Abstract
Background
While revision ACL reconstruction (rACLR) can be performed to restore knee stability and improve patient activity level, outcomes after these surgeries are reported to be inferior to primary ACL reconstruction. Further reoperation after rACLR can have an even more profound effect on patient satisfaction and outcome. However, there is a current lack of information regarding the rate and risk factors for subsequent surgery after rACLR.
Purpose
To report the rate of reoperation, procedures performed, and risk factors for reoperation two years after rACLR.
Study Design
Cohort Study, Level of Evidence 2
Methods
1205 patients who underwent rACLR were enrolled in the Multicenter ACL Revision Study (“MARS”) between 2006 and 2011, comprising the prospective cohort. Two-year questionnaire follow-up was obtained on 989 (82%), while telephone follow-up was obtained on 1112 (92%). If a patient reported having a subsequent surgery, operative reports detailing the subsequent procedure(s) were obtained and categorized. Multivariate regression analysis was performed to determine independent risk factors for reoperation.
Results
Of the 1112 patients included in the analysis, 122 patients (11%) underwent a total of 172 subsequent procedures on the ipsilateral knee at 2-year follow-up. Of the reoperation procedures, 27% were meniscus procedures (69% meniscectomy, 26% repair), 19% were subsequent rACLR, 17% were cartilage procedures (61% chondroplasty, 17% microfracture, and 13% mosaicplasty), 11% were hardware removal, and 9% were procedures for arthrofibrosis. Multivariate analysis revealed that patients under 20 years old had twice the odds of patients aged 20–29 to have a reoperation. Use of allograft at the time of rACLR (OR 1.79, p=0.007) was a significant predictor for reoperations at 2 years while staged revision (bone grafting of tunnels before rACLR) (OR 1.93, p=0.052) trended toward significance. Patients with grade IV cartilage damage seen during rACLR were 78% less likely to undergo subsequent operations within 2 years. Sex, BMI, smoking history, Marx activity score, technique for femoral tunnel placement and meniscal tear or meniscal treatment at the time of rACLR showed no significant effect on reoperation rate.
Conclusion
There is a significant reoperation rate following rACLR at two years (11%) with meniscal procedures most commonly involved. Independent risk factors for subsequent surgery on the ipsilateral knee include age<20 years old and use of allograft tissue at the time of rACLR.
Keywords: revision anterior cruciate ligament reconstruction, subsequent surgery, reoperation, risk factors, outcomes
Introduction
Anterior cruciate ligament (ACL) ruptures can be devastating injuries, leading to joint instability, meniscal tears33, and subsequent osteoarthritis16. Primary ACL reconstruction provides increased stability to the knee and aids in returning patients to sports and activity17. Recent studies have further demonstrated a significant overall increase in the diagnosis of ACL injury and treatment with ACL reconstruction in both adult and pediatric populations18, 19, 30, 32, 34. As ACL reconstruction (ACLR) has become more widely utilized there has been a concomitant increase in failure of the surgery with estimated graft failure rates ranging from 1.8% to 10.4%12–14, 37. In fact, a recent meta-analysis by Wiggins et al. estimated an overall graft failure rate of 7% with failures upwards of 10% in a younger (age<25) population34.
The increased number of ACLR has therefore amplified the need for revision ACL reconstruction (rACLR) which may present a challenging dilemma for both the surgeon and patient as several studies have shown inferior clinical outcomes after rACLR compared to primary ACLR4, 15, 17, 26, 35, 36. Studies by Wright et al.35 and Spindler et al.26 showed that Marx activity, International Knee Documentation Committee (IKDC), and median Knee injury and Osteoarthritis Outcome Score (KOOS) subscale Knee Related Quality of Life (KRQOL) scores were significantly decreased in rACLR compared to primary ACL reconstruction at 2-year follow-up.
Reoperation rates after primary ACLR are reported as high as 27.6% and have a profound effect on patient outcome and satisfaction29. Younger age at the index surgery, female sex, and the use of allografts have been reported as risk factors for subsequent surgery12, 17, 24. In fact, younger patients who undergo ACL reconstruction had significant increases in incidence of concomitant meniscal and cartilage procedures which can portend worse clinical outcomes32. Revision ACLR can be difficult and by definition involves a knee that already has had multiple traumatic episodes.
Currently, there is a lack of information concerning rates and risk factors for further reoperation after rACLR. The development of the Multicenter ACL Revision Study (MARS) group led to a prospective longitudinal cohort of patients to evaluate these factors as well as outcomes of reoperation after rACLR. This is the first multicenter, prospectively collected cohort study looking at rACLR and detailing the results and factors associated with reoperations. The purpose of this study was to report the rate of reoperation in this cohort; the procedures performed, and identify potential risk factors for reoperation two years after rACLR. Our null hypothesis was that no variable was a risk factor for reoperation.
Methods
Setting and Study Population
The MARS Study is an academic and private practice multicenter consortium funded by the National Institutes of Health and sponsored by American Orthopaedic Society for Sports Medicine (AOSSM)10. This prospective cohort consisted of 1205 patients enrolled between 2006 and 2011 who had undergone rACLR following previously failed primary ACLR. All enrolled patients signed informed consent and were required to complete a series of previously validated patient-reported outcome questionnaires both prior to surgery and then again at 2-yearfollow-up. Exclusion criteria were inability or unwillingness to complete a 2-year follow-up survey, graft failure secondary to prior intra-articular infection, arthrofibrosis, or complex regional pain syndrome.
All participating sites obtained local institutional review board approval before enrolling subjects and complied with a standardized manual of operations. Participating surgeons were required to complete a training session that integrated articular cartilage and meniscus agreement studies, review of the study design, patient inclusion criteria, a practice intra-articular grading sheet, and a trial surgeon questionnaire. The surgeon questionnaire was completed at the time of surgery and included sections on history of knee injury and/or surgery on both knees, the results of the general knee examination done under anesthesia, recording of all previous and new intra-articular injuries and treatments to the meniscus and articular cartilage, and the surgical technique used for the revision ACL reconstruction.
Data Sources
Completed baseline data forms were mailed from the participating sites to the data-coordinating center. Data from both the patient and surgeon questionnaires were subsequently scanned and read with Teleform software (Cardiff Software Inc, Vista, California) using optical character recognition to avoid manual data entry, and the scanned data were then verified and exported to a master database. A series of logical error and quality control checks were subsequently performed prior to data analysis.
