Abstract
Background
The nitric oxide synthase gene (NOS1) exon 1f (ex1f) VNTR is a known genetic risk factor for Attention-Deficit/Hyperactivity Disorder (ADHD), particularly in females. NOS1 plays an important role in neurite outgrowth and may thus influence brain development, specifically white matter (WM) microstructure, which is known to be altered in ADHD. The current study aimed to investigate whether NOS1 is associated with WM microstructure in (female) individuals with and without ADHD.
Methods
Diffusion Tensor Imaging (DTI) scans were collected from 187 participants with ADHD (33% female) and 103 controls (50% female), aged 8–26 years, and NOS1-ex1f VNTR genotype was determined. Whole-brain analyses were conducted for fractional anisotropy (FA) and mean diffusivity (MD) to examine associations between NOS1 and WM microstructure, including possible interactions with gender and diagnosis.
Results
Consistent with previous literature, NOS1-ex1f was associated with total ADHD and hyperactivity-impulsivity symptoms, but not inattention; this effect was independent of gender. NOS1-ex1f was also associated with MD values in several major WM tracts in females, but not males. In females, homozygosity for the short allele was linked to higher MD values than carriership of the long allele. MD values in these regions did not correlate with ADHD symptoms. Results were similar for participants with and without ADHD.
Conclusions
NOS1-ex1f VNTR is associated with WM microstructure in females in a large sample of participants with ADHD and healthy controls. Whether this association is part of a neurodevelopmental pathway from NOS1 to ADHD symptoms should be further investigated in future studies.
Keywords: ADHD, NOS1, imaging genetics, DTI
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders (Faraone et al., 2015) and is thought to be caused by a combination of genetic and environmental risk factors, through different causal or neurobiological pathways (Sonuga-Barke, 2005, Coghill et al., 2005). Despite its high heritability, only a few genetic risk factors have been identified, all with small effects (Franke et al., 2009, Franke et al., 2012, Faraone et al., 2015). Moreover, it is still unknown how these genetic risk variants lead to the clinical symptoms of ADHD. One approach to better understand the function of genetic risk factors in psychiatric disorders is to investigate whether they affect brain structure and/or function (Dresler et al., 2014, Durston, 2010), which can help to identify neurobiological mechanisms underlying ADHD.
Converging evidence indicates that ADHD is associated with strong and widespread alterations in the microstructure and organization of brain white matter (WM) tracts, i.e. the structural connectivity between brain regions. In children and adults with ADHD, regions of elevated as well as reduced fractional anisotropy (FA) and mean diffusivity (MD) values have been reported, measured with Diffusion Tensor Imaging (DTI) (van Ewijk et al., 2012, Cortese et al., 2013, Konrad et al., 2010, Onnink et al., 2015). FA represents the direction of water diffusion in the brain, whereas MD represents the strength of diffusion, both being indicative of the organization and microstructural integrity of brain WM tracts. Altered WM connectivity in ADHD has been associated with dysfunctions in cognitive control, as well as clinical symptoms of inattention and impulsivity (Konrad et al., 2010, Casey et al., 2007, Liston et al., 2011, Nagel et al., 2011, Onnink et al., 2015).
WM alterations in ADHD are likely to have a strong genetic basis. A large study comprising data from the Human Connectome Project and the ENIGMA consortium showed heritability estimates ranging from 0.53 to 0.90 for eleven major WM tracts analysed using tract-based spatial statistics (Kochunov et al., 2015), and genetic effects on WM microstructure have been found to be much stronger than shared environmental factors (Jahanshad et al., 2010). Furthermore, a comparison of properties of WM microstructure between parents and their children with ADHD revealed that WM properties in frontostriatal regions were highly correlated between parents and their offspring, suggesting a genetic basis for decreased frontostriatal connectivity in ADHD (Casey et al., 2007). Moreover, two DTI studies showed that unaffected siblings of subjects with ADHD had similar WM alterations as their affected siblings, suggesting that WM changes observed in ADHD have a shared familial basis (Lawrence et al., 2013, van Ewijk et al., 2014). Its strong genetic basis makes WM microstructure an interesting candidate for imaging genetics studies in ADHD. For example, in Alzheimer’s Disease, associations have been found between FA values and several common variants, including variants in the genes encoding the brain-derived neurotrophic factor (BDNF), clusterin (CLU), and catechol-O-methyl transferase (COMT) (Wu et al., 2014). No studies to date have specifically investigated associations of WM properties with ADHD risk genes.
An interesting candidate gene for such studies is NOS1 (Nitric Oxide Synthase 1). NOS1 is located on chromosome 12 and encodes an enzyme that synthesizes nitric oxide (NO), which plays an important role in early brain development. It has been shown that neuronal nitric oxide is strongly involved in the differentiation of neurons in the brain, as well as in neurite outgrowth (Chen et al., 2006). NOS1 inhibitors have been found to block neurite outgrowth, and can cause aberrant outgrowth of axons in the brain (Cramer et al., 1996, Kalisch et al., 2002, Park et al., 2016). Variation in this gene has been linked to several psychiatric disorders. Association studies and functional studies identified a link between single nucleotide polymorphisms (SNPs) in this gene and schizophrenia (Weber et al., 2014). Furthermore, this gene was among the (suggestive) top-findings of a genome wide association study (GWAS) in children with ADHD (Franke et al., 2009, Lasky-Su et al., 2008), while another study found that NOS1 genotype was associated with ADHD symptoms in adults, but not children, with ADHD (Salatino-Oliveira et al., 2016). A different type of genetic variant, a variable number of tandem repeats (VNTR), in exon 1f of the gene, was linked to impulsivity disorders, including (adult) ADHD (Reif et al., 2009). Of the multiple alternative first exons of the gene, the NOS1 exon 1f is highly expressed in the brain, specifically in frontostriatal regions. The VNTR in this exon has multiple alleles, which have been grouped into long (L) and short (S). Homozygosity for the short alleles (S/S) can result in decreased gene expression compared to carriership of long alleles (S/L or L/L) (Reif et al., 2009). A significant excess of S alleles has been found in adults with ADHD (Reif et al., 2009). Homozygosity for the short allele appears to be specifically related to hyperactive-impulsive behaviour, and the hyperactive-impulsive and combined types of ADHD (Reif et al., 2009, Tanda et al., 2009, Weber et al., 2015). The association between the NOS1 VNTR and ADHD symptoms appears to be gender-specific; both the original study as well as a recent meta-analysis showed that the association of NOS1 with ADHD and impulsive behaviour was only present in females (Weber et al., 2015, Reif et al., 2009).
