholar]
- 44.Buchner T, Berdel WE, Schoch C, et al. Double induction containing either two courses or one course of high-dose cytarabine plus mitoxantrone and postremission therapy by either autologous stem-cell transplantation or by prolonged maintenance for acute myeloid leukemia. J Clin Oncol. 2006;24:2480–9. doi: 10.1200/JCO.2005.04.5013. [DOI] [PubMed] [Google Scholar]
- 45.Mattison RJ, Luger SM, Lazarus HM. New strategies for the evaluation of the nadir bone marrow following induction in acute myeloid leukemia. Curr Opin Hematol. 2013;20:93–9. doi: 10.1097/MOH.0b013e32835d8207. [DOI] [PubMed] [Google Scholar]
- 46.Hussein K, Jahagirdar B, Gupta P, Burns L, Larsen K, Weisdorf D. Day 14 bone marrow biopsy in predicting complete remission and survival in acute myeloid leukemia. Am J Hematol. 2008;83:446–50. doi: 10.1002/ajh.21133. [DOI] [PubMed] [Google Scholar]
- 47.Yanada M, Borthakur G, Ravandi F, Bueso-Ramos C, Kantarjian H, Estey E. Kinetics of bone marrow blasts during induction and achievement of complete remission in acute myeloid leukemia. Haematologica. 2008;93:1263–5. doi: 10.3324/haematol.12825. [DOI] [PubMed] [Google Scholar]
- 48.Morris TA, DeCastro CM, Diehl LF, et al. Re-induction therapy decisions based on day 14 bone marrow biopsy in acute myeloid leukemia. Leuk Res. 2013;37:28–31. doi: 10.1016/j.leukres.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yezefski T, Xie H, Walter R, et al. Value of routine ‘day 14’ marrow exam in newly diagnosed AML. Leukemia. 2015;29:247–9. doi: 10.1038/leu.2014.268. [DOI] [PubMed] [Google Scholar]
- 50.Norkin M, Chang M, An Q, et al. A new model to predict remission status in AML patients based on day 14 bone marrow biopsy. Leuk Res. 2016;46:69–73. doi: 10.1016/j.leukres.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 51.Ueda M, Xie H, Sandhu RK, Walter RB, Pagel JM, Estey EH. Factors associated with early reinduction chemotherapy for adults with acute myeloid leukemia. Leuk Lymphoma. 2015;56:782–4. doi: 10.3109/10428194.2014.928931. [DOI] [PubMed] [Google Scholar]
- 52.Estey E. Management of persistent AML at day 14. Best Pract Res Clin Haematol. 2014;27:235–40. doi: 10.1016/j.beha.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Bisel HF. Criteria for the Evaluation of Response to Treatment in Acute Leukemia. Blood. 1956;11:673–7. [Google Scholar]
- 54.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 55.Estey E. Acute myeloid leukemia: 2016 Update on risk-stratification and management. Am J Hematol. 2016;91:824–46. doi: 10.1002/ajh.24439. [DOI] [PubMed] [Google Scholar]
- 56.Buckley SA, Appelbaum FR, Walter RB. Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia. Bone Marrow Transplant. 2013;48:630–41. doi: 10.1038/bmt.2012.139. [DOI] [PubMed] [Google Scholar]
- 57.Venditti A, Buccisano F, Del Poeta G, et al. Level of minimal residual disease after consolidation therapy predicts outcome in acute myeloid leukemia. Blood. 2000;96:3948–52. [PubMed] [Google Scholar]
- 58.Araki D, Wood BL, Othus M, et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? J Clin Oncol. 2016;34:329–36. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goswami M, McGowan KS, Lu K, et al. A multigene array for measurable residual disease detection in AML patients undergoing SCT. Bone Marrow Transplant. 2015;50:642–51. doi: 10.1038/bmt.2014.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thepot S, Itzykson R, Seegers V, et al. Azacitidine in untreated acute myeloid leukemia: a report on 149 patients. Am J Hematol. 2014;89:410–6. doi: 10.1002/ajh.23654. [DOI] [PubMed] [Google Scholar]