At 2-year follow-up, patients were mailed the same questionnaire, which they had completed at baseline and were asked to complete and send back. At the same time, patients were also contacted by telephone and asked if any subsequent surgeries had occurred on either knee since their rACLR. If they responded affirmatively, either on the questionnaire and/or by telephone, attempts were made to obtain the operative report. Operative reports were analyzed by a single MARS physician so as to ensure consistency, and all procedures were categorized and recorded along with the surgical date. If multiple procedures were performed during surgery, all procedures were recorded. Because one of our goals was to assess the impact of individual procedures on future outcomes in a multivariate analysis, all procedures were listed - not only whether the patient had any subsequent surgery. Subsequent procedures encompassed hardware removal, arthroscopic scar debridement/synovectomy/manipulation, loose body removal, debridement for infection, articular cartilage procedures (chondroplasty, microfracture, autologous chondrocyte implantation, osteochondral autograft transplantation, and/or osteochondral allografts), meniscal procedures (meniscectomy, repair, and/or meniscal transplants), revision ACL reconstruction, and total knee arthroplasty.
Statistical Analysis
Assuming normal distribution of the data on the basis of the large sample size (n=1112 with 2 year follow-up), we used the Pearson chi-squared test for analysis of categorical data and the independent-samples t-test for continuous data. Multivariable binary logistic regression analysis was performed to determine factors associated with reoperations. Results were reported as odds ratios (OR) with 95% confidence intervals (CI). A repeated measure ANOVA was used to assess for changes in patient-reported outcome scores comparing rACLR patients who had subsequent surgery and those who did not. Statistical significance was set for all analyses to P < .05. SAS version 9.3 software (SAS Institute Inc, Cary, NC, USA) was used for statistical analyses and data modeling.
Results
A total of 1205 patients who underwent rACLR were enrolled from 2006–2011. Two-year questionnaire follow-up was obtained on 989 (82%), while telephone follow-up was obtained on 1112 (92%) which comprised the study population (Table 1). One hundred and twenty-two patients (11% of the cohort) underwent a total of 172 subsequent procedures on the ipsilateral knee at 2-year follow-up. Of the reoperation procedures, 27% were meniscus procedures (69% meniscectomy, 26% repair, 5% meniscal transplant), 19% were subsequent rACLR, 17% were cartilage procedures (61% chondroplasty, 17% microfracture, 13% mosaicplasty, 9% cell based cartilage restoration), 11% were hardware removal, and 9% were procedures for arthrofibrosis such has lysis of adhesions and synovectomy (Figure 1).
Table 1.
Patient Characteristics of the Study Population
| NO Reoperations | Reoperations | Total | |
|---|---|---|---|
| Total Patients | 1083 | 122 | 1205 |
| Sex | |||
| Female | 446 (41%) | 62 (51%) | 508 (42%) |
| Male | 637 (58%) | 60 (49%) | 697 (58%) |
| Age Group (years) | |||
| <20 | 249 (23%) | 43 (35%) | 292 (24%) |
| 20–29 | 418 (39%) | 33 (27%) | 451 (37%) |
| 30–39 | 254 (24%) | 33 (27%) | 287 (24%) |
| 40–49 | 129 (12%) | 9 (7%) | 138 (12%) |
| >50 | 33 (3%) | 4 (3%) | 37 (3%) |
| Body Mass Index (BMI) | |||
| Normal (18.5–24) | 494 (46%) | 62 (51%) | 556 (46%) |
| Overweight (25–29) | 385 (36%) | 47 (39%) | 432 (36%) |
| Obese (30–34) | 151 (14%) | 9 (7%) | 160 (13%) |
| Morbidly Obese (>35) | 53 (5%) | 4 (3%) | 57 (5%) |
| Smoking Status | |||
| Never | 824 (76%) | 99 (81%) | 923 (77%) |
| Quit | 140 (13%) | 14 (12%) | 154 (13%) |
| Current | 101 (9%) | 8 (7%) | 109 (9%) |
| Unknown | 18 (2%) | 1 (1%) | 19 (2%) |
| Baseline Marx Activity Level (0–16) | |||
| 0–4 | 301 (28%) | 35 (29%) | 336 (28%) |
| 5–8 | 131 (12%) | 11 (9%) | 142 (12%) |
| 9–12 | 246 (23%) | 24 (20%) | 270 (22%) |
| 13–16 | 398 (37%) | 51 (42%) | 449 (37%) |
Figure 1.
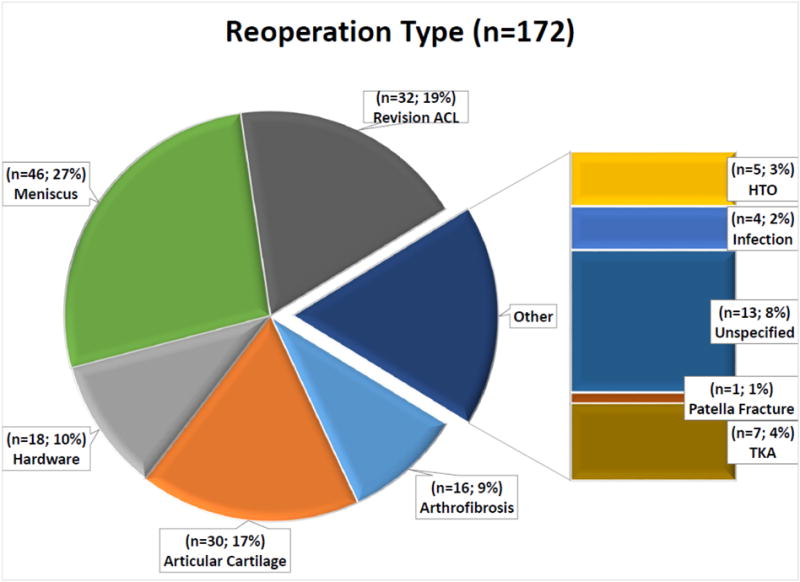
Reoperation types that were performed within 2 years of rACLR
Of the patients who underwent reoperations, there were 62 females (51%) and 60 males (49%). Reoperations occurred more frequently in patients aged 20 or younger compared with the overall cohort of patients over 20 years of age (35% reoperations vs. 24% in general cohort). The majority of the reoperation group had normal body mass index (BMI) levels (51%), with only 11% being defined as obese or morbidly obese. The overwhelming majority of the patients never smoked (99 patients, 81%) while only 8 (7%) of the patients were current smokers. Baseline surgical characteristics between the groups who underwent subsequent reoperations compared with the group who didn’t also revealed some differences (Table 2). Staged revisions (bone grafting of tunnels before rACLR) occurred more frequently in the group who subsequently had a reoperation (13% vs. 8%). Allografts (59% vs. 49%) and meniscal repairs (25% vs 17%) were more common in the reoperation group, while Grade IV articular cartilage lesions were much less common (13% vs. 27%).