Nitric oxide plays an important role in the differentiation of neurons in the brain, as well as in neurite outgrowth (Chen et al., 2006). This suggests early influences of NOS1 on brain development, through neurogenesis and WM outgrowth and organization. In addition, NOS1 has been implicated in a neurobiological network involved in neurite outgrowth, which was found when linking the top-results of five early ADHD GWASs (Poelmans et al., 2011). Consequently, NOS1 may influence early WM development in the brain, yet no studies to date have used DTI to investigate this.
The current study aimed to investigate the association between the ex1f VNTR in NOS1 and WM microstructure, as measured by DTI, in a large sample of subjects with ADHD and healthy controls. In particular, we investigated the role of gender, given previous reports of gender-specificity of NOS1’s association with ADHD. Exploratory analyses were aimed at examining whether this association would be different between individuals with ADHD and controls. Given that we are the first to investigate the association between NOS1 and WM microstructure using DTI, and the scarcity of previous literature in this area, we did not formulate specific hypotheses about the expected effects of NOS1 on DTI parameters or specific WM tracts.
Methods
Participants
The current study was part of the NeuroIMAGE project, the Dutch follow-up of the IMAGE cohort, which has been described in detail elsewhere (Von Rhein et al., 2014). In short, inclusion criteria for the NeuroIMAGE cohort were: age between 6–30 years, European Caucasian descent, IQ ≥ 70, and no known neurological or genetic disorder. Individuals with comorbid psychiatric disorders reported by parents were excluded, except for oppositional defiant disorder (ODD), conduct disorder (CD), and pervasive developmental disorder not otherwise specified (PDD-NOS), given their high co-occurrence in ADHD. Complete data (i.e. a DTI scan, NOS1 genotyping, and full diagnostic information) were available for 187 participants with ADHD and 103 healthy controls.
Procedure
The current study made use of a DTI scan that was part of a comprehensive assessment protocol, see (van Ewijk et al. 2014). Testing was carried out at two locations in The Netherlands (Amsterdam and Nijmegen), using identical protocols. Participants were asked to withhold the use of psychoactive medication for 48 hours before measurement. IQ was estimated by a short form of the Wechsler Intelligence Scale for Children–III (WISC- III) or Wechsler Adult Intelligence Scale–III (WAIS-III; for participants ≥17 years of age) containing the Vocabulary and Block Design subtests. This study was approved by the regional ethics committee (Centrale Comissie Mensgebonden Onderzoek: CMO Regio Arnhem Nijmegen; 2008/163; ABR: NL23894.091.08) and the medical ethical committee of the VU University Medical Center. Informed consent was signed by all participants (≥12 years) and/or their parents (for subjects under 18 years). Participants received 50 euros and a copy of their MRI scan after completion of the study.
Diagnostic Assessment
For a full description see (Von Rhein et al., 2014). In short, all participants were assessed with a combination of a semi-structured diagnostic interview (Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version; K-SADS-PL) (Kaufman et al., 1997) and Conners’ ADHD questionnaires from different informants (Conners et al., 1999, Conners et al., 1998a, Conners et al., 1998b). Information was combined using an algorithm, to create a combined symptom count from all informants. Symptom counts were created for inattentive symptoms and hyperactive-impulsive symptoms separately, as well as a total symptom count (sum of both symptom dimensions). ADHD diagnoses were based on full DSM-IV-TR (American Psychiatric Association, 2000) criteria, using the combined symptom count. Control participants were required to score ≤3 symptoms on both symptom dimensions. Criteria were slightly adapted for young adults (≥18 years), such that a combined symptom count of 5 symptoms was sufficient for a diagnosis (American Psychiatric Association, 2013), and ≤2 symptoms on both symptom dimensions were required for controls.
Genotyping
Genotyping was performed on DNA isolated from blood or saliva using standard protocols. Generally, 5% blanks as well as duplicates in and between plates were included as quality controls during genotyping. All genetic analyses were performed at the Department of Human Genetics of the Radboudumc in Nijmegen.
Sequence length analysis of the NOS1-ex1f VNTR was performed for genetic analysis with a genetic analyzer (GeneScan) (for genotyping details, see Supplementary Online Material: Appendix S1). Fragment length analysis was performed on the ABI prism 3730 Genetic Analyzer (Applied Biosystems), and GeneMapper Software version 4.0 (Applied Biosystems) was used to determine the different VNTR allele lengths. Alleles were grouped into short (S) and long (L) alleles in line with previous studies (Reif et al., 2009, Hoogman et al., 2011) (see Table S1).
Imaging acquisition and (pre-)processing
MRI scanning was carried out on a 1.5 Tesla Sonata or Avanto MRI scanner (Siemens, Erlangen, Germany), using the same Siemens 8-channel head coil. Whole-brain, high-resolution T1-weighted anatomical images were acquired in the sagittal plane (MP-RAGE, 176 slices, acquisition matrix 256 × 256, voxel size 1×1×1 mm; TE/TR=2.95/2730 ms, TI=1000 ms, FA=7°, GRAPPA-acceleration). Eddy current-compensated diffusion-weighted SE-EPI images were collected during one acquisition consisting of five volumes without directional weighting (b=0), followed by 60 volumes with non-collinear gradient directions (60 interleaved slices, matrix 64 × 64, voxel size 2 × 2 × 2.2 mm, TE/TR = 97/8500 ms, b-value 1000 s/mm2, GRAPPA-acceleration 2).
Pre-processing included eddy current correction, realignment (using affine transformations and mutual information as a cost function), unwarping image distortions, and correction for motion-induced artefacts, using SPM8 (Wellcome Trust Centre for Neuroimaging) functionality and in-house developed methods. Tensor images were estimated, and FA and MD maps were derived for each participant, which were further processed using FSL’s Tract-Based Spatial Statistics (TBSS) (Smith et al., 2006) using standard settings with a threshold of FA>0.3. For a full description of image (pre)processing, see Appendix S2.