- 61.Maurillo L, Venditti A, Spagnoli A, et al. Azacitidine for the treatment of patients with acute myeloid leukemia: report of 82 patients enrolled in an Italian Compassionate Program. Cancer. 2012;118:1014–22. doi: 10.1002/cncr.26354. [DOI] [PubMed] [Google Scholar]
- 62.Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–9. doi: 10.1182/blood-2015-01-621664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kadia TM, Thomas XG, Dmoszynska A, et al. Decitabine improves outcomes in older patients with acute myeloid leukemia and higher blast counts. Am J Hematol. 2015;90:E139–41. doi: 10.1002/ajh.24036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Estey E, Levine RL, Lowenberg B. Current challenges in clinical development of “targeted therapies”: the case of acute myeloid leukemia. Blood. 2015;125:2461–6. doi: 10.1182/blood-2015-01-561373. [DOI] [PubMed] [Google Scholar]
- 65.Stone RM, Mandrekar S, Sanford BL, et al. The Multi-Kinase Inhibitor Midostaurin (M) Prolongs Survival Compared with Placebo (P) in Combination with Daunorubicin (D)/Cytarabine (C) Induction (ind), High-Dose C Consolidation (consol), and As Maintenance (maint) Therapy in Newly Diagnosed Acute Myeloid Leukemia (AML) Patients (pts) Age 18-60 with FLT3 Mutations (muts): An International Prospective Randomized (rand)-Controlled Double-Blind Trial (CALGB 10603/RATIFY [Alliance]) Blood (ASH Annual Meeting Abstracts) 2015;126:6. [Google Scholar]
- 66.Paschka P, Schlenk RF, Gaidzik VI, et al. IDH1 and IDH2 mutations are frequent genetic alterations in acute myeloid leukemia and confer adverse prognosis in cytogenetically normal acute myeloid leukemia with NPM1 mutation without FLT3 internal tandem duplication. J Clin Oncol. 2010;28:3636–43. doi: 10.1200/JCO.2010.28.3762. [DOI] [PubMed] [Google Scholar]
- 67.Kc B, DiNardo CD. Evidence for Clinical Differentiation and Differentiation Syndrome in Patients With Acute Myeloid Leukemia and IDH1 Mutations Treated With the Targeted Mutant IDH1 Inhibitor, AG-120. Clin Lymphoma Myeloma Leuk. 2016 doi: 10.1016/j.clml.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gianfaldoni G, Mannelli F, Baccini M, Antonioli E, Leoni F, Bosi A. Clearance of leukaemic blasts from peripheral blood during standard induction treatment predicts the bone marrow response in acute myeloid leukaemia: a pilot study. Br J Haematol. 2006;134:54–7. doi: 10.1111/j.1365-2141.2006.06100.x. [DOI] [PubMed] [Google Scholar]
- 69.Gianfaldoni G, Mannelli F, Bencini S, Leoni F, Baldini S, Bosi A. Peripheral blood blast clearance during induction therapy in acute myeloid leukemia. Blood. 2008;111:1746–7. doi: 10.1182/blood-2007-10-121103. [DOI] [PubMed] [Google Scholar]
- 70.Arellano M, Pakkala S, Langston A, et al. Early clearance of peripheral blood blasts predicts response to induction chemotherapy in acute myeloid leukemia. Cancer. 2012;118:5278–82. doi: 10.1002/cncr.27494. [DOI] [PubMed] [Google Scholar]
- 71.Elliott MA, Litzow MR, Letendre LL, et al. Early peripheral blood blast clearance during induction chemotherapy for acute myeloid leukemia predicts superior relapse-free survival. Blood. 2007;110:4172–4. doi: 10.1182/blood-2007-07-104091. [DOI] [PubMed] [Google Scholar]
- 72.Lacombe F, Arnoulet C, Maynadie M, et al. Early clearance of peripheral blasts measured by flow cytometry during the first week of AML induction therapy as a new independent prognostic factor: a GOELAMS study. Leukemia. 2009;23:350–7. doi: 10.1038/leu.2008.296. [DOI] [PubMed] [Google Scholar]
