Abstract
Background
Many studies in multiple sclerosis (MS) have investigated the retina. Little however is known about the effect of MS on the cornea, which is innervated by the trigeminal nerve. It is the site of neural-immune interaction with local dendritic cells reacting in response- to environmental stimuli.
Objective
This study aims to investigate the effect of MS on- corneal nerve fibres and dendritic cells in the subbasal nerve plexus using in vivo confocal microscopy (IVCM).
Methods
We measured the corneal nerve fibre and dendritic cell density in 26 MS patients and matched healthy controls using a Heidelberg Retina Tomograph with Cornea Module. Disease severity was assessed with the Multiple Sclerosis Functional Composite, Expanded Disability Status Scale, visual acuity and retinal optical coherence tomography.
Results
We observed significant reduction in total corneal nerve fibre density in MS patients compared to controls. Dendritic cell density was similar in both groups. Reduced total nerve fibre density was associated with worse clinical severity, but not with previous clinical trigeminal symptoms, retinal neuroaxonal damage, visual acuity, or disease duration.
Conclusion
Corneal nerve fibre density is a promising new imaging marker for the assessment of disease severity in MS and should be investigated further.
Keywords: Multiple Sclerosis, Cornea, Subbasal nerve plexus, Trigeminal Neuralgia, Peripheral nerves, Retina
Introduction
Multiple Sclerosis (MS) is the most common autoimmune disorder of the central nervous system (CNS). The cause of MS is unknown, but suggested factor in accruing clinical disability in MS are the myelin and axonal damage as well as neurodegeneration caused by an autoimmune reaction against CNS-specific myelin and myelin-forming oligodendrocytes 1.
Changes in the eye’s retina have been intensely studied in MS (for a recent review see 2). The retina is affected by retrograde damage from acute optic neuritis 3, and shows chronic axonal and ganglion cell degeneration also without clinically overt optic neuritis 4–6. Imaging the retina using optical coherence tomography (OCT) has therefore been suggested as potential surrogate marker of disease severity in clinical trials 7. In contrast to the retina, the cornea has not been investigated in MS.
Axons of the trigeminal nerve’s third terminal branch, the ophthalmic nerve, form the subbasal nerve plexus (SNP) in the human cornea. Imaging of nerve fibres in the SNP is possible with corneal in vivo confocal microscopy (IVCM), a non-invasive imaging technique providing high-resolution real-time images of corneal tissue at cellular resolution 8.
Next to nerve fibres, dendritic cells (DC) can be analyzed using IVCM. These cells usually respond to external stimuli, e.g. from contact lenses or dirt, and maintain a healthy immune state of the cornea on the outer surface to the environment. IVCM, thus, provides a unique opportunity for analysing immune and peripheral nerve system interactions with microscopic resolution in vivo 9–14.
Our study explored the potential of IVCM imaging of the SNP as a tool in the assessment of MS-related clinical parameters. First and foremost, we aimed to assess nerve fibre and DC differences in the corneal SNP in patients with MS compared to HC. We also investigated the association between corneal SNP differences and measures of clinical disability, as well as neuro-axonal damage in the retina assessed by optical coherence tomography (OCT).
Material and Methods
Patients and controls
Twenty-six MS patients and 26 healthy controls (HC) were initially enrolled. Patients with relapsing-remitting MS (RRMS) were recruited from the neuroimmunology outpatient clinic of the Charité – Universitätsmedizin Berlin. Inclusion criteria were age between 18 and 65 years, diagnosis of MS according to the 2010 revised McDonald criteria 15 and stable immunomodulatory therapy for at least six months. Exclusion criteria were disease attacks and administration of intravenous corticosteroids within six months prior to study recruitment, any known neurologic or ophthalmologic disorder unrelated to MS, diabetes mellitus, previous refractive surgery, pathological cornea changes due to corneal dystrophy or keratoconus, history of corneal transplantation and any other form of ocular surgery. Healthy controls (HC) were recruited from volunteers. All participants were surveyed regarding eye dryness, specifically epiphora or burning and aching, and usage of artificial tears and contact lenses to account for exogenous factors influencing SNP 16. Exclusion criteria for healthy controls were corneal DC density exceeding 137.1 cells/mm2 corresponding to two standard deviations of published reference data 17. All MS patients were clinically scored using the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) with its components Timed 25ft Walk Test (T25FW), 9-Hole Peg Test (9-HPT) and Paced Auditory Serial Additions Test (PASAT) 18. Multiple Sclerosis Severity Scores (MSSS) were calculated from disease duration and EDSS 19.