Table 2.
Knee characteristics and types of procedures performed at the time of rACLR
| NO Reoperations | Reoperations | Total | |
|---|---|---|---|
| Total Patients | 1083 | 122 | 1205 |
| REVISION # | |||
| First | 953 (88%) | 102 (84%) | 1055 (88%) |
| Multiple | 130 (12%) | 20 (16%) | 150 (12%) |
| STAGING | |||
| One-Stage | 1004 (93%) | 106 (87%) | 1110 (92%) |
| Two-Stage | 79 (7%) | 16 (13%) | 95 (8%) |
| Femoral Tunnel Technique | |||
| Transtibial drilling | 345 (32%) | 43 (35%) | 388 (32%) |
| Anteromedial portal drilling | 442 (41%) | 53 (43%) | 495 (41%) |
| Two-incision outside-in drilling | 155 (14%) | 26 (21%) | 181 (15%) |
| Revision ACL graft type | |||
| Autograft BTB | 308 (28%) | 28 (23%) | 336 (28%) |
| Autograft soft tissue | 227 (21%) | 17 (14%) | 244 (20%) |
| Allograft BTB | 252 (23%) | 35 (29%) | 287 (24%) |
| Allograft soft tissue | 262 (24%) | 36 (30%) | 298 (25%) |
| Hybrid (autograft + allograft) | 33 (3%) | 6 (5%) | 39 (3%) |
| Meniscus Tears | |||
| Complete | 489 (45%) | 56 (46%) | 545 (45%) |
| Partial | 187 (17%) | 25 (21%) | 212 (18%) |
| None | 407 (38%) | 41 (34%) | 448 (37%) |
| Meniscal Treatment | |||
| Normal | 404 (37%) | 41 (34%) | 445 (37%) |
| No treatment | 44 (4%) | 5 (4%) | 49 (4%) |
| Repair | 180 (17%) | 30 (25%) | 210 (17%) |
| Meniscectomy | 436 (40%) | 44 (36%) | 480 (40%) |
| Other | 19 (2%) | 2 (2%) | 21 (2%) |
| Concomitant Cartilage Operations | |||
| None | 660 (61%) | 75 (62%) | 735 (61%) |
| Chondroplasty | 341 (32%) | 37 (30%) | 378 (31%) |
| Microfracture | 74 (7%) | 10 (8%) | 84 (7%) |
| Other (e.g., OATS, ACI, osteochondral allograft) | 8 (1%) | 0 (0%) | 8 (1%) |
| HIGHEST CARTILAGE GRADE | |||
| Grade 1 | 307 (28%) | 38 (31%) | 345 (29%) |
| Grade 2 | 352 (33%) | 48 (39%) | 400 (33%) |
| Grade 3 | 110 (10%) | 20 (16%) | 130 (11%) |
| Grade 4 | 313 (29%) | 16 (13%) | 329 (27%) |
Multivariate analysis revealed that patients under 20 years old had an odds ratio of 2.1 (95% CI:1.2,3.7) for reoperation compared to patients aged 20–29 (Table 3). Use of allograft at the time of rACLR (OR 1.79 [95% CI: 1.17, 2.73], p=0.007) was a significant predictor for reoperations at 2 years. Staged revision (bone grafting of tunnels before rACLR) (OR 1.93 [95% CI: 0.99, 3.75], p=0.052) and the use of a hybrid auto-allograft (OR 2.48 [95% CI: 0.92, 6.65], p=0.07) did not reach significance. Patients with grade IV cartilage damage seen during rACLR were 4.5 times (OR 0.22 [95% CI: 0.09, 0.53], p=0.018) less likely to undergo subsequent operations within 2 years. Sex, BMI, smoking history, Marx activity score, technique for femoral tunnel placement (anteromedial vs trans-tibial drilling), number of previous revision surgeries, and meniscal tear or meniscal treatment at the time of rACLR showed no significant effect on reoperation rate.
Table 3.
