Abstract
Social housing of rhesus macaques (Macaca mulatta) is considered to be the cornerstone of behavioral management programs in biomedical facilities. However, it also involves the risk of socially inflicted trauma. The ability to avoid such trauma would contribute to the animals' well-being and alleviate staff's concerns, thus paving the path for more introductions. Here, we sought to address the conflict between the need to socially house rhesus macaques and the need to bring social wounding to a minimum by identifying behaviors expressed early in social introductions that may serve as predictors of later wounding events. We employed logistic regression analysis to predict the occurrence of wounding for 39 iso-sexual, adult pairs in the 30 days following the introduction into full contact using the levels of behaviors that were observed at the onset of the introduction. The results show that the levels of submissive behaviors were the only significant predictor to later stage wounding. Higher levels of submissive behaviors expressed during the early phases of the introduction were associated with a decreased likelihood of wounding. Interestingly, levels of affiliative behaviors have not added any power to the predictability of the statistical model. Therefore, it may be suggested that the exchange of submissive signals at the earliest stages of the introduction is critical in the determination of relative rank and preclude the need to establish dominance via aggression when allowed full contact. While the observation of clear-cut dominance relationships is commonly considered a harbinger of success, our findings suggest that it is the acknowledgement of subordination, rather than the expression of dominance that underlies this observed pattern. The value of our findings for guiding social housing decision-making may be strongest in situations in which the composition of potential partners is constrained, and therefore requiring that wise decisions be relied upon early behaviors.
Keywords: social introductions, submissive behaviors, social wounding
Introduction
Group living among wild nonhuman-primates (NHP) is maintained by social interactions that are pivotal for the animals' fitness [Baker, Coleman, Bloomsmith, McCowan and Truelove, 2014; Oettinger, Baker, Neu et al., 2008; Silk, 2007]. For example, strong social bonds have been shown to confer fitness benefits in male Assamese macaques (Macaca assamensis) [Schülke, Bhagavatula, Vigilant and Ostner, 2010], and among female baboons (Papio ssp.), individuals who were more socially integrated survived longer [Schülke, Bhagavatula, Vigilant and Ostner, 2010] and had offspring with relatively higher survival rate compared to less socially integrated females [Silk, Alberts and Altmann, 2003]. Moreover, in rhesus macaques (Macaca mulatta), sociality, both affiliative and agonistic, has been demonstrated to be highly heritable by several measures [Brent, Heilbronner, Horvath et al., 2013]. The expressions of behaviors with such dramatic influence on the animals' fitness has been suggested to be accompanied by high motivation [Dawkins, 1990]. For example, when tested on a series of preference tests, capuchin monkeys (Cebus apella) equally valued social companionship and food [Dettmer and Fragaszy, 2000]. Accordingly, among zoo-housed NHP the chronic inability to express highly motivated behaviors have been linked to poor welfare [Pomerantz, Meiri and Terkel, 2013]. Indeed, sociality is considered to be a fundamental feature in NHP life, and in accordance with that many studies have found that social-housing of NHP promotes their welfare. For instance, pair-housing rhesus macaques resulted in lower levels of anxiety-related behaviors and abnormal behaviors [Baker, Bloomsmith, Oettinger et al., 2014; Gottlieb, Maier and Coleman, 2015], induced a buffer effect against aversive stimuli [Gilbert and Baker, 2011], and was generally associated with superior immunity in comparison to single-housing [Schapiro, Nehete, Perlman and Sastry, 2000]. Therefore, the importance of maintaining compatible social groups (pairs and above) has been recognized by the United States Department of Agriculture [United States Department of Agriculture, 2013], Public Health Service Policy [United States Department of Health and Human Services, 2002], and the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) [National Research Council, 2011]. Social housing is considered to be the cornerstone of most behavioral management programs in biomedical research facilities [Baker, 2007, 2016; Truelove, Martin, Perlman, Wood and Bloomsmith, 2015]. However, there are also risks that are involved when maintaining animals together, in particular the risk of socially inflicted trauma [Hannibal, Bliss-Moreau, Vandeleest, McCowan and Capitanio, 2016; Reinhardt, 1994; Reinhardt and Reinhardt, 2000]. In the wild, rhesus macaques incorporate aggressive and submissive behaviors as a means to establish and maintain highly linear dominance hierarchies [Cooper and Bernstein, 2008; Thierry, 2004]. However, while wild NHP can cope with aggression by avoiding the aggressor [Aureli, 1992] or enlisting the help of allies [Maestripieri, 2007; van Schaik and Aureli, 2000], this is greatly restricted when housed in cages [Novak and Suomi, 1989]. Consequently, NHP are less likely to be able to cope successfully with aggression that is directed at them, which may lead to distress and/or injury. Therefore, wounding is to be avoided as much as possible since it negatively affects the welfare of the animals [Fraser, Weary, Pajor and Milligan, 1997]. In addition, among laboratory-housed animals, trauma may disrupt research, and the required treatment of injuries may not be compatible with the requirements of certain research project protocols (e.g. when animals are assigned to a research study that prohibits the use of antibiotics), and can be costly [Hannibal, Bliss-Moreau, Vandeleest, McCowan and Capitanio, 2016].