Single eyes of two patients and two controls were excluded after IVCM measurement due to insufficient image quality. Both eyes of three HC and single eyes of four further HC were excluded because DC density exceeded 137.1 cells/mm2 in each eye, leaving 23 subjects in the HC cohort and the initial 26 subjects in the patient cohort. A demographic and clinical overview of the cohort after application of exclusion criteria is given in Table 1.
Table 1. Cohort description.
| MS | HC | p | ||
|---|---|---|---|---|
| Subjects | N | 26 | 23 | |
| Sex | Male/Female (N) | 7 / 19 | 7 / 16 | >0.999 (Chi2) |
| Age / years | Mean ± SD (Range) | 42.8 ± 9.5 (28-62) | 38.2 ± 13.7 (21-63) | 0.135 (MWU) |
| Use of contact lenses | Yes | 5 | 4 | >0.999 (Chi2) |
| No | 21 | 19 | ||
| Eyes with previous ON | Yes/No (N) | 23/27 | ||
|
Time since diagnosis in
months |
Mean ± SD (Range) | 121 ± 64 (33 – 286) | ||
| EDSS | Median (Range) | 2.5 (1 - 6.5) | ||
| MSSS | Median (Range) | 3.1 (0.64 - 7.14) | ||
| 9-HPT / s | Mean ± SD (Range) | 20.1 ± 3.6 (15.0 – 31.1) |
||
| T25FW / s | Mean ± SD (Range) | 7.2 ± 11.4 (3.5 - 62.9) |
||
| PASSAT | Mean ± SD (Range) | 49.5 ± 11.1 (19 – 60) |
Abbreviations: MS – Multiple sclerosis patients, HC – Healthy controls, ON – optic neuritis, EDSS - Expanded Disability Status Scale, MSSS – Multiple Sclerosis Severity Scale, 9-PHT – Nine-Hole Peg Test (component of the Multiple Sclerosis Functional Composite), T25FW – Timed 25-foot Walk (component of the Multiple Sclerosis Functional Composite), PASSAT - Paced Auditory Serial Addition Test (component of the Multiple Sclerosis Functional Composite)
The study was approved by the ethics committee of the Charité – Universitätsmedizin Berlin and was conducted in conformity with the 1954 Declaration of Helsinki in its currently applicable version and applicable German laws. All study participants gave written informed consent.