Multivariate Regression Predicting Reoperation after rACL
| OR (95% CI) | P | |
|---|---|---|
| GENDER: Male vs. Female | 1.30 (0.85, 1.99) | 0.229 |
| AGE: ref.=<20 yrs | ||
| 20–29 yrs | 0.47 (0.27, 0.81) | 0.007 |
| 30–39 yrs | 0.87 (0.478, 1.57) | 0.640 |
| 40–49 yrs | 0.58 (0.25, 1.36) | 0.212 |
| 50–59 yrs | 0.99 (0.29, 3.40) | 0.989 |
| BMI: ref.= Normal (17–25) | ||
| Overweight (25–29) | 1.33 (0.85, 2.09) | 0.211 |
| Obese (30–34) | 0.53 (0.25, 1.15) | 0.107 |
| Morbidly Obese (35–40) | 0.68 (0.23, 2.04) | 0.490 |
| SMOKING HISTORY: ref = Never | ||
| Current | 0.72 (0.33, 1.59) | 0.417 |
| Quit | 0.90 (0.48, 1.70) | 0.752 |
| MARX SCORE: ref = 0–4 | ||
| 5–8 | 0.67 (0.32, 1.40) | 0.285 |
| 9–12 | 0.86 (0.48, 1.54) | 0.611 |
| 13–16 | 0.88 (0.52, 1.51) | 0.649 |
| STAGING: ref.=single stage revision | ||
| Two-stage revision (bone grafting before revision) | 2.08 (1.12, 3.88) | 0.021 |
| ACL GRAFT: ref.= Autograft | ||
| Allograft | 1.83 (1.21, 2.78) | 0.004 |
| Hybrid (auto-allograft) | 2.53 (0.96, 6.65) | 0.060 |
| HIGHEST CARTILAGE GRADE: ref = Grade 1 | ||
| Grade 2 | 1.16 (0.72, 1.89) | 0.539 |
| Grade 3 | 1.59 (0.84, 3.02) | 0.155 |
| Grade 4 | 0.45 (0.24, 0.87) | 0.018 |
| FEMORAL TUNNEL TECHNIQUE: ref = Transtibial drilling | ||
| Anteromedial portal drilling | 0.97 (0.63, 1.51) | 0.905 |
| Two-incision outside-in drilling | 1.38 (0.80, 2.38) | 0.250 |
| MENISCUS: ref.= No tear | ||
| Partial tear | 2.47 (0.12, 50.9) | 0.559 |
| Complete tear | 2.13 (0.11, 42.8) | 0.622 |
| MENISCUS TREATMENT: ref.= normal meniscus | ||
| No treatment for partial tear | 0.51 (0.02, 11.6) | 0.670 |
| Meniscectomy | 0.49 (0.02, 9.94) | 0.642 |
| Repair | 0.81 (0.04, 16.6) | 0.890 |
| Other (Transplant) | 0.72 (0.03, 15.8) | 0.836 |
When analyzing the 989 patients who completed patient reported outcome surveys, while patients in both the reoperation and no reoperation group improved from baseline, patients who had not undergone reoperations showed significantly greater improvements in IKDC (p=0.005), KOOS Symptoms (p=0.001), and KOOS Pain (p=0.034) compared to the reoperation group (Table 4). In addition, WOMAC stiffness (p=0.02) scores improved more in the reoperation cohort as the baseline WOMAC stiffness scores (62 [95% CI:50, 87]) were lower in the reoperations cohort than the no reoperations cohort (75 [95% CI 50, 87]) (p=0.01).
Table 4.
Patient-Reported Median (25%, 75% quartile) Outcome Scores over Time
| Total | NO Reoperations | Reoperations | P-Value | ||||
|---|---|---|---|---|---|---|---|
| Baseline | 2-year | Baseline | 2 Year | Baseline | 2 Year | ||
| IKDC | 51(37, 63) | 77(60, 86) | 51(37, 63) | 78(63, 87) | 50(38, 64) | 66(48, 81) | 0.005 |
| KOOS | |||||||
| Symptoms | 67(53, 82) | 78(64, 89) | 67(53, 82) | 82(67, 92) | 64(50, 78) | 71(57, 82) | 0.001 |
| Pain | 75(58, 86) | 88(75, 94) | 75(58, 86) | 91(77, 97) | 72(58, 86) | 83(69, 91) | 0.034 |
| ADL | 86(69, 95) | 97(88, 100) | 86(69, 95) | 97(89, 100) | 83(64, 95) | 94(83, 98) | 0.157 |
| Sports | 45(25, 65) | 75(55, 90) | 45(25, 65) | 75(55, 90) | 45(25, 65) | 65(37, 80) | 0.063 |
| Quality of Life | 31(18, 43) | 56(37, 75) | 31(18, 43) | 62(43, 75) | 37(18, 50) | 50(31, 68) | 0.248 |
| WOMAC | |||||||
| Stiffness | 75(50, 87) | 75(62, 100) | 75(50, 87) | 75(62, 100) | 62(50, 87) | 75(62, 87) | 0.020 |
| Pain | 85(70, 95) | 95(80, 100) | 85(70, 95) | 95(80, 100) | 80(70, 95) | 90(75, 95) | 0.089 |
| ADL | 86(69, 95) | 97(88, 100) | 86(69, 95) | 97(89, 100) | 83(64, 95) | 94(83, 98) | 0.157 |
| Marx Activity Score | 11(4, 16) | 7(2, 12) | 11(4, 16) | 7(2, 12) | 11(4, 16) | 6(3, 12) | 0.529 |
Discussion
Our results showed that after rACLR, the rate of reoperation at a short-term follow-up of 2 years was 11% overall with 27% of reoperations consisting of meniscus operations, 19% undergoing another rACLR, and 17% having subsequent articular cartilage operations. These findings are consistent with previous studies reporting reoperations after primary ACLR. Lyman et al. reported a 6.5% reoperation rate on either knee after primary ACLR within one year using the New York SPARCS database17. Dunn et al.’s epidemiologic study on US Army personnel reported a 12.7% rate of reoperation following ACLR with 56% meniscal operations and 35% articular cartilage operations6. Hettrich and the MOON group reported an 18.9% rate of subsequent surgery on the ipsilateral knee at 6 years, of which there was a 7.7% rate of ACL revisions, a 13.3% rate of cartilage procedures, a 5.4% rate of arthrofibrosis procedures, and a 2.4% rate of procedures related to hardware12.
Reoperations were associated with younger aged patients, as our under 20-year-old population had a 2.1 times higher risk of reoperation compared to the patients in their 20’s. Paterno et al. showed an increased risk of repeat ACL tears after ACLR—up to six times more likely than a young, healthy cohort without ACLR21. Additionally, Hettrich et al. found that after ACLR, a 17-year-old patient had an over two-fold greater risk of reoperation compared to a 34-year-old patient12. This has been reiterated by literature showing the rate of subsequent surgery to the ACL to be age dependent, with the risk decreasing approximately 10% with each successive year14, 24. Similarly, Webster et al. found a 6-fold increase in ipsilateral ACL graft rupture in patients younger than 20 years at the age of surgery31. This was correlated with our study which showed that of the 32 rACLR in the reoperations cohort, 20 (62.5%) were performed in patients under 20 years old. Possible causes include younger patients who rupture their ACL may be likely to return to more aggressive cutting and pivoting sports, be less compliant with postoperative instructions, and/or have a genetic predisposition to collagen disruption impacting on their risk for ACL retear as well as meniscal and cartilage damage1, 24, 29. Additionally, in older patients, further surgery, especially those with long recovery like revision-rACLR, may be discouraged by the surgeon.