In the current study, we sought to address the conflict between the need to socially house rhesus macaques and the need to bring social wounding to a minimum by identifying behaviors expressed early in social introductions that may serve as predictors of later wounding events. At the Tulane National Primate Research Center (TNPRC), the introduction of adult rhesus macaques into pairs begins by introducing animals in adjacent cages that are separated by a barred panel, termed protected contact. This type of setting allows the animals to interact with each other without gaining full access to one another and thereby decreasing the risk of immediate severe aggression. The protected contact period additionally enables behavioral management personnel to assess the compatibility of prospective partners. Animals that are deemed to be initially compatible are then allowed full contact to each other by removing the barred panel. However, the initial assessment does not guarantee that wounding will not occur. More specifically, we hypothesized that the expression of affiliative, aggressive, dominant and submissive behaviors by the animals during the initial protected contact phase would all affect the likelihood of social trauma in the 30 days following introduction into full contact.
Methods
All aspects of management and research use conformed to applicable US federal regulations and the guidelines described in the Guide for the Care and Use of Laboratory Animals [National Research Council, 2011] and the US Department of Agriculture's Animal Welfare regulations [United States Department of Agriculture, 2013], and adhered to the study's protocol as approved by the TNPRC Institutional Animal Care and Use Committee. In addition, methods adhered to the guidelines and principles of the American Society of Primatologists for the ethical treatment of non-human primates [American Society of Primatology, 2014].
Subjects and Housing
Subjects of this study consisted of a set of adult rhesus monkeys (Macaca mulatta) that were involved in 39 isosexual social pairing attempts (21 female pairs, 18 male pairs, N = 39) implemented between April 2015 and May 2016 at the TNPRC. An initial set of subjects (8 male and 10 female pairs) were selected on the basis of injury incurred by at least one of the partners in the 30 days following introduction into full contact. A set of 21 pairs (10 male and 11 female pairs), matched for age, age difference between pair members, and weight difference between pair members comprised the second set. Most subjects (97.3%) were reared at the TNPRC either by their mothers, or in social groups containing the mothers and at least one other adult during their first six months of life. Early rearing of two subjects involved some period of nursery rearing in the first six months, and the rearing of two subjects was unknown. A total of 76 unique animals (41 females, 35 males) were included in the analysis. All but two animals were represented in only one pair, with the remaining having been introduced to two different partners. At the time of social introduction, animals were a mean of 7.3 years of age ± 2.4 (SD). Females ranged in age from 4.2 to 17.1 years (mean ± SD = 7.8 ± 2.4) and males from 4.2 to 12.0 years (mean ± SD = 6.7 ± 2.2).
The subjects were housed indoors in rooms maintained on a 12:12-hr light:dark cycle and ambient temperature between 18–22°C with a relative humidity of 30–70%. Stainless steel cages had a height of 0.8–0.9 m and floor space of 0.4–0.8 m2 which met or exceeded federal animal welfare regulations. Caging was designed so that adjacent cages could interconnect to expand space for individual animals or for social groups. The side walls of each cage were fitted with two sliding panels, including a solid panel on one side and a barred panel on the other. This design allows two degrees of access between adjacent cages (see Figure 1). The barred panel consisted of a 37.5 cm-deep by 92 cm-high area containing vertical bars, 2 cm apart. This barred area was located in the front half of the cages' side walls, leaving a 34-cm-deep solid area in the back of the cage to serve as a visual barrier. Study subjects were provided with various manipulanda as well as foraging boards. Subjects were fed twice daily with commercial monkey biscuits. Feeding enrichment including fresh fruits, nuts or seeds were also provided daily. Water was provided ad libitum.
Figure 1.

A view of the side wall with a barred panel separating the two cages during the protected contact phase. The barred panel can be slid in, in order to allow for full contact between prospective partners.
All subjects were also assigned to research or breeding protocols. For thirty of the thirty-nine pairs studied, the identity of the pair-mate was entirely dictated by research constraints (i.e., pairs could only be derived from the same research project and treatment group). However, for 18 subjects, there was some latitude in the composition of pairs. Pairings were chosen so that individuals were not closely matched in body weight, since greater weight disparity has been associated with a higher success rate in some studies [Capitanio and Cole, 2015] and in the authors' personal experience.