Data analysis
Group differences in sample characteristics were investigated using analysis of variance and Chi square tests in SPSS (version 21, IBM, Chicago, IL, USA). Symptom count differences between NOS1-ex1f genotype groups were investigated using Mann-Whitney U tests in SPSS (version 21, IBM, Chicago, IL, USA), given the non-normality of our symptom count data. Voxelwise TBSS analyses on FA and MD values were performed in FSL using the randomise tool, based on permutation testing (Smith et al. 2006). A General Linear Model (GLM) was built with the predictors diagnostic group (ADHD versus controls), genotype (risk [S/S] versus other [S/L, L/L]), and gender, and covariates for age and scan site. Dependent variables were FA and MD values. To test whether the association between NOS1 genotype and WM properties differed between participants with ADHD and controls, or between males and females, 3- and 2-way interactions were tested between genotype, diagnostic group, and gender. Results were obtained using Threshold-Free Cluster Enhancement (TFCE) (Smith and Nichols, 2009), providing results at p<0.05 using family-wise error (FWE) correction for multiple testing. Anatomical labels of significant clusters were identified using built-in Harvard-Oxford and JHU atlases for WM tracts in FSLview.
Mean FA or MD values were extracted from significant clusters to explore associations between significant clusters and ADHD symptoms. Pearson correlations between FA or MD values and normalized symptom count were conducted for total ADHD symptoms, as well as the two separate symptom domains (hyperactivity/impulsivity and inattention symptoms).
Results
Sample characteristics
The final sample included in the analyses consisted of a total of 290 individuals between 8 and 24 years of age, including 170 children (under 18 years of age) and 120 young adults (18–25 years). Sample characteristics are summarized in Table 1. Taking into account the three main factors that were included in the analyses (genotype, gender, and diagnostic group), subgroups consisted of 60 individuals with the risk genotype (including 26 ADHD males, 14 ADHD females, 9 control males, and 11 control females) and 230 individuals with other genotypes (including 100 ADHD males, 47 ADHD females, 42 control males, and 41 control females). For a schematic overview of the sample, see Figure 1. Motion parameters were recorded for all subjects during the scan. The average displacement (translation) over all directions was compared between groups, to ensure that motion differences between groups did not confound results of the analyses. Motion parameters did not differ between diagnostic groups (F(1,286)= .408, p=.524), genotypes (F(1, 286)=1.891, p=.170), or gender (F(1, 286)=.019, p=.891).
Table 1.
Sample characteristics.
| Risk genotype (S/S) | Other genotypes (S/L, L/L) | Test statistics | p-value | Contrasts | |||
|---|---|---|---|---|---|---|---|
| (n=60) | (n=230) | ||||||
| Gender (% male) | 58.3% | 61.7% | χ2(1)=.232 | .367 | – | ||
| ADHD (% diagnosed) | 66.7% | 63.9% | χ2(1)=.158 | .406 | – | ||
| Number of ADHD symptoms (M, SD) | |||||||
| Total | 9.75 (6.76) | 8.26 (6.32) | H(3)=3.956, w2=.01 | .047 | Risk > other | ||
| Inattention | 5.13 (3.55) | 4.66 (3.62) | H(3)=.884, w2=.00 | .347 | |||
| Hyperactivity-impulsivity | 4.62 (3.53) | 3.60 (3.20) | H(3)=4.414, w2=.02 | .036 | Risk > other | ||
|
| |||||||
| Risk males | Risk females | Other males | Other females | Test statistics | p-value | Contrastsa | |
| (n=35) | (n=25) | (n=142) | (n=88) | ||||
|
| |||||||
| Age, years (M, SD) | 17.80 (3.29) | 16.76 (3.72) | 17.22 (2.84) | 16.57 (3.51) | F(3,289)=1.52 | .208 | – |
| IQ (M, SD) | 102.08 (14.97) | 100.15 (14.98) | 98.83 (14.80) | 101.99 (13.95) | F(3,288)=1.05 | .373 | – |
| Scan site (% Amsterdam) | 48.6% | 36.0% | 53.3% | 34.1% | χ2(3)=9.35 | .025 | 2 < 3, 3 < 4 |
| Number of ADHD symptoms (M, SD) | |||||||
| Total | 10.74 (6.45) | 8.36 (7.06) | 9.04 (6.04) | 7.00 (6.60) | H(3)=10.020, w2=.03 | <.018 | 1 > 4, 3 > 4 |
| Inattention | 5.54 (3.36) | 4.56 (3.79) | 5.24 (3.48) | 3.73 (3.67) | H(3)=10.358, w2=.04 | <.016 | 1 > 4, 3 > 4 |
| Hyperactivity-impulsivity | 5.20 (3.52) | 3.80 (3.45) | 3.8 (3.08) | 3.27 (3.38) | H(3)=9.093, w2=.03 | <.028 | 1 > 3, 1 > 4 |
| Autism spectrum scorec | 10.29 (9.11) | 8.80 (8.73) | 8.47 (8.27) | 7.26 (8.35) | F(3,285)=1.13 | .337 | – |
| ODD/CD comorbidityd | 20.0% | 16.0% | 20.4% | 15.9% | χ2(3)=0.89 | .828 | – |
| History of medication use (%) | 71.0% | 63.2% | 64.2% | 50.0% | χ2(3)=5.70 | .127 | – |
1=males with risk genotype; 2=females with risk genotype; 3=males with other genotype; 4=females with other genotype
Combined symptom count from K-SADS-PL and Conners’ ADHD questionnaires
Using the Children’s Social Behaviour Questionnaire
Comorbid diagnosis of oppositional defiant disorder (ODD) or conduct disorder (CD), as determined by the K-SADS interview
Figure 1.
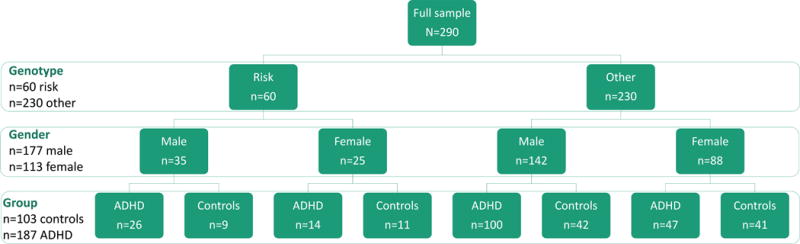
Schematic overview of the sample, stratified by the main predictors in the analyses: Diagnostic group (ADHD versus controls), genotype (risk [S/S] versus other [S/L and L/L]), and gender (male versue female).