In our analysis, use of allograft was shown to be a significant risk factor for reoperation at two years. The risk of ACL graft rupture with regards to graft choice has been extensively reported in the literature. Risk of rupture with allograft was seen to be up to 5 times greater compared to that of bone-tendon-bone autograft14. Other authors have noted that use of allograft significantly increases the risk for hardware removal reoperations3. In a previous MARS group manuscript, allograft was confirmed to have an increased incidence of re-rupture and lower outcome measures9. In addition to showing that patients undergoing rACLR using autograft tissue were 2.78 times less likely to sustain a subsequent graft rupture compared to allograft, the group showed that the use of autograft resulted in improved IKDC scores, KOOS sports and recreation and quality of life subscores, as well as increased Marx activity level scores9. While previous articles reported better outcomes with bone-tendon-bone autografts18, 23, in our analysis, the choice of a specific type of allograft or autograft (hamstring, bone-tendon-bone, or quadriceps tendon) was not a significant risk factor for reoperations. When allografts were taken as a whole, they showed a 1.8-times increase in reoperations compared to autograft. Additionally, while using a hybrid auto-allograft did not reach significance (p=0.06), it showed a 2.5-times higher risk of reoperations compared to using autograft. This was likely due to the low numbers of hybrid grafts (39 total, 6 requiring reoperations) despite our large database.
A two-staged revision (bone grafting of tunnels before rACLR) had a OR of 1.93 (95% CI 0.99, 3.75 p=0.052) compared to a single stage revision for reoperation at 2 years. In our data collection, the 2nd stage of the revision itself is not counted as a reoperation. Shortcomings of two-staged revisions (increased costs, morbidity, and rehabilitation) notwithstanding, the increase in reoperations at 2 years may be due to increased Fairbanks changes that occur after a bone grafting procedure7 and worsening meniscus pathology during the staged process. Typically, the time between bone grafting and the rACLR is between 4–5 months and during this time between surgeries, it is possible that the patient may sustain additional meniscal and chondral damage from ambulation on an unstable knee, or subtle microinstability. Additionally, various methods of bone grafting have been described2, 7, 27, 28 and it is possible that despite our attempts to restore native bony anatomy to the knee, the previous tunnels remain a source of continued frailty for graft stability. While our numbers are low, 5 of the 95 two-staged patients (5%) sustained another ACL rupture compared to only 25 of the 1110 single-staged (2%) at 2 years. Further study is needed, however, as patients who underwent 2 stage revision might have done even worse with single stage revision surgery. Presumably these patients were bone grafted because one or both tunnels were very enlarged. Our findings emphasize the challenge of taking care of patients with failed ACL reconstruction and enlarged tunnels, and the importance of studying this issue further to better define the optimal treatment protocol.
At the time of the baseline rACLR, concomitant injuries such as meniscal tears and chondral damage were commonly present: 63% of the patients had meniscal tears noted during surgery and 39% of patients had concomitant cartilage procedures performed. This is similar to Widener et al. who reported a 74% rate of concomitant meniscal pathology at the time of rACLR33. Our results demonstrated that grade 4 chondral damage noted at the time of initial surgery was associated with fewer reoperations within 2 years. This may be related to a decrease in activity with increasing chondral damage as patients develop more painful joints. These patients have lower IKDC knee scores and lower Marx activity level proportional to their Outerbridge classification22. Furthermore, there may also be the added impact of physician counseling to decrease activity with severe cartilage loss following rACLR and a decreased proclivity of surgeons to recommend further procedures in these patients.
Interestingly, meniscal pathology and meniscal surgery (either repair or meniscectomy) at the time of rACLR did not portend future reoperations. Previous studies have shown mixed results: some have demonstrated a correlation with meniscal surgery and future reoperations3 while others studies have found no correlation12. This may be due in part to the philosophy of the operating surgeon with regards to meniscal pathology at the time of rACLR. Meniscal pathology, such as posterior lateral meniscal tears5, 25 and small medial meniscal tears5, can be left in situ with very low rates of reoperations at greater than 6 year follow-up following ACLR.
Female sex also was not an independent predictor for future reoperations, which at first seems contradictory to previous studies that suggest that females are more prone to arthrofibrosis and stiffness-related reoperations3, 20. However, our study focused on revision ACL surgeries. Patients who have already undergone previous ACL surgery may be more knowledgeable and compliant with the post-operative rehabilitation protocols. As a result, these patients may be more vigilant for prevention of arthrofibrosis compared to those undergoing primary ACLR. Alternatively, underlying biological differences that make patients more likely to undergo rACLR may make them less likely to develop scar tissue, arthrofibrosis and stiffness.
In our study, several knee function scores were relatively lower in the reoperations group. These included the IKDC, KOOS Symptoms, KOOS Pain, as well as their WOMAC stiffness scores. Similarly, Granan et al. found a correlation between lower KOOS scores and ACL graft failure8. The median IKDC score of our rACLR patients who did not undergo reoperations was 78 at 2 years, while the median IKDC score of patients who underwent reoperation was significantly lower at 66. While our study is the first to note decreasing patient-reported outcomes with reoperations after rACLR, van Dijck et al. reported significantly lower Lysholm scores in patients who underwent reoperation after primary ACLR in comparison with the patients who did not need additional surgery29.
Our study has strengths as well as limitations. This is the largest prospective longitudinal cohort to analyze the outcomes of rACLRs. The 50:50 mix of academic and private practice surgeons makes the results generalizable to the sports medicine fellowship–trained community. The use of validated patient-reported outcome measures allowed us to compare this study with previous studies that have used these measures in other settings. The large number of patients enrolled allowed us to perform sophisticated statistical analyses controlling for a large number of variables to understand the predictors of inferior outcomes noted in rACLRs. Our study design is limited in that it currently precludes on-site follow-up which may lead to recall bias and is limited to 2-year follow-up. It is also possible that important risk factors or confounders were not realized and not included in the multivariate regression. Long-term studies such as those by van Dijck et al.29 and Hanypsiak et al.11 show reoperation rates as high as 34% with greater than 7 year follow-up. Future follow-up studies, including continued follow-up of our current cohort, may show comparable incidence of reoperations.
Conclusion
There is a significant reoperation rate following rACLR at two years (11%). The most prevalent reoperations involved meniscal procedures. Independent risk factors for subsequent surgery on the ipsilateral knee include age<20 years old and use of allograft tissue at the time of rACLR. Knowledge of these facts will allow physicians to better council their patients appropriately before surgery.