Social Introduction Procedure
A minimum of 24 hrs prior to the onset of the introduction process, the prospective partners were placed in adjacent cages. Social introductions between potential pair mates were conducted between Mondays and Wednesdays in the mornings (9:00-11:00 AM) after husbandry work had been completed in the rooms. Behavioral management staff began introductions by pulling the solid panel, permitting limited contact through the barred panel, thus entering a protected contact phase. In protected contact, individuals could touch, communicate and interact through the panel. At a minimum, partners were observed immediately following the pulling of the solid panel, an additional time in the afternoon, twice (morning and afternoon) during the following day, and finally, once a day for the remaining weekdays in this setting. Additional observations were conducted based on the judgment of the behavioral management staff who introduced the animals. Observations and checks included visual inspection of the animals and the cage for blood and injuries. If during the protected contact phase no wounding was observed and no obvious signs of incompatibility such as overt, persistent, bi-directional aggression occurred, animals were then allowed full access to one another by pulling the barred panel. This step was taken after a mean of 11.8 ±10.9 days in protected contact. The duration of this phase was influenced by two factors: 1) the schedule of upcoming anesthetic or husbandry events that might negatively influence introduction outcomes by increasing the stress experienced by the animals and 2) observed behavior. Only pairs that progressed into the full contact phase were included in the current study.
Behavioral Data Collection
All data were collected during the first minutes of the initial protected contact introduction. Each pair was observed by one of five behavioral management technicians who were trained by the authors of this study to identify conspicuous affiliative, aggressive, dominant and submissive behaviors (see Table I). The behavioral management technicians entered the room and waited approximately 10 minutes prior to the onset of data collection which began immediately following the pulling of the solid panel (the onset of protected contact). Observations lasted for a mean of 15.1±5.3 minutes. A total of 589 minutes of data were recorded in person on a tablet computer, using instantaneous scan sampling with 30 second intervals [Martin and Bateson, 2007]. We focused on clear, distinctive and easily-identified behaviors since we were interested in detecting behavioral markers that were practical to train multiple individuals to recognize.
Table 1.
A list of the observed behaviors divided into four categories: affiliative, aggressive, dominant and submissive behaviors
| Affilative Behaviors | |
|---|---|
| Co-threatening/solicit co-threatening | alternating threats and glancing at the partner, who may or may not join in the threatening. |
| Grooming | manipulation, brushing, or licking of fur (or eyes, wounds) of another animal with the mouth and/or both hands. |
| Lip-smacking | bringing the lips together rapidly, resulting in a smacking sound; teeth are covered. |
| Mounting through the panel | common usage, with or without pelvic thrusting and penetration and with or without foot clasp. |
| Playing | Non-aggressive, lively actions performed with another individual with or without direct physical contact. Play should not be accompanied by pilo-erection. Play can be accompanied by a play face and/or laughing or other vocalizations. |
| Aggressive Behaviors | |
| Aggressive contact | physical contact that may or may not result in injury ( (e.g. mouth fight, pushing, pulling, grabbing, minor scratching). |
| Lunging | high-speed aggressive intentional movement toward another animal. |
| Threatening partner | at least one of the following: 1) stare (visual fixation in an aggressive context. Body appears rigid. Often accompanied by pilo-erection. May also include ears flattened against the head, brow retracted, round mouth, teeth partially exposed [open-mouth stare]). May also include head bobbing 2) threat gesture: slap surface while oriented toward partner or slap at the partner without making contact. |
| Dominant Behaviors | |
| Displaying | vigorous shaking, slamming, or bouncing off of the cage. |
| Supplanting | animal approaches within proximity of another individual, resulting in the latter's movement away from the current space. |
| Submissive Behaviors | |
| Avoiding | immediate movement away from an approaching animal once the approach begins. |
| Cowering | cringe in a crouched position with limbs held beneath the body and head lowered. |
| Eye Averting | avoidance of eye contact with animal with whom one is directly engaged in a social interaction (may include an approach, threat, grooming bout). Body of the actor is typically oriented toward the social partner while the gaze is oriented away. |
| Fearful grimacing | grin-like facial expression involving retraction of the lips, exposing teeth. May be accompanied by vocalizations, such as: shrill, high-pitched calls, including screeching, squealing, and squeaking. |
| Rump presenting | a posture involving a stance on all fours with the hind quarters elevated and the tail raised. In some animals the tail may be lifted to the side rather than raised. In some instances, animals may place their heads between their legs. |
All incidences of injury in the 30 days following introduction into full contact were recorded. Among the pairings involving wounding, ten of the eighteen wounding incidents required veterinary intervention due to lacerations and puncture wounds. The remaining eight wounding incidents involved scratches and other superficial injuries.
Statistical Analysis
For all statistical tests, the pair was the unit of analysis. All pairs were dichotomized according to whether or not any wounding occurred. Behavioral data for each observation were expressed as percentage of observation data points in which the behavior was expressed by either partner and recorded. The percent of scans during which a behavior was observed was calculated across both members of the pair.
As the preliminary step, potential correlations between the outcome variable (occurrence or absence of wounding in the period of up to 30 days following introduction into full contact) and the covariates: the percentage of affiliative, aggressive, dominant and submissive behaviors that were recorded at the onset of protected contact and the sex and absolute weight difference between partners were examined using Pearson correlation in order to assess the nature of their relationship.
We then employed binary logistic regression analysis in order to predict the occurrence of wounding for 39 iso-sexual pairs using the above mentioned covariates. Statistical analyses were done using IBM SPSS Statistics version 22. For all analyses an alpha value of p < 0.05 was considered significant.