Genotype and ADHD symptoms
Genotype was equally distributed over the two diagnostic groups. We did observe differences between genotypes in the number of ADHD symptoms: subjects with the risk genotype (S/S) (n=60) had higher hyperactive-impulsive and total ADHD symptom scores compared with subjects with the other genotypes (n=230) (see Table 1 for details). When the analysis was repeated in subgroups stratified by gender, no clear gender-specific pattern emerged (see Table 1), suggesting that the association between NOS1 and ADHD symptoms was not female-specific. Of note, it is possible that stratification by gender and diagnostic group would show a different pattern, since it is possible that this association is only or mostly present in females with ADHD. However, the current sample was too small for such subgroup analyses (smallest group: n=9 male control subjects with the risk genotype).
White matter microstructure, NOS1 and ADHD
First, we tested the 3-way interaction between genotype, gender and diagnostic group. We found no significant clusters for a 3-way interaction on FA or MD values (all p>.228). Of note, this finding should be interpreted with care given that the smallest group in this analysis consisted of only n=9 individuals (control males with the risk genotype).
Second, we investigated the genotype by gender interaction. We found a significant interaction on MD values in several major WM tracts, captured in four clusters (see Table 2 and Figure 2). Clusters of significant voxels were located in the bilateral anterior, superior, and posterior corona radiata, the body of the corpus callosum, the right superior longitudinal fasciculus and the posterior internal capsule. No significant results were found for FA values (all p>.538). To further interpret the genotype by gender interaction, mean MD values from the significant voxels were plotted, stratified by gender, for visualisation purposes (see Figure 2), and TBSS analyses were re-run in males (n=177) and females (n=113) separately as a post-hoc analysis. Results revealed a main effect of genotype on MD in females (n=25 risk genotype versus n=88 other genotypes) in the right anterior, superior, and posterior corona radiata, anterior and posterior internal capsule, external capsule, genu of the corpus callosum, and superior longitudinal fasciculus (see Figure 3). The risk genotype was associated with higher MD values compared with the other genotypes (p=.013, d=0.57). Exploratory correlation analyses were conducted to examine whether these clusters were associated with ADHD symptoms. No significant correlations were found between mean MD values from the significant clusters and the number of ADHD symptoms (−.09 > rs > −.12. and all p>.216 for inattention, hyperactive-impulsive, and total symptoms). No genotype effects on MD values were found in males (n=35 risk genotype versus n=142 other genotypes) (all p≥.165).
Table 2.
White matter tracts showing a significant association between NOS1 genotype and mean diffusivity (MD) values.
| MNI coordinatesa
|
|||||||
|---|---|---|---|---|---|---|---|
| cluster # | x | y | Z | n Voxels | p-value | Hemisphere | Anatomical labels |
|
Full sample - gender by genotype interaction
| |||||||
| 1 | 28 | −7 | 26 | 6319 | 0.017 | Right | Anterior/superior/posterior corona radiata, posterior internal capsule, superior longitudinal fasciculus, body of the corpus callosum |
| 2 | −23 | 18 | 13 | 2319 | 0.041 | Left | Anterior/superior corona radiata |
| 3 | −19 | −56 | 44 | 1848 | 0.036 | Left | Posterior corona radiata, splenium of the corpus callosum |
| 4 | −18 | 44 | 18 | 656 | 0.047 | Left | Anterior corona radiata |
|
Females - main effect of genotype | |||||||
| 1 | 24 | −9 | 13 | 8659 | 0.013 | Right | Anterior/superior/posterior corona radiata, anterior/posterior internal capsule, external capsule, genu of the corpus callosum, superior longitudinal fasciculus |
Note. The table shows significant clusters with cluster size ≥ 100 voxels at p < .05 (family-wise error corrected for multiple comparisons), controlling for age, ADHD status, and scan center.
MNI coordinates represent maximum intensity voxel.
Figure 2.
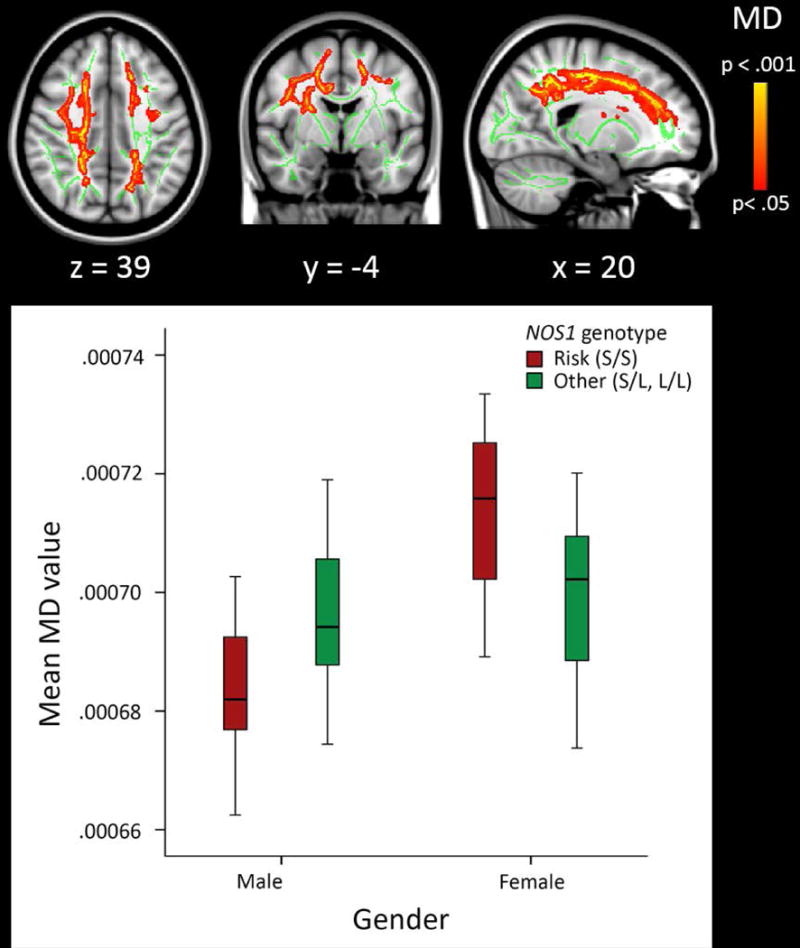
Main results from the voxelwise tract-based spatial statistics (TBSS) analysis. For visualisation of the interaction between NOS1 genotype and gender, mean MD value derived from all significant clusters are plotted, stratified by gender.