What is known about the subject
Previous MARS studies have evaluated outcomes after primary ACL reconstruction including rates and risk factors for further reoperations. However, revision ACL reconstructions present a uniquely difficult problem for orthopedic surgeons.
What this study adds to existing knowledge
Currently, there is a lack of information concerning rates and risk factors for further reoperation after revision ACL reconstruction. This is the first multicenter, prospectively collected cohort study looking at rACLR and detailing the results and factors associated with reoperations.
David Y. Ding MD (University of California, San Francisco, San Francisco, California USA), Alan L. Zhang MD (University of California, San Francisco, San Francisco, California USA), Christina R. Allen, MD (University of California, San Francisco, San Francisco, California USA); Allen F. Anderson, MD (Tennessee Orthopaedic Alliance, Nashville, TN USA); Daniel E. Cooper, MD (W.B. Carrell Memorial Clinic, Dallas, TX USA); Thomas M. DeBerardino, MD (The San Antonio Orthopaedic Group, San Antonio, TX USA); Warren R. Dunn, MD, MPH (Reedsburg Area Medical Center, Reedsburg, WI USA); Amanda K. Haas, MA (Washington University in St. Louis, St. Louis, MO USA); Laura J. Huston, MS (Vanderbilt University, Nashville, TN USA); Brett (Brick) A. Lantz, MD (Slocum Research and Education Foundation, Eugene, OR USA); Barton Mann, PhD (deceased) (AOSSM, Rosemont, IL USA); Kurt P. Spindler, MD (Cleveland Clinic, Cleveland, OH USA); Michael J. Stuart, MD (Mayo Clinic, Rochester, MN USA); Rick W. Wright, MD (Washington University in St. Louis, St. Louis, MO USA); John P. Albright, MD (University of Iowa Hospitals and Clinics, Iowa City, IA USA), Annunziato (Ned) Amendola, MD (Duke University, Durham, NC USA); Jack T. Andrish, MD (Cleveland Clinic, Cleveland, OH USA); Christopher C. Annunziata, MD (Commonwealth Orthopaedics & Rehabilitation, Arlington, VA USA); Robert A. Arciero, MD (University of Connecticut Health Center, Farmington, CT USA); Bernard R. Bach Jr, MD (Rush University Medical Center, Chicago, IL USA); Champ L. Baker III, MD (The Hughston Clinic, Columbus, GA USA); Arthur R. Bartolozzi, MD (3B Orthopaedics, University of Pennsylvania Health System, Philadelphia, PA USA); Keith M. Baumgarten, MD (Orthopedic Institute, Sioux Falls, SD USA); Jeffery R. Bechler, MD (University Orthopaedic Associates LLC, Princeton, NJ USA); Jeffrey H. Berg, MD (Town Center Orthopaedic Associates, Reston, VA USA); Geoffrey A. Bernas, MD (State University of New York at Buffalo, Buffalo, NY); Stephen F. Brockmeier, MD (University of Virginia, Charlottesville, VA USA); Robert H. Brophy, MD (Washington University in St. Louis, St. Louis, MO USA); Charles A. Bush-Joseph, MD (Rush University Medical Center, Chicago, IL USA); J. Brad Butler V, MD (Orthopedic and Fracture Clinic, Portland, OR USA); John D. Campbell, MD (Bridger Orthopedic and Sports Medicine, Bozeman, MT USA); James L. Carey, MD, MPH (University of Pennsylvania, Philadelphia, PA USA); James E. Carpenter, MD (University of Michigan, Ann Arbor, MI USA); Brian J. Cole, MD (Rush University Medical Center, Chicago, IL USA); Jonathan M. Cooper, DO (HealthPartners Specialty Center, St. Paul, MN USA); Charles L. Cox, MD, MPH (Vanderbilt University, Nashville, TN USA); R. Alexander Creighton, MD (University of North Carolina Medical Center, Chapel Hill, NC USA); Diane L. Dahm, MD (Mayo Clinic, Rochester, MN USA); Tal S. David, MD (Synergy Specialists Medical Group, San Diego, CA USA); David C. Flanigan, MD (The Ohio State University, Columbus, OH USA); Robert W. Frederick, MD (The Rothman Institute/Thomas Jefferson University, Philadelphia, PA USA); Theodore J. Ganley, MD (Children’s Hospital of Philadelphia, Philadelphia, PA USA); Elizabeth A. Garofoli (Washington University in St. Louis, St. Louis, MO USA); Charles J. Gatt Jr, MD (University Orthopaedic Associates LLC, Princeton, NJ USA); Steven R. Gecha, MD (Princeton Orthopaedic Associates, Princeton, NJ USA); James Robert Giffin, MD (Fowler Kennedy Sport Medicine Clinic, University of Western Ontario, London Ontario, Canada); Sharon L. Hame, MD (David Geffen School of Medicine at UCLA, Los Angeles, CA USA); Jo A. Hannafin, MD, PhD (Hospital for Special Surgery, New York, NY USA); Christopher D. Harner, MD (University of Texas Health Center, Houston, TX USA); Norman Lindsay Harris Jr, MD (Orthopaedic Associates of Aspen & Glenwood, Aspen, CO USA); Keith S. Hechtman, MD (UHZ Sports Medicine Institute, Coral Gables, FL USA); Elliott B. Hershman, MD (Lenox Hill Hospital, New York, NY USA); Rudolf G. Hoellrich, MD (Slocum Research and Education Foundation, Eugene, OR USA); Timothy M. Hosea, MD (deceased) (University Orthopaedic Associates LLC, Princeton, NJ USA); David C. Johnson, MD, Timothy S. Johnson, MD (National Sports Medicine Institute, Leesburg, VA USA); Morgan H. Jones, MD (Cleveland Clinic, Cleveland, OH USA); Christopher C. Kaeding, MD (The Ohio State University, Columbus, OH USA); Ganesh V. Kamath, MD (University of North Carolina Medical Center, Chapel Hill, NC USA); Thomas E. Klootwyk, MD (Methodist Sports Medicine, Indianapolis, IN USA); Bruce A. Levy, MD (Mayo Clinic Rochester, MN USA); C. Benjamin Ma, MD (University of California, San Francisco, CA USA); G. Peter Maiers II, MD (Methodist Sports Medicine Center, Indianapolis, IN USA); Robert G. Marx, MD (Hospital for Special Surgery, New York, NY USA); Matthew J. Matava, MD (Washington University in St. Louis, St. Louis, MO USA); Gregory M. Mathien, MD (Knoxville Orthopaedic Clinic, Knoxville, TN USA); David