Corneal in vivo Confocal Microscopy
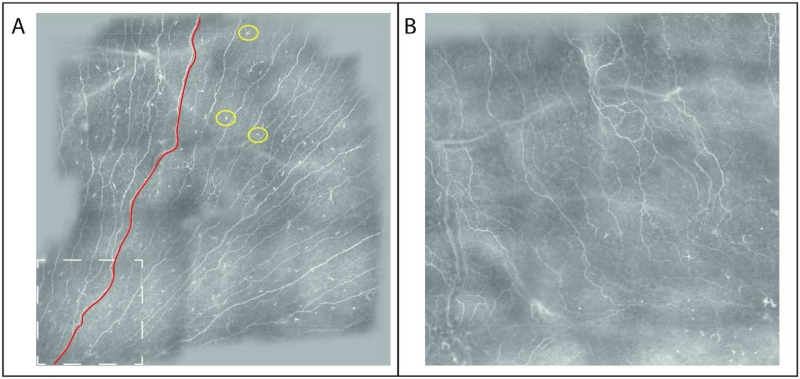
Corneal laser IVCM to analyse SNP nerve density and immune DCs was performed using the Rostock Cornea Module as add-on to the Heidelberg Retina Tomograph 3 (Heidelberg Engineering, Germany). Prior to examination, topical anaesthesia with oxybuprocainhydrochloride 4.0 g (Conjuncain® EDO®; Dr. Gerhard Mann, Chem.pharm. Fabrik GmbH, Germany) as active ingredient was applied to both eyes, followed by a drop of lubricant 2 mg/g carbomer-containing gel (Vidisic gel®; Bausch & Lomb, Heidelberg, Germany). The IVCM imaging using “composite” mode was then performed as previously described in detail 20–22. The maximum possible corneal scan area in composite mode (3.2 mm × 3.2 mm) was acquired wherever possible (Figure 1). DCs were identified by the morphology of cell bodies surrounded by dendritiform structures, which were clearly distinguishable from the linear structures of corneal nerve fibres. To calculate the density of DCs, ImageJ (National Institutes of Health, USA) was used. For the composite IVCM images, the surface area of the image was first measured using ImageJ in mm2. The cell number of DCs in the entire image was counted using ImageJ’s Cell Counter plug-in. The DC density was then expressed as cells/mm2. To measure subbasal nerve fibre density, nerves were traced using NeuronJ software (http://www.imagescience.org/meijering/software/neuronj/), which is a semi-automated nerve analysis plug-in of ImageJ that traces all visible nerve fibres in the image and calculates their total length in millimetres. Nerve fibre density was then expressed in μm/mm2 in relation to the composite image’s surface area. All nerve measurements were performed by two independent blinded observers.
Figure 1. Composite image of the corneal subbasal nerve plexus.
The device’s software automatically fuses repeated section measurements in a composite image. A) Sample image of a healthy control subject. The dashed white box depicts the size of one section image. A sample corneal nerve segment is delineated in red, sample dendritic cells shown by a yellow circle. B) Sample image of a multiple sclerosis patient.
Optical coherence tomography
Retinal examination of all patients was performed using spectral domain OCT (Spectralis, Heidelberg Engineering, Germany). Peripapillary nerve fibre layer thickness (pRNFL) was determined from a ring scan around the optic nerve head using the OCT device’s standard protocol with a 12° circular scan, resulting in 3.4 mm diameter, and with activated eye tracker. Whenever possible, the maximum 100 averaging frames in the automatic-real-time mode (ART) were used. Macular scans were acquired using a custom protocol generating 61 vertical slices (B-scans) focusing on the fovea at 30°×25° scanning angle with resolution of 768 A-scans per B-scan and ART 13. All scans were evaluated for sufficient signal strength, correct centring and segmentation. Intraretinal segmentation was performed using the above OCT manufacturer’s semiautomatic beta software (Heidelberg Eye Explorer V1.8.6.0 with Spectralis Viewing Module V6.0.0.2). The latter software detects and verifies boundaries between retinal layers automatically, but necessitates manual error correction by an experienced grader. Based on the intraretinal segmentation, ganglion cell and inner plexiform layer thickness (GCIP) and inner nuclear layer thickness (INL) were determined as volume within the standard 6 mm ETDRS ring around the fovea. Whereas pRNFL and GCIP are established parameters of neuro-axonal degeneration in MS, INL has been suggested as a correlate of neuroinflammation 23.