- 73.Vainstein V, Buckley SA, Shukron O, et al. Rapid rate of peripheral blood blast clearance accurately predicts complete remission in acute myeloid leukemia. Leukemia. 2014;28:713–6. doi: 10.1038/leu.2013.341. [DOI] [PubMed] [Google Scholar]
- 74.Klco JM, Miller CA, Griffith M, et al. Association Between Mutation Clearance After Induction Therapy and Outcomes in Acute Myeloid Leukemia. JAMA. 2015;314:811–22. doi: 10.1001/jama.2015.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yanada M, Borthakur G, Garcia-Manero G, et al. Blood counts at time of complete remission provide additional independent prognostic information in acute myeloid leukemia. Leuk Res. 2008;32:1505–9. doi: 10.1016/j.leukres.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen X, Newell LF, Xie H, et al. Low platelet count reduces subsequent complete remission rate despite marrow with <5% blasts after AML induction therapy. Leukemia. 2015;29:1779–80. doi: 10.1038/leu.2015.23. [DOI] [PubMed] [Google Scholar]
- 77.Chen X, Xie H, Wood BL, et al. Relation of clinical response and minimal residual disease and their prognostic impact on outcome in acute myeloid leukemia. J Clin Oncol. 2015;33:1258–64. doi: 10.1200/JCO.2014.58.3518. [DOI] [PubMed] [Google Scholar]
- 78.Ommen HB. Monitoring minimal residual disease in acute myeloid leukaemia: a review of the current evolving strategies. Ther Adv Hematol. 2016;7:3–16. doi: 10.1177/2040620715614529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hokland P, Ommen HB, Mule MP, Hourigan CS. Advancing the Minimal Residual Disease Concept in Acute Myeloid Leukemia. Semin Hematol. 2015;52:184–92. doi: 10.1053/j.seminhematol.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ossenkoppele G, Schuurhuis GJ. MRD in AML: time for redefinition of CR? Blood. 2013;121:2166–8. doi: 10.1182/blood-2013-01-480590. [DOI] [PubMed] [Google Scholar]
- 81.Hourigan CS, Karp JE. Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol. 2013;10:460–71. doi: 10.1038/nrclinonc.2013.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hokland P, Ommen HB, Nyvold CG, Roug AS. Sensitivity of minimal residual disease in acute myeloid leukaemia in first remission--methodologies in relation to their clinical situation. Br J Haematol. 2012;158:569–80. doi: 10.1111/j.1365-2141.2012.09203.x. [DOI] [PubMed] [Google Scholar]
- 83.Wood BL. Flow cytometric monitoring of residual disease in acute leukemia. Methods Mol Biol. 2013;999:123–36. doi: 10.1007/978-1-62703-357-2_8. [DOI] [PubMed] [Google Scholar]
- 84.Othus M, Wood BL, Stirewalt DL, et al. Effect of measurable (‘minimal’) residual disease (MRD) information on prediction of relapse and survival in adult acute myeloid leukemia. Leukemia. 2016 doi: 10.1038/leu.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Freeman SD, Virgo P, Couzens S, et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol. 2013;31:4123–31. doi: 10.1200/JCO.2013.49.1753. [DOI] [PubMed] [Google Scholar]
- 86.Terwijn M, van Putten WL, Kelder A, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013;31:3889–97. doi: 10.1200/JCO.2012.45.9628. [DOI] [PubMed] [Google Scholar]