R. McAllister, MD (David Geffen School of Medicine at UCLA, Los Angeles, CA USA); Eric C. McCarty, MD (University of Colorado Denver School of Medicine, Denver, CO USA); Robert G. McCormack, MD (University of British Columbia, New Westminster, BC Canada); Bruce S. Miller, MD, MS (University of Michigan, Ann Arbor, MI USA); Carl W. Nissen, MD (Connecticut Children’s Medical Center, Hartford, CT USA); Daniel F. O’Neill, MD, EdD (Littleton Regional Healthcare, Littleton, NH USA); Brett D. Owens, MD (Warren Alpert Medical School, Brown University, Providence, RI USA); Richard D. Parker, MD (Cleveland Clinic, Cleveland, OH USA); Mark L. Purnell, MD (Orthopaedic Associates of Aspen & Glenwood, Aspen, CO USA); Arun J. Ramappa, MD (Beth Israel Deaconess Medical Center, Boston, MA USA); Michael A. Rauh, MD (State University of New York at Buffalo, Buffalo, NY USA); Arthur C. Rettig, MD (Methodist Sports Medicine, Indianapolis, IN USA); Jon K. Sekiya, MD (University of Michigan, Ann Arbor, MI USA); Kevin G. Shea, MD (Intermountain Orthopaedics, Boise, ID USA); Orrin H. Sherman, MD (NYU Hospital for Joint Diseases, New York, NY USA); James R. Slauterbeck, MD (Robert Larner College of Medicine, University of Vermont, Burlington, VT USA); Matthew V. Smith, MD (Washington University in St. Louis, St. Louis, MO USA); Jeffrey T. Spang, MD (University of North Carolina Medical Center, Chapel Hill, NC USA); LTC Steven J. Svoboda, MD (Keller Army Community Hospital, United States Military Academy, West Point, NY USA); Timothy N. Taft, MD (University of North Carolina Medical Center, Chapel Hill, NC USA); Joachim J. Tenuta, MD (Albany Medical Center, Albany, NY USA); Edwin M. Tingstad, MD (Inland Orthopaedic Surgery and Sports Medicine Clinic, Pullman, WA USA); Armando F. Vidal, MD (University of Colorado Denver School of Medicine, Denver, CO USA); Darius G. Viskontas, MD (Royal Columbian Hospital, New Westminster, BC Canada); Richard A. White, MD (St. Mary’s Audrain, Mexico, MO USA); James S. Williams Jr, MD (Cleveland Clinic, Euclid, OH USA); Michelle L. Wolcott, MD (University of Colorado Denver School of Medicine, Denver, CO USA); Brian R. Wolf, MD (University of Iowa Hospitals and Clinics, Iowa City, IA USA); James J. York, MD (Chesapeake Orthopaedic & Sports Medicine Center, Glen Burnie, MD USA).
Contributor Information
MARS Group, Department of Orthopaedic Surgery, University of California San Francisco.
David Y. Ding, 1500 Owens Street, Box 3004, San Francisco CA 94158.
References
- 1.Brophy RH, Schmitz L, Wright RW, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. Am J Sports Med. 2012;40(11):2517–2522. doi: 10.1177/0363546512459476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coats AC, Johnson DL. Two-stage revision anterior cruciate ligament reconstruction: indications, review, and technique demonstration. Orthopedics. 2012;35(11):958–960. doi: 10.3928/01477447-20121023-08. [DOI] [PubMed] [Google Scholar]
- 3.Csintalan RP, Inacio MC, Funahashi TT, Maletis GB. Risk factors of subsequent operations after primary anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):619–625. doi: 10.1177/0363546513511416. [DOI] [PubMed] [Google Scholar]
- 4.Dodwell ER, Lamont LE, Green DW, Pan TJ, Marx RG, Lyman S. 20 years of pediatric anterior cruciate ligament reconstruction in New York State. Am J Sports Med. 2014;42(3):675–680. doi: 10.1177/0363546513518412. [DOI] [PubMed] [Google Scholar]
- 5.Duchman KR, Westermann RW, Spindler KP, et al. The Fate of Meniscus Tears Left In Situ at the Time of Anterior Cruciate Ligament Reconstruction: A 6-Year Follow-up Study From the MOON Cohort. Am J Sports Med. 2015 doi: 10.1177/0363546515604622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn WR, Lyman S, Lincoln AE, Amoroso PJ, Wickiewicz T, Marx RG. The effect of anterior cruciate ligament reconstruction on the risk of knee reinjury. Am J Sports Med. 2004;32(8):1906–1914. doi: 10.1177/0363546504265006. [DOI] [PubMed] [Google Scholar]
- 7.Franceschi F, Papalia R, Del Buono A, et al. Two-stage procedure in anterior cruciate ligament revision surgery: a five-year follow-up prospective study. Int Orthop. 2013;37(7):1369–1374. doi: 10.1007/s00264-013-1886-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granan LP, Baste V, Engebretsen L, Inacio MC. Associations between inadequate knee function detected by KOOS and prospective graft failure in an anterior cruciate ligament-reconstructed knee. Knee Surg Sports Traumatol Arthrosc. 2015;23(4):1135–1140. doi: 10.1007/s00167-014-2925-5. [DOI] [PubMed] [Google Scholar]
- 9.Group M, Group M. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) Cohort. Am J Sports Med. 2014;42(10):2301–2310. doi: 10.1177/0363546514549005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group M. Wright RW, Huston LJ, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2010;38(10):1979–1986. doi: 10.1177/0363546510378645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanypsiak BT, Spindler KP, Rothrock CR, et al. Twelve-year follow-up on anterior cruciate ligament reconstruction: long-term outcomes of prospectively studied osseous and articular injuries. Am J Sports Med. 2008;36(4):671–677. doi: 10.1177/0363546508315468. [DOI] [PubMed] [Google Scholar]