Statistical analysis
Statistical analysis was performed with R version 3.1.2 and geepack 1.2-0. To account for within-subject inter-eye effects, generalized estimating equation models (GEE) with working correlation matrix “exchangeable“ were used for all group comparisons and correlations involving corneal, retinal, and visual function measurements. GEE Results are given with regression coefficient (B) and standard error (SE). In HC, higher corneal nerve fibre density and DC density showed a trend to an association with higher age (B=107.9, SE=57.2, p=0.059 and B=0.8, SE=0.4, p=0.052, respectively), which is why we included age as a covariate in all analyses. Demographic group differences between patients and healthy controls were analysed using a non-parametric Mann-Whitney-U test (MWU) for age and Pearson’s Chi2 test for sex. TN-related symptom frequency comparisons between patients with and without history of such symptoms were calculated with Pearson’s χ2 statistics. Statistical significance was established at p<0.05. No a priori sample size calculation was performed and significance levels were not corrected for multiple comparisons. The study should therefore be considered exploratory.
Results
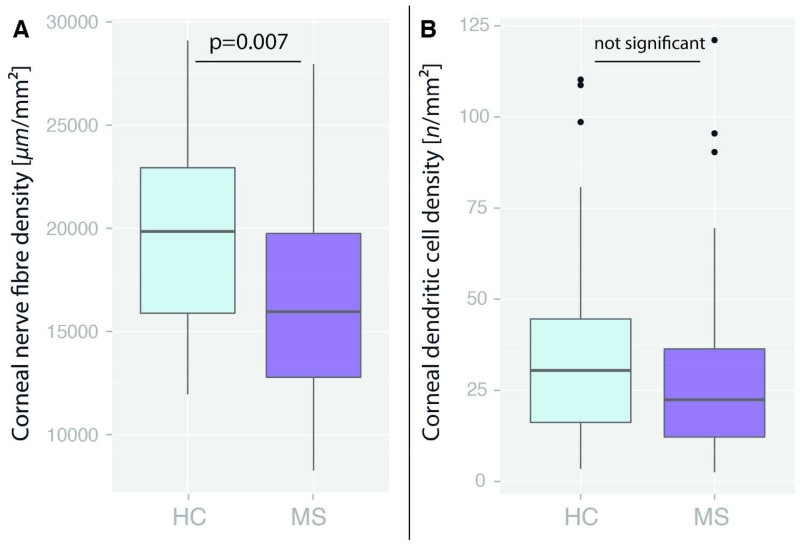
Corneal nerve fibre density was significantly lower in MS patients than in HC (16,531.7 ± 4,426.6 vs. 19,399.1 ± 4,546.1 μm/mm2, B=3,227.1, SE=1,192.0, p=0.007). The density of 13 of 50 MS eyes (26%) in 12 of 26 patients (46%) was below that of the 5th percentile of HC. In contrast, DC density was not significantly different between MS patients and HC (28.6 ± 24.5 vs. 37.0 ± 28.3 cells/mm2, B=12.2, SE=7.6, p=0.11) (Figure 2). As expected, pRNFL and GCIPL were reduced in MS patients in comparison to HC, but INL was similar (Table 2).
Figure 2. Corneal microscopy measurements.
Comparison of corneal measurements between RRMS patients and healthy controls (HC) A) Corneal nerve fibre density expressed as total nerve length in μm per mm2; B) Dendritic cell density expressed as n per mm2
Table 2. Retinal measurements.