Results
Descriptive statistics are provided in Table II.
Table 2. Descriptive Statistics.
| Variable | N | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|---|
| Mean % of affiliative behaviors | 39 | 0.00 | 84.00 | 13.15 | 16.77 |
| Mean % of aggressive behaviors | 39 | 0.00 | 22.00 | 1.74 | 4.50 |
| Mean % of dominant behaviors | 39 | 0.00 | 11.00 | 0.67 | 2.39 |
| Mean % of submissive behaviors | 39 | 0.00 | 97.00 | 23.00 | 29.36 |
| Absolute delta weight (kg) | 39 | 0.05 | 10.85 | 2.51 | 2.13 |
The preliminary Pearson correlation only detected significant correlation between the occurrence of trauma and mean percent of scans with submissive behaviors (r[37] = -0.52, P = 0.001).
Binary logistic regression analysis
A test of the full model against an intercept-only model was statistically significant, indicating that the predictors as a set (sex, absolute weight difference, and all four behavioral categories) reliably distinguished between introductions that resulted in injury and those that had not (Binary logistic regression: χ2= 14.118, P = 0.028 with df = 6; Nagelkerke's R2 = 0.406). Prediction success overall was 71.8% (compared to 53.8% in the intercept only model). The Wald criterion demonstrated that only the mean percent of scans with submissive behaviors made a significant contribution to prediction (P = 0.009). Exp(B) value (which represents the odds ratio) indicates that for every 1% increase in scans with observed submissive behaviors the odds of later stage wounding decrease by 5.8% (see Table III).
Table 3. Predictors of socially inflicted wounding.
| Predictor | B | Standard error | Wald | DF | Significance | Exp(B) | 95% C.I.for EXP(B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Sex | -0.592 | 0.794 | 0.556 | 1 | 0.456 | 0.553 | 0.117 | 2.622 |
| Absolute delta weight | -0.072 | 0.187 | 0.147 | 1 | 0.701 | 0.931 | 0.644 | 1.344 |
| Mean % of affiliative behaviors | -0.005 | 0.021 | 0.06 | 1 | 0.807 | 0.995 | 0.954 | 1.037 |
| Mean % of aggressive behaviors | -0.019 | 0.115 | 0.027 | 1 | 0.868 | 0.981 | 0.784 | 1.228 |
| Mean % of dominant behaviors | 0.128 | 0.164 | 0.61 | 1 | 0.435 | 1.137 | 0.824 | 1.569 |
| Mean % of submissive behaviors | -0.059 | 0.023 | 6.899 | 1 | 0.009 | 0.942 | 0.901 | 0.985 |
| Constant | 1.381 | 0.885 | 2.437 | 1 | 0.119 | 3.979 | ||
The following predictors were entered: Sex (0=Female, 1=Male), Delta weight (kg) at time of social introduction, mean % of scans with: affiliative, aggressive, dominant and submissive behaviors
The relationship between the mean % of scans with submissive behaviors and the predicted probability for wounding in up to 30 days following introduction into full contact can be seen in Figure 2. In order to calculate the predicted probability for later stage social trauma, we first calculated the predicted logit scores according to the following formula:
Figure 2.
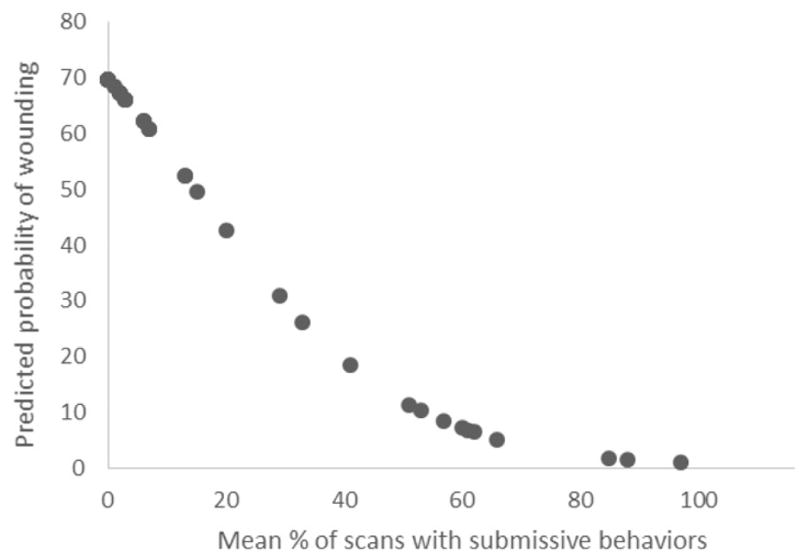
Relationship of mean % of scans with submissive behaviors to predicted probability of wounding up to 30 days following introduction into full contact.