- 12.Hettrich CM, Dunn WR, Reinke EK, Group M. Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534–1540. doi: 10.1177/0363546513490277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaeding CC, Aros B, Pedroza A, et al. Allograft Versus Autograft Anterior Cruciate Ligament Reconstruction: Predictors of Failure From a MOON Prospective Longitudinal Cohort. Sports Health. 2011;3(1):73–81. doi: 10.1177/1941738110386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaeding CC, Pedroza AD, Reinke EK, Huston LJ, Consortium M. Spindler KP. Risk Factors and Predictors of Subsequent ACL Injury in Either Knee After ACL Reconstruction: Prospective Analysis of 2488 Primary ACL Reconstructions From the MOON Cohort. Am J Sports Med. 2015;43(7):1583–1590. doi: 10.1177/0363546515578836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath GV, Redfern JC, Greis PE, Burks RT. Revision anterior cruciate ligament reconstruction. Am J Sports Med. 2011;39(1):199–217. doi: 10.1177/0363546510370929. [DOI] [PubMed] [Google Scholar]
- 16.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 17.Lyman S, Koulouvaris P, Sherman S, Do H, Mandl LA, Marx RG. Epidemiology of anterior cruciate ligament reconstruction: trends, readmissions, and subsequent knee surgery. J Bone Joint Surg Am. 2009;91(10):2321–2328. doi: 10.2106/JBJS.H.00539. [DOI] [PubMed] [Google Scholar]
- 18.Maletis GB, Chen J, Inacio MC, Funahashi TT. Age-Related Risk Factors for Revision Anterior Cruciate Ligament Reconstruction: A Cohort Study of 21,304 Patients From the Kaiser Permanente Anterior Cruciate Ligament Registry. Am J Sports Med. 2016;44(2):331–336. doi: 10.1177/0363546515614813. [DOI] [PubMed] [Google Scholar]
- 19.Nagelli CV, Hewett TE. Should Return to Sport be Delayed Until 2 Years After Anterior Cruciate Ligament Reconstruction? Biological and Functional Considerations. Sports Med. 2016 doi: 10.1007/s40279-016-0584-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nwachukwu BU, McFeely ED, Nasreddine A, et al. Arthrofibrosis after anterior cruciate ligament reconstruction in children and adolescents. J Pediatr Orthop. 2011;31(8):811–817. doi: 10.1097/BPO.0b013e31822e0291. [DOI] [PubMed] [Google Scholar]
- 21.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am J Sports Med. 2014;42(7):1567–1573. doi: 10.1177/0363546514530088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Potter HG, Jain SK, Ma Y, Black BR, Fung S, Lyman S. Cartilage injury after acute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med. 2012;40(2):276–285. doi: 10.1177/0363546511423380. [DOI] [PubMed] [Google Scholar]
- 23.Shakked R, Weinberg M, Capo J, Jazrawi L, Strauss E. Autograft Choice in Young Female Patients: Patella Tendon versus Hamstring. J Knee Surg. 2016 doi: 10.1055/s-0036-1584561. [DOI] [PubMed] [Google Scholar]
- 24.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 25.Shelbourne KD, Heinrich J. The long-term evaluation of lateral meniscus tears left in situ at the time of anterior cruciate ligament reconstruction. Arthroscopy. 2004;20(4):346–351. doi: 10.1016/j.arthro.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Spindler KP, Huston LJ, Wright RW, et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med. 2011;39(2):348–359. doi: 10.1177/0363546510383481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas NP, Kankate R, Wandless F, Pandit H. Revision anterior cruciate ligament reconstruction using a 2-stage technique with bone grafting of the tibial tunnel. Am J Sports Med. 2005;33(11):1701–1709. doi: 10.1177/0363546505276759. [DOI] [PubMed] [Google Scholar]
- 28.Tse BK, Vaughn ZD, Lindsey DP, Dragoo JL. Evaluation of a one-stage ACL revision technique using bone void filler after cyclic loading. Knee. 2012;19(4):477–481. doi: 10.1016/j.knee.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 29.van Dijck RA, Saris DB, Willems JW, Fievez AW. Additional surgery after anterior cruciate ligament reconstruction: can we improve technical aspects of the initial procedure? Arthroscopy. 2008;24(1):88–95. doi: 10.1016/j.arthro.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Webster KE, Feller JA. Exploring the High Reinjury Rate in Younger Patients Undergoing Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2016;44(11):2827–2832. doi: 10.1177/0363546516651845. [DOI] [PubMed] [Google Scholar]
- 31.Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. doi: 10.1177/0363546513517540. [DOI] [PubMed] [Google Scholar]
- 32.Werner BC, Yang S, Looney AM, Gwathmey FW., Jr Trends in Pediatric and Adolescent Anterior Cruciate Ligament Injury and Reconstruction. J Pediatr Orthop. 2015 doi: 10.1097/BPO.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 33.Widener DB, Wilson DJ, Galvin JW, Marchant BG, Arrington ED. The prevalence of meniscal tears in young athletes undergoing revision anterior cruciate ligament reconstruction. Arthroscopy. 2015;31(4):680–683. doi: 10.1016/j.arthro.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Wiggins AJ, Grandhi RK, Schneider DK, Stanfield D, Webster KE, Myer GD. Risk of Secondary Injury in Younger Athletes After Anterior Cruciate Ligament Reconstruction: A Systematic Review and Meta-analysis. Am J Sports Med. 2016;44(7):1861–1876. doi: 10.1177/0363546515621554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright R, Spindler K, Huston L, et al. Revision ACL reconstruction outcomes: MOON cohort. J Knee Surg. 2011;24(4):289–294. doi: 10.1055/s-0031-1292650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright RW, Dunn WR, Amendola A, et al. Anterior cruciate ligament revision reconstruction: two-year results from the MOON cohort. J Knee Surg. 2007;20(4):308–311. doi: 10.1055/s-0030-1248066. [DOI] [PubMed] [Google Scholar]
- 37.Wright RW, Magnussen RA, Dunn WR, Spindler KP. Ipsilateral graft and contralateral ACL rupture at five years or more following ACL reconstruction: a systematic review. J Bone Joint Surg Am. 2011;93(12):1159–1165. doi: 10.2106/JBJS.J.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]