- 87.Walter RB, Gooley TA, Wood BL, et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol. 2011;29:1190–7. doi: 10.1200/JCO.2010.31.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Walter RB, Buckley SA, Pagel JM, et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 2013;122:1813–21. doi: 10.1182/blood-2013-06-506725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walter RB, Gyurkocza B, Storer BE, et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia. 2015;29:137–44. doi: 10.1038/leu.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hourigan CS, Goswami M, Battiwalla M, et al. When the Minimal Becomes Measurable. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.67.6395. [DOI] [PubMed] [Google Scholar]
- 91.Butturini A, Klein J, Gale RP. Modeling minimal residual disease (MRD)-testing. Leuk Res. 2003;27:293–300. doi: 10.1016/s0145-2126(02)00166-2. [DOI] [PubMed] [Google Scholar]
- 92.Chen Y, Cortes J, Estrov Z, et al. Persistence of cytogenetic abnormalities at complete remission after induction in patients with acute myeloid leukemia: prognostic significance and the potential role of allogeneic stem-cell transplantation. J Clin Oncol. 2011;29:2507–13. doi: 10.1200/JCO.2010.34.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marcucci G, Mrozek K, Ruppert AS, et al. Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J Clin Oncol. 2004;22:2410–8. doi: 10.1200/JCO.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 94.Willekens C, Blanchet O, Renneville A, et al. Prospective long-term minimal residual disease monitoring using RQ-PCR in RUNX1-RUNX1T1-positive acute myeloid leukemia: results of the French CBF-2006 trial. Haematologica. 2016;101:328–35. doi: 10.3324/haematol.2015.131946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Krauter J, Gorlich K, Ottmann O, et al. Prognostic value of minimal residual disease quantification by real-time reverse transcriptase polymerase chain reaction in patients with core binding factor leukemias. J Clin Oncol. 2003;21:4413–22. doi: 10.1200/JCO.2003.03.166. [DOI] [PubMed] [Google Scholar]
- 96.Laczika K, Mitterbauer G, Mitterbauer M, et al. Prospective monitoring of minimal residual disease in acute myeloid leukemia with inversion(16) by CBFbeta/MYH11 RT-PCR: implications for a monitoring schedule and for treatment decisions. Leuk Lymphoma. 2001;42:923–31. doi: 10.3109/10428190109097711. [DOI] [PubMed] [Google Scholar]
- 97.Grimwade D, Jovanovic JV, Hills RK, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009;27:3650–8. doi: 10.1200/JCO.2008.20.1533. [DOI] [PubMed] [Google Scholar]
- 98.Ivey A, Hills RK, Simpson MA, et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N Engl J Med. 2016;374:422–33. doi: 10.1056/NEJMoa1507471. [DOI] [PubMed] [Google Scholar]
- 99.Cilloni D, Renneville A, Hermitte F, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol. 2009;27:5195–201. doi: 10.1200/JCO.2009.22.4865. [DOI] [PubMed] [Google Scholar]
- 100.Steinbach D, Bader P, Willasch A, et al. Prospective validation of a new method of monitoring minimal residual disease in childhood acute myelogenous leukemia. Clin Cancer Res. 2015;21:1353–9. doi: 10.1158/1078-0432.CCR-14-1999. [DOI] [PubMed] [Google Scholar]
- 101.Mule MP, Mannis GN, Wood BL, et al. Multigene Measurable Residual Disease Assessment Improves Acute Myeloid Leukemia Relapse Risk Stratification in Autologous Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2016 doi: 10.1016/j.bbmt.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ommen HB, Schnittger S, Jovanovic JV, et al. Strikingly different molecular relapse kinetics in NPM1c, PML-RARA, RUNX1-RUNX1T1, and CBFB-MYH11 acute myeloid leukemias. Blood. 2010;115:198–205. doi: 10.1182/blood-2009-04-212530. [DOI] [PubMed] [Google Scholar]