In the next step, the predicted probabilities were calculated according to the following formula (by using Euler's number):
Discussion
Social housing is widely considered as the foundation of welfare for naturally gregarious NHP species in captivity [e.g., National Research Council, 2011, Office of Laboratory Animal Welfare, 2014] and is therefore mandated (with some exceptions) by federal law [United States Department of Agriculture, 2013]. Indeed, comprehensive surveys from recent years demonstrate that increased use of social housing is in fact the trend among research facilities [Baker, 2007, 2016]. Nevertheless, the social introduction process and pairing of caged, adult rhesus macaques is still regarded as overly precarious by some laboratory animal staff, due to their perception regarding the likelihood of wounding [DiVincenti and Wyatt, 2011]. While this view seems to put more weight on the risks compared to the benefits [DiVincenti and Wyatt, 2011], it may serve as a significant deterrent to pairing NHP. Therefore, in order to address those concerns, and with the interest of minimizing the incidence of social trauma, it is crucial to be able, as early in the introduction process as possible, to identify incompatible prospective social partners that are likely to wound one another. Ideally, this detection should be done while the animals have restricted ability to significantly injure each other, i.e., in protected contact.
The use of protected contact as a transitory phase is almost universally employed [Baker, 2016]. This familiarization period has been suggested to be critical in many cases since it enables the animals to establish rank in a relatively safe environment, thus, forestalling the need to use aggression in order to assert dominance when allowed full-access [DiVincenti and Wyatt, 2011; Reinhardt, 1989, 1995]. The results of the current study support the use of protected contact, since clearly meaningful communication is exchanged between potential rhesus macaque partners even before they are granted full access to each other. In our study, however, we evaluated what type of initial communication most closely corresponds to outcome, and found that higher levels of submissive behaviors predict lower probability of later stage wounding. Therefore, it may be suggested that the exchange of submissive signals in rhesus macaques are critical in the determination of relative rank and preclude the need to establish dominance via aggression. While the observation of clear-cut dominance relationships is commonly considered a harbinger of success (reviewed in DiVincenti and Wyatt, 2011), our findings suggest that it is the acknowledgement of subordination, rather than the expression of dominance (e.g. supplanting) that underlies this observed pattern.
Our findings also suggest that during initial interactions, affiliative behaviors, which are traditionally regarded by staff as the gold standard for determining compatibility, have no predictive capacity for later stage social trauma. It is commonly assumed that individuals who groom one another, play and/or lip-smack will be compatible when allowed full access, and will not inflict injuries on their partner. Indeed, in approximately half of facilities in the United States, affiliative behaviors must be observed at the early stages of the introductions process in order for the introduction to progress [Baker, 2016]. While further research is required to evaluate the predictive role of affiliation later in the introduction process, we suggest the need for a shift in attention that emphasizes the significance of submissive rather than affiliative behaviors. The findings that are published herein suggest that if the predictive effect of affiliation remains lacking beyond initial protected contact, then pairing programs that require the observation of grooming in initial interactions could be unnecessarily abandoning potentially successful pairs. Our results may also be important for decision-making because repeated expression of submissive behavior could be misinterpreted by staff to indicate high levels of fear and distress, contributing to a decision to terminate an introduction attempt.
This study generated several other surprising findings. First, it was unexpected that predictors of wounding did not vary with sex, both because of behavioral sex differences in rhesus macaques, but also because predictors of pairing success have been found to vary with sex. This finding, however, is in line with several others who have found no differences in rates of aggression and wounding between male and female rhesus macaques [Oettinger, Baker, Neu et al. , 2008; Reinhardt, 1987]. Second, it is somewhat surprising that body weight differences did not contribute to the predictability of the statistical model, since it has been reported previously [Baker, 2010] at least among males (rhesus macaques [Capitanio, Blozis, Snarr, Steward and McCowan, 2017]; vervets (Chlorocebus aethiops )[Jorgensen, Lambert, Breaux et al., 2015]). The role of weight may vary by species (e.g. pigtailed macaques (Macaca nemestrina [Worlein, Kroeker, Lee et al., 2016]); cynomolgus macaques (M. fascicularis [Abney, Toscano, Poor and Moomaw, 2014]). Such analyses, however, may be very vulnerable to the inclusion of sexually-immature animals in the subject pool [Maguire-Herring, Stonemetz, Lynch and Fahey, 2013; West, Leland, Collins et al., 2009]. For some of these studies, contrasting findings may relate to the fact that the focus is on introduction success per se, rather than injury. Indeed, Worlein et al. [2016] found differences in predictors between wounding and compatibility in pigtailed macaques. It is important for the reader to be aware that the findings of the current study relate only to the former and not the latter.