Note. Results are overlaid on a standard MNI152 template with the mean skeleton in green (FA>0.3), and were “thickened” towards the full width of the tract for visualization purposes.
Figure 3.
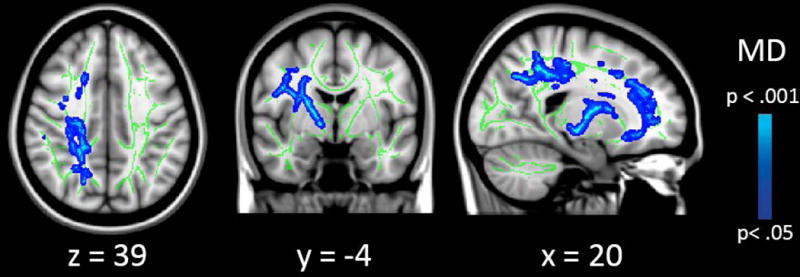
Results from the follow-up analysis, showing an association between NOS1 genotype and mean diffusivity (MD) specifically in females (n=113).
Note. Results are overlaid on a standard MNI152 template with the mean skeleton in green (FA>0.3), and were “thickened” towards the full width of the tract for visualization purposes.
Third, for reference, we tested the main effect of ADHD. Lower FA and MD values were found for ADHD compared to controls in widespread white matter tracts including, but not limited to, the bilateral internal capsule, superior longitudinal fasciculus, forceps minor, and the corpus callosum. Of note, more extensive analyses and interpretation of group differences between ADHD and control participants in this cohort have been described in detail in previously published work (van Ewijk et al., 2014), and were therefore not repeated in the current study. The majority of participants in the current study were also included in this previous publication (86% of the ADHD subjects and 97% of the controls). A comparison between the effects of genotype and group in the current sample revealed that voxels showing genotype effects on MD values overlapped for 55% with group effects for ADHD on FA values.
Of note, twelve participants were not able to comply to the 48-hour medication washout period before testing, resulting in 7 participants with a 24-hour washout and 5 participants using medication during measurement. Medication use did not differ between the genotypes (see Table 1), and results remained similar when these participants were excluded from the analyses.
Discussion and Conclusion
The current study was the first to investigate whether the NOS1 exon 1f VNTR, a known risk variant for adult ADHD in females, is associated with the microstructure and organisation of WM tracts. We found association between NOS1 genotype and ADHD symptoms, and showed that NOS1 exon 1f VNTR genotype was associated with the microstructure and organisation of brain WM tracts in individuals with and without ADHD, specifically in females.
We replicated previously observed associations of NOS1 with specifically hyperactive/impulsive and total ADHD symptoms, but not the inattentive symptoms (Reif et al., 2009, Weber et al., 2015) in an independent sample. These findings extend previous findings by showing that this association is also observed in children and adolescents.
Results from the whole-brain analyses revealed that the NOS1 risk genotype (S/S) was associated with higher MD values in several major white matter tracts in females, but not males. The strong genetic basis of the organisation and microstructure of WM tracts (Kochunov et al., 2015) suggests a directional influence of the NOS1-ex1f risk genotype on the development of brain WM. Such an effect is consistent with fundamental research that shows involvement of NOS1 in neurite outgrowth (Chen et al., 2006). Homozygosity for the short allele was associated with higher MD values in several major WM tracts, including the anterior, superior, and posterior corona radiata, superior longitudinal fasciculus, genu of the corpus callosum, anterior and posterior internal capsule, and external capsule. MD is sensitive to a wide range of tissue properties (Jones et al., 2013). Higher MD values can, for example, be indicative of demyelination, lower axonal density, or axonal degeneration. However, interpretation of diffusion tensor measures such as MD should be performed with great care, since these are complex measures that are sensitive to a wide range of tissue properties and are thus fairly nonspecific (e.g., Jones et al., 2013, Alexander et al., 2007). Despite this ambiguity in the biological interpretation of our finding, we can conclude that homozygosity for the short allele is a risk factor for alterations in WM microstructure. This could possibly indicate aberrant outgrowth and organisation of WM tracts during early brain development, or degeneration of these tracts throughout later developmental stages.
The association of NOS1 genotype and WM properties was only present in females. A female specificity of the NOS1 effects was indeed expected based on the earlier studies linking the exon 1f variant to ADHD status and ADHD-like symptoms (Reif et al., 2009, Weber et al., 2015). One possible explanation could be given by the fact that the promoter region of exon 1f harbours an oestrogen binding site (Reif et al., 2009), and a link between oestrogen and upregulation of nitric oxide synthases has indeed been found (Nuedling et al., 2001). Consequently, it is possible that oestrogen binding influences NOS1 expression, and thereby (indirectly) influences the development or degeneration of WM tracts in females. Of interest for this theory is that the age-range of the current study includes puberty, although beyond the scope of this research, a very important developmental stage considering oestrogen availability. These results underline the importance of taking gender into account in future imaging (genetics) studies of WM tracts, specifically in psychiatric disorders such as ADHD, since males and females may have different mechanisms underlying WM abnormalities.
The association of NOS1-ex1f with WM microstructure was independent of ADHD status, indicating that the relationship between NOS1 and WM development is similar in persons with and without ADHD. However, this result should be interpreted with care, given the relatively small subgroups (n≥9) included in this exploratory analysis. Replication in larger samples is warranted to determine whether the association between NOS1 and WM is indeed independent of ADHD status.
Regions showing an association of WM microstructure with NOS1 strongly overlap with those previously found altered FA and MD in individuals with ADHD (Cortese et al., 2013, Hamilton et al., 2008, Nagel et al., 2011, van Ewijk et al., 2014). WM microstructure in these regions has shown to differ between individuals with ADHD and controls (Cortese et al., 2013, Hamilton et al., 2008, Nagel et al., 2011, van Ewijk et al., 2014), and has been associated with ADHD symptoms and cognitive measures of inattention and hyperactivity-impulsivity (Konrad et al., 2010, Li et al., 2010, Witt and Stevens, 2015, Onnink et al., 2015). Furthermore, effects of NOS1 on MD overlapped for 55% with voxels that also showed an association between ADHD and FA values in our sample. However, the current study did not find a main effect or interaction with ADHD status, potentially due to our sample sizes of the subgroups. These results show that, even though FA and MD may represent different neurobiological mechanisms, NOS1 and ADHD are both associated with white matter integrity in strongly overlapping regions. We could not confirm a dimensional relationship between MD values in these regions and ADHD symptoms in the current sample. Due to the fact that our sample was recruited to have either a very low number of symptoms (control participants), or symptoms in the clinical range (ADHD participants), our data was not optimally suitable to examine such an association between white matter microstructure and the dimensional spectrum of ADHD symptoms.