| MS | HC | MS/HC group comparison |
Correlation with corneal nerve fibre density in MS |
Correlation with corneal DC density in MS |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | B | SE | P | B | SE | P | B | SE | P | |
|
pRNFL / μm
GCIP / mm3 INL / mm3 |
83.7 ± 13.7 | 96.6 ± 7.4 | 12.7 | 2.8 | <0.001 | 11.2 | 35.7 | 0.75 | 0.2 | 0.3 | 0.45 |
| 1.74 ± 0.20 | 1.97 ± 0.13 | 0.23 | 0.04 | <0.001 | 1766 | 2468 | 0.47 | 2.3 | 15.1 | 0.88 | |
| 0.96 ± 0.07 | 0.95 ± 0.07 | 0.02 | 0.02 | 0.37 | 1960 | 8864 | 0.83 | −9.3 | 54.6 | 0.87 | |
Abbreviations: MS – Multiple sclerosis, HC – Healthy control, GEE - Generalized estimating equation models, B – Coefficient, SE – Standard error, DC – Dendritic cells, pRNFL – Peripapillary retinal nerve fibre layer thickness, GCIP – Ganglion cell and inner plexiform layer volume, INL – Inner nuclear layer volume
In MS patients, lower corneal total nerve fibre density was associated with worse MSFC scores (B=1.810.4, SE=431.8, p<0.001) and worse EDSS (B=−822.9, SE=366.2, p=0.025). Corneal nerve density was not associated with time since diagnosis (B=−41.29, SE=10.67, p=0.90) but with MSSS (B=−855.6, SE=406.2, p=0.035). Analysing the individual MSFC tests, reduced corneal nerve fibre density correlated with worse T25FW times (B=−77.0, SE=15.9, p<0.001) and reduced PASAT performance (B=123.1, SE=55.1, p=0.026), but not with 9-HPT results (B=−0.538, SE=0.714, p=0.451). None of the OCT and visual function parameters correlated significantly with corneal nerve fibre density or DC density (Table 2). Likewise, there was no association with a previous optic neuritis (not shown).
We then assessed if corneal nerve fibre density was associated with a history of trigeminal symptoms. None of the MS patients had a history of diagnosed TN, however 11 out of 25 patients (for 1 patient this information was not available) had other TN-related symptoms in their medical record, i.e. facial hypaesthesia, dysaesthesia, or paraesthesia. Patients with a history of TN-related symptoms had similar corneal nerve density compared to patients without TN-related symptoms (15,731.0 ± 4,546.0 μm/mm2 vs. 17,177.0 ± 4,391.0 μm/mm2, B= 1,676.0, SE=1,561.0, p=0.28). Moreover, these patients did not show corneal nerve fibre densities below the 5th percentile of HC more frequently (p=0.74) than patients without TN-related symptoms.
Discussion
In this study we show that a) corneal nerve fibre density is reduced in MS patients, b) this reduction is associated with disease severity, c) the reduction is not associated with retinal damage, and d) the reduction is independent of a history of clinical trigeminal-related symptoms. Corneal nerve fibre density is an interesting new biomarker in MS as suggested by the consistent correlations with clinical severity. The marker was not affected by mild trigeminal symptoms, which suggests little dependency on focal symptoms or lesions. This is in contrast to OCT derived parameters, where optic neuritis causes additional damage and thus frequently interferes with the use of OCT parameters as surrogates for disease progression 24. Recent applications of OCT as disease progression biomarker have therefore focused on eyes without previous optic neuritis 25,26. The high frequency of optic neuritis in MS patients (confirmed in our random sample) thus limits these novel OCT applications. In contrast, no patient reported a history of trigeminal neuralgia, which is in line with its low prevalence.
The corneal SNP comprises terminal nerve endings from pseudo-unipolar sensory neurons originating in the trigeminal ganglion. Cell bodies from these neurons reside in the ganglion, connecting the cornea with peripheral axonal branches and the thalamic trigeminal nuclei with central axonal branches. Comparable to dorsal root ganglia in structure and function, these neurons are part of the PNS and are void of CNS-specific myelin from oligodendrocytes. Instead, Schwann cells ensheath both the peripheral and central axonal branches with peripheral myelin, which is composed of disparate cellular components and does not incorporate antigen targets thought to be relevant in MS 27. However, the trigeminal nerve is the 5th cranial/brain nerve and the trigeminal ganglion receives direct input from brainstem nuclei. As such the nerve is anatomically considered to be part of the central nervous system, despite belonging to the PNS from its cellular composition. The trigeminal ganglion and nerve thus represent an interesting target at the interface between central and peripheral nervous system. The trigeminal nerve is myelinated outside the cornea, but the terminal nerve endings in the corneal SNP are unmyelinated. The corneal SNP is highly dynamic and changes its fibre layout over a 6-week period 28. The neuronal regulators of this dynamic turnover are enigmatic in humans. Trigeminal, sympathetic or parasympathetic modulators have been shown in animal studies.