- 103.Hokland P, Ommen HB. Towards individualized follow-up in adult acute myeloid leukemia in remission. Blood. 2011;117:2577–84. doi: 10.1182/blood-2010-09-303685. [DOI] [PubMed] [Google Scholar]
- 104.Zeijlemaker W, Kelder A, Oussoren-Brockhoff YJ, et al. Peripheral blood minimal residual disease may replace bone marrow minimal residual disease as an immunophenotypic biomarker for impending relapse in acute myeloid leukemia. Leukemia. 2016;30:708–15. doi: 10.1038/leu.2015.255. [DOI] [PubMed] [Google Scholar]
- 105.Rubnitz JE, Inaba H, Dahl G, et al. Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial. Lancet Oncol. 2010;11:543–52. doi: 10.1016/S1470-2045(10)70090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hills RK, Ivey A, Grimwade D Group UKNCRIAW. Assessment of Minimal Residual Disease in Standard-Risk AML. N Engl J Med. 2016;375:e9. doi: 10.1056/NEJMc1603847. [DOI] [PubMed] [Google Scholar]
- 107.Estey E, Pierce S. Routine bone marrow exam during first remission of acute myeloid leukemia. Blood. 1996;87:3899–902. [PubMed] [Google Scholar]
- 108.Stolzel F, Rollig C, Radke J, et al. (1)(8)F-FDG-PET/CT for detection of extramedullary acute myeloid leukemia. Haematologica. 2011;96:1552–6. doi: 10.3324/haematol.2011.045047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cribe AS, Steenhof M, Marcher CW, Petersen H, Frederiksen H, Friis LS. Extramedullary disease in patients with acute myeloid leukemia assessed by 18F-FDG PET. Eur J Haematol. 2013;90:273–8. doi: 10.1111/ejh.12085. [DOI] [PubMed] [Google Scholar]
- 110.Cunningham I, Kohno B. (18) FDG-PET/CT: 21st century approach to leukemic tumors in 124 cases. Am J Hematol. 2016;91:379–84. doi: 10.1002/ajh.24287. [DOI] [PubMed] [Google Scholar]
- 111.Shields AF, Grierson JR, Dohmen BM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998;4:1334–6. doi: 10.1038/3337. [DOI] [PubMed] [Google Scholar]
- 112.Buck AK, Bommer M, Juweid ME, et al. First demonstration of leukemia imaging with the proliferation marker 18F-fluorodeoxythymidine. J Nucl Med. 2008;49:1756–62. doi: 10.2967/jnumed.108.055335. [DOI] [PubMed] [Google Scholar]
- 113.Vanderhoek M, Juckett MB, Perlman SB, Nickles RJ, Jeraj R. Early assessment of treatment response in patients with AML using [(18)F]FLT PET imaging. Leuk Res. 2011;35:310–6. doi: 10.1016/j.leukres.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shih TT, Hou HA, Liu CY, et al. Bone marrow angiogenesis magnetic resonance imaging in patients with acute myeloid leukemia: peak enhancement ratio is an independent predictor for overall survival. Blood. 2009;113:3161–7. doi: 10.1182/blood-2008-08-173104. [DOI] [PubMed] [Google Scholar]
- 115.Hou HA, Shih TT, Liu CY, et al. Changes in magnetic resonance bone marrow angiogenesis on day 7 after induction chemotherapy can predict outcome of acute myeloid leukemia. Haematologica. 2010;95:1420–4. doi: 10.3324/haematol.2009.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lazarus HM, Litzow MR, Gale RP. Improving survival in acute myeloid leukemia: pick the best subjects? J Clin Oncol. 2013;31:3854–6. doi: 10.1200/JCO.2013.52.0296. [DOI] [PubMed] [Google Scholar]
- 117.Mengis C, Aebi S, Tobler A, Dahler W, Fey MF. Assessment of differences in patient populations selected for excluded from participation in clinical phase III acute myelogenous leukemia trials. J Clin Oncol. 2003;21:3933–9. doi: 10.1200/JCO.2003.03.186. [DOI] [PubMed] [Google Scholar]