Several caveats must be noted. First, the subject pool was relatively small and the significance of our conclusions is tempered by this fact. Second, no inter-observer reliability testing was performed. However, the data collection system was specifically designed to be readily learnable and feasible for large teams of employees. Third, our analyses rested on the presence or absence of an interaction type within a pair, and did not address whether one or both individuals expressed a behavioral category or the preponderance of directionality between partners. All could potentially yield insights into the factors underlying later social trauma. Fourth, our findings pertain only to initial interactions during the protected contact phase of introductions. Predictors may very well differ once animals are allowed to share enclosures (i.e., be housed in full contact. Fifth, study subjects were followed for only 30 days following introduction into full contact. Compatibility in the first 30 days does not of course guarantee long-term compatibility. However, at the TNPRC, after the 30 day period, only 3% of individuals incur wounds over the full tenure of their pair, suggesting that our findings are relevant beyond the initial period (unpublished data). Sixth, as with much of the behavioral management literature, it is important to avoid assuming that the findings of studies of rhesus macaques generalize to other (even closely related) species. There is emerging evidence that there are relevant behavioral contrasts even within the macaque genus. For example, a recent study of pair introductions of pigtailed macaques found that aggression during the initial day of introduction predicted injuries, and, rather than having no effect, grooming was actually positively correlated with later injuries [Worlein, Kroeker, Lee et al. , 2016].
Last, it must be noted that common practice at the many facilities relies heavily on the expression of aggressive behaviors in determining when to abandon pair introduction attempts. While it would not be ethical to do otherwise, it prevents us from fully examining the predictive role in aggression for later wounding. Nonetheless, our findings suggest that our level of tolerance for some aggression is appropriate, because the aggression observed among pairs whose introduction is allowed to continue does not predict later wounding. Evaluations such as those described herein may be particularly valuable to disseminate to other stakeholders involved in rhesus macaque care and use. Injury following any observation of any degree of aggression may be assumed both within behavioral management departments and between stakeholders to represent mistakes in judgement, undermining the confidence in behavioral management programs necessary for collaborative care and use of laboratory primates. Furthermore, some forms of aggression and even wounding do not always necessitate the separation of the pair [Baker, Coleman, Bloomsmith, McCowan and Truelove, 2014]. In fact, assessing the correspondence between aggression and introduction outcome could be an important test in evaluating program performance. A negative finding would suggest that introduction decision-making involves an appropriate level of tolerance for aggression. Should a facility find that the level of aggression that they tolerate predicts later social trauma, then managers may want to refine decision rules and truncate introduction attempts at a lower severity level of aggression.
While it is always desirable to avoid wounding, each case should be examined in light of the severity of the wound and the context in which it occurred, in order to reach an informed decision regarding the fate of the pair. It is important to note that the social behavior of rhesus macaques is complex, and may involve more than just submissive behaviors when clear rank relationships are set. For example, captive rhesus macaques use aggression when new groups are formed in order to establish social order [Bernstein, Gordon and Rose, 1974], behavioral displays, such as branch shaking are employed by wild male rhesus macaques in unstable groups when competing for dominance [Milich and Maestripieri, 2016], and affiliative behaviors play an important role in maintaining dominance hierarchies [Snyder-Mackler, Kohn, Barreiro et al., 2016]. Nevertheless, the significance of the current study is in identifying behavioral indicators in the context of the initial familiarization process, while housed in protected contact, and when additional clear signals such as overt, persistent aggression are lacking. We found that levels of submissive behaviors that are expressed during the onset of the introduction process are the best predictor to later stage social wounding, but they should not be the only factor to be taken into account when determining whether a pair should remain together or separated. Because this study has limits, we are not suggesting that the affiliation, aggression, and dominance behaviors should be ignored during introduction observations. However, we suggest using submissive behaviors as a stronger signal than the other behavioral categories. We also strongly suggest that the observation of affiliation not be mandatory for continuing introduction attempts.
These findings have significant implications for the welfare of rhesus macaques in biomedical facilities. While it has been firmly recognized that social housing of rhesus macaques is critical to their welfare [Hannibal, Bliss-Moreau, Vandeleest, McCowan and Capitanio, 2016], it is also known that pathological states (that can result from injuries) decrease welfare levels since they limit the animals' capability to cope with their environment, and often involve pain [Broom, 2006]. Thus, being able to separate animals before they have full capacity to injure each other, while at the same time keeping social partners with little risk of incurring damage together, is likely to be a major refinement. Moreover, in some cases, the injury may be an acute manifestation of social tension that has been building-up in the period proceeding to it. In cases such as these, the animals' welfare is being affected not just by the stress associated with the actual trauma, but also by the detrimental psychological effects of behavioral incompatibility [Novak and Suomi, 1989; Truelove, Martin, Perlman, Wood and Bloomsmith, 2015]. Early separation of ultimately incompatible partners will spare them unnecessary stress. Finally, the ability to predict the likelihood of wounding by using clearly identifiable behavioral signals should alleviate some of the concerns that stakeholders may have and pave a path for more social introductions. The value of our findings for guiding social housing decision-making may be strongest in situations in which the composition of potential partners is somewhat to entirely constrained. Literature is available for guiding partner choice (for a review, see Truelove et al 2015) but when choice is not available, wise decisions based upon early behaviors must be relied upon. Our findings therefore underscore the need for considerable early monitoring of social introductions.
Research Highlights.
Among rhesus macaques, higher levels of submissive behaviors expressed during the early phases of the introduction were associated with a decreased likelihood of wounding at later stages.
Levels of affiliative behaviors have not added any power to the predictability of the statistical model.