The current results should be viewed in light of some strengths and limitations. We are the first to study the role of the ADHD genetic risk factor NOS1-ex1f on WM microstructure using DTI, and this combination might elucidate mechanisms contributing to the clinical symptoms of ADHD more completely than the analysis of any of these parameters in isolation. Another strength is that our relatively large sample size allowed us to investigate possible differential effects of ADHD status and gender, resulting in our female-specific finding, potentially overlooked in other studies, specifically as most ADHD studies have an excess of male participants. A limitation is that the subgroups in our sample were too small for detailed subgroup analyses or interactions, as the expected genetic effect of the VNTR in NOS1 for complex phenotypes is small to moderate. Therefore, we are not able to draw strong conclusions regarding whether the association between NOS1 and WM microstructure is independent of ADHD status, whether the association between genotype and ADHD symptoms was gender-specific, and whether there may be an association between MD values and ADHD symptoms in the regions where we found a NOS1 effect. Larger female samples in future studies with a more representative distribution of ADHD symptoms across the entire spectrum are needed to further explore this. Larger samples could also provide the opportunity to study the role of age, as it is possible that the role of NOS1 on neurite outgrowth could have different expression during different periods of development.
In sum, we here show that the exon 1f VNTR in NOS1 is associated with ADHD symptoms and, specifically in females, with the microstructure and organisation of several major WM tracts that are also implicated in ADHD. These findings may elucidate (part of) a neurobiological pathway from NOS1 variation to ADHD, through WM development. Of note, the effect was observed in subjects with ADHD as well as controls. As ADHD is a multifactorial disorder, this may indicate that NOS1 - and its effect on WM microstructure - is one of many possible risk factors underlying the disorder, and that a combination with other risk factors is needed for clinical manifestation of the disorder. Our findings provide new insights into the neurobiological mechanisms underlying ADHD, as well as the involvement of NOS1 in the development and organisation of brain WM tracts in individuals with and without ADHD, warranting replication and further exploration in large independent studies.
Supplementary Material
Appendix S1. Genotyping details.
Appendix S2. Imaging details.
Table S1. NOS1–ex1f VNTR allele sizes in number of tandem repeats in the current sample.
Key points.
This study replicates associations of NOS1 with hyperactive/impulsive and total ADHD symptoms, but not the inattentive symptoms, and shows that this association is also observed in children and adolescents with ADHD.
For the first time an ADHD candidate genetic variant ([S/S] of NOS1-ex1f), was associated with differences in several major WM tracts in females, but not males.
Female specificity for the NOS1-ex1f ADHD association was known. We showed additionally female specificity for NOS1 and WM. These results underline the importance of gender in future imaging (genetics) studies, since males and females may have different mechanisms underlying WM abnormalities.
These findings may elucidate (part of) a neurobiological pathway from NOS1 variation to ADHD, through WM development.
Acknowledgments
This work was supported by NIH Grant R01MH62873 and R01MH081803, NWO Large Investment Grant 1750102007010, ZonMW Grant 60-60600-97-193 and NWO Brain and Cognition grant 433-09-242 to J.K.B., and grants from Radboud University Nijmegen Medical Center, University Medical Center Groningen and Accare, and VU University Amsterdam. The research leading to these results also received funding from the European Community’s Seventh Framework Programme (FP7/2007–2013) under grant agreement n° 278948 (TACTICS), n° 305697 (OPTIMISTIC), n° 602805 (Aggressotype), n° 603036 (MATRICS), and n° 602450 (IMAGEMEND) and from the Horizon2020 Marie-Curie ETN grant n° 643051 (MiND). B.F. is supported by a Vici grant from NWO (grant number 016-130-669) and she and J.K.B. received funding from the National Institutes of Health (NIH) Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centers of Excellence (BD2K). The authors thank all families that participated in this study and all researchers who collected the data. Furthermore, we acknowledge the department of Pediatrics of the VU university Medical Center for having the opportunity to use the mock scanner for preparation of our participants. J.O. has received unrestricted investigator initiated research grants from Shire pharmaceuticals. B.F. received an educational speaking fee from Merz. J.K.B. has been in the past 3 years a consultant to / member of advisory board of / and/or speaker for Janssen Cilag BV, Eli Lilly, Lundbeck, Shire, Roche, Novartis, Medice and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties. P.J.H. has in the past three years been an advisory board member of Shire.
Footnotes
Supporting information
Additional Supporting Information may be found in the online version of this article:
The remaining authors declare no competing or potential conflicts of interest.