In our study, almost half of all investigated MS patients (42%) and 26% of all analysed eyes from these patients exhibited a corneal nerve fibre density below the 5th percentile of that of HC corneas. Few previous studies have suggested that the PNS might be affected in up to 5% of MS patients 29–33. However, the mechanisms underlying this MS-related PNS involvement and the contribution to overall clinical disability in MS are yet to be determined. Transsynaptic neurodegeneration after CNS nerve cell damage is most likely, but also primary neurodegeneration of peripheral neurons, have been discussed in studies of PNS impairment 33.
Trigeminal neuralgia (TN), a painful affection of the trigeminal nerve, affects 2 to 6% of MS patients, which is a 20-fold increased risk of developing TN compared to the general population 34–36. Our study shows that the trigeminal nerve can be affected in MS without a history of clinically diagnosed TN. However, such impairment might render the nerve susceptible to further damage and eventually trigger TN. Our random sample of MS patients did not include any patients with previous TN, thus a follow-up study of patients with diagnosed TN is needed to investigate this notion.
MS patients had similar DC density in the corneal SNP as that of the study’s HC and that of previously published controls 37. Local corneal inflammation, usually caused by exogenous influences like microbes, pollen or desiccating stress, leads to DCs migrating into the central part of the cornea, where they can be found up to six weeks after an inflammatory response 38. Previous studies have suggested that resident corneal DCs are always present in the central cornea but increase rapidly in response to various exogenous factors 39. It is therefore likely, that influences of exogenous factors on the corneal DC presence outweigh effects potentially attributable to MS. Thus, dendritic cell count at one single time point might not be a reliable marker to draw any firm conclusion regarding differences in DC dynamics in MS in comparison to HC.
This study is an exploratory pilot study, and results should be replicated in an independent study.
Our study shows that corneal SNP nerve fibre density is substantially reduced in MS patients in comparison to healthy subjects. The association of reduced corneal SNP density with higher clinical disability prompts further investigations on the applicability of this new measure as potential imaging biomarker for disease severity and progression in MS.
Acknowledgements
We thank the Departments of Ophthalmology at the Heidelberg University Hospital and the University of Rostock for training in IVCM.
Funding
This study was partially funded by a German Research Foundation (DFG Exc. 257) grant to FP. PH’s contribution was funded by the NIH (NIH R01-EY022695), the Falk Medical Research Foundation and the MEEI Foundation.
Footnotes
Declaration of Conflicting Interests
The Authors declare that there is no conflict of interest.
References
- 1.Compston A, Coles A. Multiple sclerosis. The Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Balcer LJ, Miller DH, Reingold SC, et al. Vision and vision-related outcome measures in multiple sclerosis. Brain. 2014 doi: 10.1093/brain/awu335. awu335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabilondo I, Martínez-Lapiscina EH, Fraga-Pumar E, et al. Dynamics of retinal injury after acute optic neuritis. Ann Neurol. 2015;77:517–528. doi: 10.1002/ana.24351. [DOI] [PubMed] [Google Scholar]