Exchanging submissive signals at the earliest stages of the introduction is critical in the determination of relative rank and preclude the need to establish dominance via aggression when allowed full contact.
Acknowledgments
All research complied with the US Department of Agriculture Animal Welfare Regulations, and was supported by the National Center for Research Resources and the Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health through grant number P51 OD011104 to the Tulane National Primate Research Center. We would like to acknowledge the behavioral management team that paired the research subjects and collected behavioral data.
References
- Abney D, Toscano J, Poor L, Moomaw H. Successful social housing of adult male cynomolgus macaques with similar bodyweights. American Journal of Primatology. 2014;76:86. [Google Scholar]
- [January 2017];American Society of Primatologists Social housing for nonhuman primates used in biomedical or behavioral research in the United States. 2014 Retrieved from www.asp.org/society/resolutions/socialhousing.cfm.
- Aureli F. Post-conflict behaviour among wild long-tailed macaques (Macaca fascicularis) Behavioral Ecology and Sociobiology. 1992;31(5):329–337. [Google Scholar]
- Baker K. Retrospective assessment of pair formation in laboratory rhesus macaques: refining partner selection American Journal of Primatology. WILEY-LISS DIV JOHN WILEY & SONS INC; 111 RIVER ST, HOBOKEN, NJ 07030 USA: 2010. pp. 26–27. [Google Scholar]
- Baker KC. Enrichment and Primate Centers: Closing the Gap Between Research and Practice. Journal of Applied Animal Welfare Science. 2007;10(1):49–54. doi: 10.1080/10888700701277618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC. Survey of 2014 behavioral management programs for laboratory primates in the United States. American Journal of Primatology. 2016;78(7):780–796. doi: 10.1002/ajp.22543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof VAM. Comparing options for pair housing rhesus macaques using behavioral welfare measures. American Journal of Primatology. 2014;76(1):30–42. doi: 10.1002/ajp.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Coleman K, Bloomsmith MA, McCowan B, Truelove MA. Pairing rhesus macaques (Macaca mulatta): methodology and outcomes at four national primate research centers. American Society of Primatologists; Decatur, GA: 2014. p. 104. [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and Social Controls in Rhesus Monkey <i>(Macaca mulatta) </i>Groups Revealed in Group Formation Studies. Folia Primatologica. 1974;21(2):81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Brent LJN, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, Skene JHP, Platt ML. Genetic origins of social networks in rhesus macaques. Scientific Reports. 2013;3:1042. doi: 10.1038/srep01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom DM. Behaviour and welfare in relation to pathology. Applied Animal Behaviour Science. 2006;97(1):73–83. [Google Scholar]
- Capitanio JP, Blozis SA, Snarr J, Steward A, McCowan BJ. Do “birds of a feather flock together” or do “opposites attract”? Behavioral responses and temperament predict success in pairings of rhesus monkeys in a laboratory setting. American Journal of Primatology. 2017;79(1):1–11. doi: 10.1002/ajp.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Bernstein IS. Evaluating Dominance Styles in Assamese and Rhesus Macaques. International Journal of Primatology. 2008;29(1):225–243. [Google Scholar]
- Dawkins MS. From an animal's point of view: Motivation, fitness, and animal welfare. Behavioral and Brain Sciences. 1990;13(01):1–9. [Google Scholar]
- Dettmer E, Fragaszy D. Determining the Value of Social Companionship to Captive Tufted Capuchin Monkeys (Cebus apella) Journal of Applied Animal Welfare Science. 2000;3(4):293–304. [Google Scholar]
- DiVincenti L, Wyatt JD. Pair Housing of Macaques in Research Facilities: A Science-Based Review of Benefits and Risks. Journal of the American Association for Laboratory Animal Science : JAALAS. 2011;50(6):856–863. [PMC free article] [PubMed] [Google Scholar]
- Fraser D, Weary DM, Pajor EA, Milligan BN. A Scientific Conception of Animal Welfare that Reflects Ethical Concerns. Animal Welfare. 1997;6(3):187–205. [Google Scholar]
- Gilbert MH, Baker KC. Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. Journal of Medical Primatology. 2011;40(2):71–78. doi: 10.1111/j.1600-0684.2010.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Maier A, Coleman K. Evaluation of environmental and intrinsic factors that contribute to stereotypic behavior in captive rhesus macaques (Macaca mulatta) Applied Animal Behaviour Science. 2015;171:184–191. doi: 10.1016/j.applanim.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, Capitanio J. Laboratory rhesus macaque social housing and social changes: Implications for research. American Journal of Primatology. 2016 doi: 10.1002/ajp.22528. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen MJ, Lambert KR, Breaux SD, Baker KC, Snively BM, Weed JL. Pair housing of vervets/African green monkeys for biomedical research. American Journal of Primatology. 2015 doi: 10.1002/ajp.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D. Macachiavellian Intelligence: How Rhesus Macaques and Humans Have Conquered the World. Chicago: University of Chicago Press; 2007. [Google Scholar]
- Maguire-Herring V, Stonemetz KM, Lynch LJ, Fahey MA. The effect of weight on the compatibility of isosexual pairs of captive rhesus macaques (Macaca Mulatta) American Journal of Primatology. 2013;75(S1):77. [Google Scholar]
- Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. Cambridge, UK: Cambridge University Press; 2007. [Google Scholar]
- Milich KM, Maestripieri D. Sex or power? The function of male displays in rhesus macaques. Behaviour. 2016;153(3):245–261. [Google Scholar]
- National Research Council (Institute of Laboratory Animal Resources) Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 2011. [Google Scholar]
- Novak MA, Suomi SJ. Psychological Weil-Being of Primates in Captivity. ILAR Journal. 1989;31(3):5–15. [Google Scholar]
- Oettinger BC, Baker KC, Neu K, Griffis C, Schoof V, Maloney M, Clay A, Bloomsmith MA. Wounding incidence in isosexual pairs of adult rhesus macaques (Macaca mulatta) during introduction and in varying pair housing conditions. American Journal of Primatology. 2008;70(Suppl 1):71. [Google Scholar]
- Office of Laboratory Animal Welfare position statement on nonhuman primate housing. 2014 http://grants.nih.gov/grants/olaw/positionstatement_guide.htm#nonhuman.