References
- ALEXANDER AL, LEE JE, LAZAR M, FIELD AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMERICAN PSYCHIATRIC ASSOCIATION. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- AMERICAN PSYCHIATRIC ASSOCIATION. The Diagnostic and Statistical Manual of Mental Disorders: DSM 5. bookpointUS; 2013. [Google Scholar]
- CASEY BJ, EPSTEIN JN, BUHLE J, LISTON C, DAVIDSON MC, TONEV ST, SPICER J, NIOGI S, MILLNER AJ, REISS A, GARRETT A, HINSHAW SP, GREENHILL LL, SHAFRITZ KM, VITOLO A, KOTLER LA, JARRETT MA, GLOVER G. Frontostriatal connectivity and its role in cognitive control in parent-child dyads with ADHD. American Journal of Psychiatry. 2007;164:1729–1736. doi: 10.1176/appi.ajp.2007.06101754. [DOI] [PubMed] [Google Scholar]
- CHEN J, ZACHAREK A, LI Y, LI A, WANG L, KATAKOWSKI M, ROBERTS C, LU M, CHOPP M. N-cadherin mediates nitric oxide-induced neurogenesis in young and retired breeder neurospheres. Neuroscience. 2006;140:377–388. doi: 10.1016/j.neuroscience.2006.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COGHILL D, NIGG J, ROTHENBERGER A, SONUGA-BARKE E, TANNOCK R. Whither causal models in the neuroscience of ADHD? Developmental Science. 2005;8:105–114. doi: 10.1111/j.1467-7687.2005.00397.x. [DOI] [PubMed] [Google Scholar]
- CONNERS CK, ERHARDT D, SPARROW EP. Conner’s Adult ADHD Rating Scales: CAARS. North Tonawanda, NY: Multi-Health Systems; 1999. [Google Scholar]
- CONNERS CK, SITARENIOS G, PARKER JDA, EPSTEIN JN. The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998a;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- CONNERS CK, SITARENIOS G, PARKER JDA, EPSTEIN JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998b;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- CORTESE S, IMPERATI D, ZHOU J, PROAL E, KLEIN RG, MANNUZZA S, RAMOS-OLAZAGASTI MA, MILHAM MP, KELLY C, CASTELLANOS FX. White matter alterations at 33-year follow-up in adults with childhood attention-deficit/hyperactivity disorder. Biological Psychiatry. 2013;74:591–598. doi: 10.1016/j.biopsych.2013.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAMER KS, ANGELUCCI A, HAHM JO, BOGDANOV MB, SUR M. A role for nitric oxide in the development of the ferret retinogeniculate projection. Journal of Neuroscience. 1996;16:7995–8004. doi: 10.1523/JNEUROSCI.16-24-07995.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESLER T, BARTH B, ETHOFER T, LESCH KP, EHLIS AC, FALLGATTER AJ. Imaging genetics in adult attention-deficit/hyperactivity disorder (ADHD): a way towards pathophysiological understanding? Borderline Personality Disorder and Emotion Dysregulation. 2014;1:6. doi: 10.1186/2051-6673-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DURSTON S. Imaging genetics in ADHD. Neuroimage. 2010;53:832–838. doi: 10.1016/j.neuroimage.2010.02.071. [DOI] [PubMed] [Google Scholar]
- FARAONE SV, ASHERSON P, BANASCHEWSKI T, BIEDERMAN J, BUITELAAR J, RAMOS-QUIROGA JA, ROHDE LA, SONUGA-BARKE EJS, TANNOCK R, FRANKE B. Attention-deficit/hyperactivity disorder. Nature Reviews. 2015;1:1–23. doi: 10.1038/nrdp.2015.20. [DOI] [PubMed] [Google Scholar]
- FRANKE B, FARAONE SV, ASHERSON P, BUITELAAR J, BAU CH, RAMOS-QUIROGA JA, MICK E, GREVET EH, JOHANSSON S, HAAVIK J, LESCH KP, CORMAND B, REIF A, INTERNATIONAL MULTICENTRE PERSISTENT AC The genetics of attention deficit/hyperactivity disorder in adults, a review. Molecular Psychiatry. 2012;17:960–987. doi: 10.1038/mp.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKE B, NEALE BM, FARAONE SV. Genome-wide association studies in ADHD. Human Genetics. 2009;126:13–50. doi: 10.1007/s00439-009-0663-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMILTON LS, LEVITT JG, O’NEILL J, ALGER JR, LUDERS E, PHILLIPS OR, CAPLAN R, TOGA AW, MCCRACKEN J, NARR KL. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19:1705–1708. doi: 10.1097/WNR.0b013e3283174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOOGMAN M, AARTS E, ZWIERS M, SLAATS-WILLEMSE D, NABER M, ONNINK M, COOLS R, KAN C, BUITELAAR J, FRANKE B. Nitric oxide synthase genotype modulation of impulsivity and ventral striatal activity in adult ADHD patients and healthy comparison subjects. American Journal of Psychiatry. 2011;168:1099–1106. doi: 10.1176/appi.ajp.2011.10101446. [DOI] [PubMed] [Google Scholar]
- JAHANSHAD N, LEE AD, BARYSHEVA M, MCMAHON KL, DE ZUBICARAY GI, MARTIN NG, WRIGHT MJ, TOGA AW, THOMPSON PM. Genetic influences on brain asymmetry: a DTI study of 374 twins and siblings. Neuroimage. 2010;52:455–469. doi: 10.1016/j.neuroimage.2010.04.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES DK, KNOSCHE TR, TURNER R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- KALISCH BE, BOCK NA, DAVIS WL, RYLETT RJ. Inhibitors of nitric oxide synthase attenuate nerve growth factor-mediated increases in choline acetyltransferase expression in PC12 cells. Journal of Neurochemistry. 2002;81:624–635. doi: 10.1046/j.1471-4159.2002.00854.x. [DOI] [PubMed] [Google Scholar]
- KAUFMAN J, BIRMAHER B, BRENT D, RAO U, FLYNN C, MORECI P, WILLIAMSON D, RYAN N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- KOCHUNOV P, JAHANSHAD N, MARCUS D, WINKLER A, SPROOTEN E, NICHOLS TE, WRIGHT SN, HONG LE, PATEL B, BEHRENS T. Heritability of fractional anisotropy in human white matter: A comparison of Human Connectome Project and ENIGMA-DTI data. Neuroimage. 2015;111:300–311. doi: 10.1016/j.neuroimage.2015.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONRAD A, DIELENTHEIS TF, EL MASRI D, BAYERL M, FEHR C, GESIERICH T, VUCUREVIC G, STOETER P, WINTERER G. Disturbed structural connectivity is related to inattention and impulsivity in adult attention deficit hyperactivity disorder. European Journal of Neuroscience. 2010;31:912–919. doi: 10.1111/j.1460-9568.2010.07110.x. [DOI] [PubMed] [Google Scholar]
- LASKY-SU J, NEALE BM, FRANKE B, ANNEY RJ, ZHOU K, MALLER JB, VASQUEZ AA, CHEN W, ASHERSON P, BUITELAAR J. Genome-wide association scan of quantitative traits for attention deficit hyperactivity disorder identifies novel associations and confirms candidate gene associations. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147:1345–1354. doi: 10.1002/ajmg.b.30867. [DOI] [PubMed] [Google Scholar]