- 4.Oberwahrenbrock T, Schippling S, Ringelstein M, et al. Retinal damage in multiple sclerosis disease subtypes measured by high-resolution optical coherence tomography. Mult Scler Int. 2012;2012:530305. doi: 10.1155/2012/530305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saidha S, Al-Louzi O, Ratchford JN, et al. Optical coherence tomography reflects brain atrophy in MS: A four year study. Ann Neurol. doi: 10.1002/ana.24487. Epub ahead of print 18 July 2015. DOI: 10.1002/ana.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balk LJ, Cruz-Herranz A, Albrecht P, et al. Timing of retinal neuronal and axonal loss in MS: a longitudinal OCT study. J Neurol. 2016;263:1323–1331. doi: 10.1007/s00415-016-8127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barkhof F, Calabresi PA, Miller DH, et al. Imaging outcomes for neuroprotection and repair in multiple sclerosis trials. Nat Rev Neurol. 2009;5:256–266. doi: 10.1038/nrneurol.2009.41. [DOI] [PubMed] [Google Scholar]
- 8.Lemp MA, Dilly PN, Boyde A. Tandem-scanning (confocal) microscopy of the full-thickness cornea. Cornea. 1985;4:205–209. [PubMed] [Google Scholar]
- 9.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the Nervous System: The Connection in the Cornea in Patients with Infectious Keratitis. Investig Opthalmology Vis Sci. 2011;52:5136. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagasato D, Araki-Sasaki K, Kojima T, et al. Morphological changes of corneal subepithelial nerve plexus in different types of herpetic keratitis. Jpn J Ophthalmol. 2011;55:444–450. doi: 10.1007/s10384-011-0068-5. [DOI] [PubMed] [Google Scholar]
- 12.Hamrah P, Cruzat A, Dastjerdi MH, et al. Unilateral herpes zoster ophthalmicus results in bilateral corneal nerve alteration: an in vivo confocal microscopy study. Ophthalmology. 2013;120:40–47. doi: 10.1016/j.ophtha.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhivov A, Winter K, Hovakimyan M, et al. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PloS One. 2013;8:e52157. doi: 10.1371/journal.pone.0052157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Resch MD, Marsovszky L, Németh J, et al. Dry Eye and Corneal Langerhans Cells in Systemic Lupus Erythematosus. J Ophthalmol. 2015 doi: 10.1155/2015/543835. Epub ahead of print 2015. DOI: 10.1155/2015/543835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhivov A, Stave J, Vollmar B, et al. In Vivo Confocal Microscopic Evaluation of Langerhans Cell Density and Distribution in the Corneal Epithelium of Healthy Volunteers and Contact Lens Wearers. Cornea. 2007;26:47–54. doi: 10.1097/ICO.0b013e31802e3b55. [DOI] [PubMed] [Google Scholar]
- 17.Colon CM, Cavalcanti BM, Cruzat A, et al. In Vivo Confocal Microscopy of Immune Cells in the Cornea of Normal Subjects Demonstrates Irregular Peripheral Distribution of Dendritic Cells. Invest Ophthalmol Vis Sci. 2012;53:94–94. [Google Scholar]
- 18.Cutter GR, Baier ML, Rudick RA, et al. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain J Neurol. 1999;122(Pt 5):871–882. doi: 10.1093/brain/122.5.871. [DOI] [PubMed] [Google Scholar]
- 19.Roxburgh RHSR, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology. 2005;64:1144–1151. doi: 10.1212/01.WNL.0000156155.19270.F8. [DOI] [PubMed] [Google Scholar]
- 20.Hamrah P, Cruzat A, Dastjerdi MH, et al. Corneal Sensation and Subbasal Nerve Alterations in Patients with Herpes Simplex Keratitis: An In Vivo Confocal Microscopy Study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cruzat A, Witkin D, Baniasadi N, et al. Inflammation and the Nervous System: The Connection in the Cornea in Patients with Infectious Keratitis. Invest Ophthalmol Vis Sci. 2011;52:5136–5143. doi: 10.1167/iovs.10-7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kheirkhah A, Muller R, Mikolajczak J, et al. Comparison of Standard Versus Wide-Field Composite Images of the Corneal Subbasal Layer by In Vivo Confocal Microscopy. Invest Ophthalmol Vis Sci. 2015;56:5801–5807. doi: 10.1167/iovs.15-17434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saidha S, Sotirchos ES, Ibrahim MA, et al. Microcystic macular oedema, thickness of the inner nuclear layer of the retina, and disease characteristics in multiple sclerosis: a retrospective study. Lancet Neurol. 