- Pomerantz O, Meiri S, Terkel J. Socio-ecological factors correlate with levels of stereotypic behavior in zoo-housed primates. Behavioural Processes. 2013;98:85–91. doi: 10.1016/j.beproc.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Are male rhesus monkeys more aggressive than females? Primates. 1987;28(1):123–125. [Google Scholar]
- Reinhardt V. Behavioral responses of unrelated adult male rhesus monkeys familiarized and paired for the purpose of environmental enrichment. American Journal of Primatology. 1989;17(3):243–248. doi: 10.1002/ajp.1350170305. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Pair-housing rather than single-housing for laboratory rhesus macaques. Journal of Medical Primatology. 1994;23(8):426–431. doi: 10.1111/j.1600-0684.1994.tb00131.x. [DOI] [PubMed] [Google Scholar]
- Reinhardt V. Social housing of previously single-caged macaques: what are the options and the risks? Animal Welfare. 1995;4:307–328. [Google Scholar]
- Reinhardt V, Reinhardt A. Meeting the “social space” requirements of pair-housed primates. Laboratory Primate Newsletter. 2000;39(1):7–8. [Google Scholar]
- Schapiro SJ, Nehete PN, Perlman JE, Sastry KJ. A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Applied Animal Behaviour Science. 2000;68(1):67–84. doi: 10.1016/s0168-1591(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Schülke O, Bhagavatula J, Vigilant L, Ostner J. Social Bonds Enhance Reproductive Success in Male Macaques. Current Biology. 2010;20(24):2207–2210. doi: 10.1016/j.cub.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Silk JB. Social Components of Fitness in Primate Groups. Science. 2007;317(5843):1347–1351. doi: 10.1126/science.1140734. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social Bonds of Female Baboons Enhance Infant Survival. Science. 2003;302(5648):1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Snyder-Mackler N, Kohn JN, Barreiro LB, Johnson ZP, Wilson ME, Tung J. Social status drives social relationships in groups of unrelated female rhesus macaques. Animal Behaviour. 2016;111:307–317. doi: 10.1016/j.anbehav.2015.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry B. Social epigenesis. In: Thierry B, Singh M, Kaumanns W, editors. Macaque societies: a model for the study of social organization. New York: Cambridge University Press; 2004. pp. 267–290. [Google Scholar]
- Truelove MA, Martin AL, Perlman JE, Wood JS, Bloomsmith MA. Pair housing of macaques: A review of partner selection, introduction techniques, monitoring for compatibility, and methods for long-term maintenance of pairs. American Journal of Primatology. 2015 doi: 10.1002/ajp.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Animal Welfare Act and Animal Welfare Regulations(“Blue book”) Sectopns 3.81 -Environmental enhancement to Promote psychological well-being U.S Department of Agriculture. [Google Scholar]
- amended 2002. Public Health Service Policy 507on Humane Care and Use of Laboratory Animals; Washington, DC: 1986. U.S Department of Health and Human Services. [Google Scholar]
- van Schaik CP, Aureli F. The Natural History of Valuable Relationships in Primates. In: Aureli F, De Waal FBM, editors. Natural Conflict Resolution. Berkeley, CA, US: University of California Press; 2000. pp. 307–333. [Google Scholar]
- West AM, Leland SP, Collins MW, Welty TM, Wagner WL, Erwin JM. Pair formation in laboratory rhesus macaques (Macaca mulatta): a retrospective assessment of a compatibility testing procedure. American Journal of Primatology. 2009;71(S1):37. [Google Scholar]
- Worlein JM, Kroeker R, Lee GH, Thom JP, Bellanca RU, Crockett CM. Socialization in pigtailed macaques (Macaca nemestrina) American Journal of Primatology. 2016 doi: 10.1002/ajp.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]