- LAWRENCE KE, LEVITT JG, LOO SK, LY R, YEE V, O’NEILL J, ALGER J, NARR KL. White matter microstructure in subjects with attention-deficit/hyperactivity disorder and their siblings. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:431–440. doi: 10.1016/j.jaac.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI Q, SUN J, GUO L, ZANG Y, FENG Z, HUANG X, YANG H, LV Y, HUANG M, GONG Q. Increased fractional anisotropy in white matter of the right frontal region in children with attention-deficit/hyperactivity disorder: a diffusion tensor imaging study. Neuro Endocrinol Lett. 2010;31:747–753. [PubMed] [Google Scholar]
- LISTON C, COHEN MM, TESLOVICH T, LEVENSON D, CASEY B. Atypical prefrontal connectivity in attention-deficit/hyperactivity disorder: pathway to disease or pathological end point? Biological Psychiatry. 2011;69:1168–1177. doi: 10.1016/j.biopsych.2011.03.022. [DOI] [PubMed] [Google Scholar]
- NAGEL BJ, BATHULA D, HERTING M, SCHMITT C, KROENKE CD, FAIR D, NIGG JT. Altered white matter microstructure in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:283–292. doi: 10.1016/j.jaac.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUEDLING S, KARAS RH, MENDELSOHN ME, KATZENELLENBOGEN JA, KATZENELLENBOGEN BS, MEYER R, VETTER H, GROHÉ C. Activation of estrogen receptor β is a prerequisite for estrogen-dependent upregulation of nitric oxide synthases in neonatal rat cardiac myocytes. FEBS Letters. 2001;502:103–108. doi: 10.1016/s0014-5793(01)02675-8. [DOI] [PubMed] [Google Scholar]
- ONNINK AM, ZWIERS MP, HOOGMAN M, MOSTERT JC, DAMMERS J, KAN CC, VASQUEZ AA, SCHENE AH, BUITELAAR J, FRANKE B. Deviant white matter structure in adults with attention-deficit/hyperactivity disorder points to aberrant myelination and affects neuropsychological performance. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2015;63:14–22. doi: 10.1016/j.pnpbp.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK SY, KANG MJ, HAN JS. Neuronal NOS Induces Neuronal Differentiation Through a PKCalpha-Dependent GSK3beta Inactivation Pathway in Hippocampal Neural Progenitor Cells. Molecular Neurobiology. 2016 doi: 10.1007/s12035-016-0110-1. [DOI] [PubMed] [Google Scholar]
- POELMANS G, BUITELAAR JK, PAULS DL, FRANKE B. A theoretical molecular network for dyslexia: integrating available genetic findings. Molecular Psychiatry. 2011;16:365–382. doi: 10.1038/mp.2010.105. [DOI] [PubMed] [Google Scholar]
- REIF A, JACOB CP, RUJESCU D, HERTERICH S, LANG S, GUTKNECHT L, BAEHNE CG, STROBEL A, FREITAG CM, GIEGLING I. Influence of functional variant of neuronal nitric oxide synthase on impulsive behaviors in humans. Archives of General Psychiatry. 2009;66:41–50. doi: 10.1001/archgenpsychiatry.2008.510. [DOI] [PubMed] [Google Scholar]
- SALATINO-OLIVEIRA A, AKUTAGAVA-MARTINS GC, BRUXEL EM, GENRO JP, POLANCZYK GV, ZENI C, KIELING C, KARAM RG, ROVARIS DL, CONTINI V, CUPERTINO RB, MOTA NR, GREVET EH, BAU CH, ROHDE LA, HUTZ MH. NOS1 and SNAP25 polymorphisms are associated with Attention-Deficit/Hyperactivity Disorder symptoms in adults but not in children. Journal of Psychiatric Research. 2016;75:75–81. doi: 10.1016/j.jpsychires.2016.01.010. [DOI] [PubMed] [Google Scholar]
- SMITH SM, JENKINSON M, JOHANSEN-BERG H, RUECKERT D, NICHOLS TE, MACKAY CE, WATKINS KE, CICCARELLI O, CADER MZ, MATTHEWS PM, BEHRENS TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- SMITH SM, NICHOLS TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- SONUGA-BARKE EJ. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- TANDA K, NISHI A, MATSUO N, NAKANISHI K, YAMASAKI N, SUGIMOTO T, TOYAMA K, TAKAO K, MIYAKAWA T. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Molecular Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN EWIJK H, HESLENFELD DJ, ZWIERS MP, BUITELAAR JK, OOSTERLAAN J. Diffusion tensor imaging in attention deficit/hyperactivity disorder: a systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2012;36:1093–1106. doi: 10.1016/j.neubiorev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- VAN EWIJK H, HESLENFELD DJ, ZWIERS MP, FARAONE SV, LUMAN M, HARTMAN CA, HOEKSTRA P, FRANKE B, BUITELAAR JK, OOSTERLAAN J. Different mechanisms of white matter abnormalities in Attention-Deficit/Hyperactivity Disorder: a Diffusion Tensor Imaging study. Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:790–799.e793. doi: 10.1016/j.jaac.2014.05.001. [DOI] [PubMed] [Google Scholar]
- VON RHEIN D, MENNES M, VAN EWIJK H, GROENMAN AP, ZWIERS MP, OOSTERLAAN J, HESLENFELD D, FRANKE B, HOEKSTRA PJ, FARAONE SV. The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder. Design and descriptives. European Child and Adolescent Psychiatry. 2014;24:265–281. doi: 10.1007/s00787-014-0573-4. [DOI] [PubMed] [Google Scholar]
- WEBER H, KITTEL-SCHNEIDER S, HEUPEL J, WEISSFLO L, KENT L, FREUDENBERG F, ALTTOA A, POST A, HERTERICH S, HAAVIK J. On the role of NOS1 ex1f-VNTR in ADHD—allelic, subgroup, and meta-analysis. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2015;168:445–458. doi: 10.1002/ajmg.b.32326. [DOI] [PubMed] [Google Scholar]
- WEBER H, KLAMER D, FREUDENBERG F, KITTEL-SCHNEIDER S, RIVERO O, SCHOLZ CJ, VOLKERT J, KOPF J, HEUPEL J, HERTERICH S. The genetic contribution of the NO system at the glutamatergic post-synapse to schizophrenia: further evidence and meta-analysis. European Neuropsychopharmacology. 2014;24:65–85. doi: 10.1016/j.euroneuro.2013.09.005. [DOI] [PubMed] [Google Scholar]
- WITT ST, STEVENS MC. Relationship between white matter microstructure abnormalities and ADHD symptomatology in adolescents. Psychiatry Research. 2015;232:168–174. doi: 10.1016/j.pscychresns.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU Z, YANG L, WANG Y. Applying imaging genetics to ADHD: the promises and the challenges. Molecular Neurobiology. 2014;50:449–462. doi: 10.1007/s12035-014-8683-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Genotyping details.
Appendix S2. Imaging details.
Table S1. NOS1–ex1f VNTR allele sizes in number of tandem repeats in the current sample.