2012;11:963–972. doi: 10.1016/S1474-4422(12)70213-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zimmermann H, Freing A, Kaufhold F, et al. Optic neuritis interferes with optical coherence tomography and magnetic resonance imaging correlations. Mult Scler Houndmills Basingstoke Engl. doi: 10.1177/1352458512457844. Epub ahead of print 30 August 2012. DOI: 10.1177/1352458512457844. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15:574–584. doi: 10.1016/S1474-4422(16)00068-5. [DOI] [PubMed] [Google Scholar]
- 26.Knier B, Schmidt P, Aly L, et al. Retinal inner nuclear layer volume reflects response to immunotherapy in multiple sclerosis. Brain. 2016 doi: 10.1093/brain/aww219. aww219. [DOI] [PubMed] [Google Scholar]
- 27.Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995–1999. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 28.Patel DV, McGhee CNJ. In vivo laser scanning confocal microscopy confirms that the human corneal sub-basal nerve plexus is a highly dynamic structure. Invest Ophthalmol Vis Sci. 2008;49:3409–3412. doi: 10.1167/iovs.08-1951. [DOI] [PubMed] [Google Scholar]
- 29.Di Trapani G, Carnevale A, Cioffi P, et al. Multiple sclerosis associated with peripheral demyelinating neuropathy. Clin Neuropathol. 1995;15:135–138. [PubMed] [Google Scholar]
- 30.Misawa S, Kuwabara S, Mori M, et al. Peripheral nerve demyelination in multiple sclerosis. Clin Neurophysiol. 2008;119:1829–1833. doi: 10.1016/j.clinph.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Pirko I, Kuntz NL, Patterson M, et al. Contrasting effects of IFNβ and IVIG in children with central and peripheral demyelination. Neurology. 2003;60:1697–1699. doi: 10.1212/01.wnl.0000064163.94122.eb. [DOI] [PubMed] [Google Scholar]
- 32.Tachi N, Ishikawa Y, Tsuzuki T, et al. A case of childhood multiple sclerosis with peripheral neuropathy. Neuropediatrics. 1985;16:231–234. doi: 10.1055/s-2008-1059543. [DOI] [PubMed] [Google Scholar]
- 33.Vogt J, Paul F, Aktas O, et al. Lower motor neuron loss in multiple sclerosis and experimental autoimmune encephalomyelitis. Ann Neurol. 2009;66:310–322. doi: 10.1002/ana.21719. [DOI] [PubMed] [Google Scholar]
- 34.Hooge JP, Redekop WK. Trigeminal neuralgia in multiple sclerosis. Neurology. 1995;45:1294–1296. doi: 10.1212/wnl.45.7.1294. [DOI] [PubMed] [Google Scholar]
- 35.Putzki N, Pfriem A, Limmroth V, et al. Prevalence of migraine, tension-type headache and trigeminal neuralgia in multiple sclerosis. Eur J Neurol. 2009;16:262–267. doi: 10.1111/j.1468-1331.2008.02406.x. [DOI] [PubMed] [Google Scholar]
- 36.van Hecke O, Austin SK, Khan RA, et al. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. doi: 10.1016/j.pain.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 37.Zhivov A, Stave J, Vollmar B, et al. In vivo confocal microscopic evaluation of Langerhans cell density and distribution in the normal human corneal epithelium. Graefes Arch Clin Exp Ophthalmol. 2005;243:1056–1061. doi: 10.1007/s00417-004-1075-8. [DOI] [PubMed] [Google Scholar]
- 38.Niederkorn J, Peeler J, Mellon J. Phagocytosis of particulate antigens by corneal epithelial cells stimulates interleukin-1 secretion and migration of Langerhans cells into the central cornea. Reg Immunol. 1988;2:83–90. [PubMed] [Google Scholar]
- 39.Vantrappen L, Geboes K, Missotten L, et al. Lymphocytes and Langerhans cells in the normal human cornea. Invest Ophthalmol Vis Sci. 1985;26:220–225. [PubMed] [Google Scholar]