Abstract
Meta-C–H amination and meta-C–H alkynylation of aniline and phenol substrates using a modified norbornene (methyl bicyclo[2.2.1]hept-2-ene-2-carboxylate) as a transient mediator has been developed for the first time. Both the identification of a mono-protected 3-amino-2-hydroxypyridine/pyridone type ligand and the use of a modified norbornene as a mediator are crucial for the realization of these two unprecedented meta-C–H transformations. A variety of substrates are compatible with both meta-C–H amination and meta-C–H alkynylation. Amination and alkynylation of heterocyclic substrates including indole, indoline, and indazole afford the desired products in moderate to high yields.
TOC Graphic

1. Introduction
Meta-C–H functionalization of arenes poses a particularly interesting challenge due to the distal and geometric relationship between the meta-C–H bond and the existing functional group.1 Previously, meta-selectivity has been obtained using sterically guided catalysts when concerning 1,2- and 1,3-disubstituted arenes.2 Limited success on meta-selective C–H olefination of mono-substituted electron-deficient arenes using electronic bias has also been reported.3 Inspired by the numerous reports concerning directed ortho-C–H activation of arenes, various strategies have been developed that use existing functional groups to direct meta-C–H activation.4–6 For example, by engineering the spatial relationship of a directing group to a meta-C–H bond, our group and others have achieved a number of meta-C–H functionalization reactions using a U-shaped template.4 More recently, our group7a and Dong’s group7b have utilized directed ortho-palladation to achieve meta-C–H activation reactions7 by synchronizing the palladacycle intermediate with Catellani’s norbornene-mediated relay process (Figure 1a).8 Owing to the development of improved norbornene mediators and ligands, the scope of meta-C–H arylation and alkylation using this approach has been substantially expanded.7c,7e In principle, this approach should be compatible with any substrate containing an effective ortho-directing group, thus rendering this approach potentially broadly applicable. However, the transformations which have been reported using this approach are currently limited to either alkylation or arylation. To address the feasibility of overcoming this limitation, we initiated efforts to develop other transformations using this approach. Herein, we disclose the first protocol for meta-C–H amination and meta-C–H alkynylation (Figure 1b). To date, ortho-C–H alkynylation has not been demonstrated in the Catellani type reactions.8 Key to the success of these transformations is use of a modified norbornene and mono-protected 3-amino-2-hydroxy pyridine ligands. These results indicate that a transient mediator can be used in combination with the proper ligand and palladium catalyst to achieve a broad range of meta-C–H functionalization reactions that are rich with diversity in both substrate and transformation.
Figure 1.

Meta-C‒H Amination and Alkynylation
2. Results and Discussion
2.1 meta-C–H amination
Catalytic C‒H amination has attracted much attention due to the importance of amines in medicinal and materials chemistry.9 Several transition metals, such as Ir, Ru, Rh, Pd, Fe, Co, and Cu, have been used for this purpose to afford amines and amides directly from C–H bonds.10 However, direct C‒H amination at remote positions has not yet been reported. Based on the previous finding that 3-acetylamino-2-hydroxy pyridine based ligands can promote the meta-arylation of anilines with a broad substrate scope, we chose the aniline 1a as a model substrate to examine the feasibility of developing a meta-C–H amination reaction using norbornene as a transient mediator. 3-Amino anilines provided by this methodology are potentially useful synthetic intermediates for many drug molecules (Figure 2).11
Figure 2.

Biologically active 3-amino anilines scaffold
Based on our previous work concerning ortho-C–H amination using O-benzoyl hydroxylmorpholine as the aminating reagent,12 we found that reaction of 1a with this aminating reagent in the presence of 10 mol% Pd(OAc)2, 20 mol% 3-acetylamino-2-hydroxy pyridine ligand (L1), K3PO4 (3.0 equiv.) and AgOAc (3.0 equiv.) in 1,2-dichloroethane using 2-norbornene as a mediator afforded meta-aminated product 3a in 13% yield. It is worth noting that no meta-product was observed in the absence of ligand or base under similar conditions (see SI for more information). Unfortunately, further screening of bases, solvents, and oxidants did not significantly improve the yield (see SI for more information). Next, we screened various norbornene derivatives in an attempt to improve the efficiency of this reaction and found a modified norbornene, NBE-CO2Me (methyl bicyclo[2.2.1]hept-2-ene-2-carboxylate),7c to be the most efficient mediator. Using this modified norbornene, dichloromethane was found to be a superior solvent affording the amination product 3a in 52% yield in the presence of 10 mol% of ligand (L1, Table 1). The significant increase in yield observed with this modified norbornene is likely due to suppression of the competitively formed benzocyclobutane side product, as previously reported.7c Under these newly established conditions, we performed the reaction in the absence of ligand and observed the aminated product 3a could be obtained in 19% NMR yield. This result clearly demonstrates the 3-acetylamino-2-hydroxy pyridine ligand (L1) dramatically increases the efficiency of this reaction.
Table 1.

|
Reaction conditions: 1a (35.6 mg, 0.1 mmol), 2a (41.4 mg, 0.2 mmol), Pd(OAc)2 (10 mol%), Ligand (10 mol%), NBE-CO2Me (21.4 mg, 1.5 equiv.), AgOAc (50.1 mg, 3.0 equiv.), CH2Cl2 (1.0 mL), 100 °C, Air, 24 h.
Yield was determined by 1H NMR using benzyl acetate as internal standard.
2a (31.1 mg, 0.15 mmol), AgOAc (33.4 mg, 2.0 equiv.) were used.
To facilitate systematic investigations of the influence of 3-amino-2-hydroxy pyridine-based ligands on this reaction, we developed a more practical synthetic procedure to obtain multiple grams of NBE-CO2Me from 5-norbornene-2,3-dicarboxylic anhydride ($68.75/500 gram, from TCI) (See SI, 65% yield over four steps). With the modified norbornene in hand, a wide range of 2-hydroxy pyridine ligands were examined. Replacement of the OH group on the ligand with an SH group (L4) gave only 5% yield of the desired product, which we hypothesize is due to the strong coordination of sulfur to palladium. Methyl substitution at the 4- or 5-position of the ligand did not drastically alter the efficiency of the ligand, while substitution at the 6 position (L5) led to a significant decrease in the activity. A slightly lower yield was observed when the ligand with fluorine at the 5-postion (L8) was tested. A trifluoromethyl group at the 5-position of the ligand improved the yield of the reaction to 63% (L9). 3-amino-2-hydroxy quinoline (L10) afforded the product in 37% yield, presumably due to the steric similarity of L10 and L5, further confirming the intolerance of substitution at the 6-position. Interestingly, 3-acetylamino-4-hydroxy pyridine (L11) can also promote this reaction, though to a lesser extent, affording the desired product in 30% yield. Notably, the protecting group on the amine slightly affects the activity of the ligand (L1 vs L2 and L3). With L9 being the most promising ligand, we used this scaffold to further screen various protecting groups on the amine. Carbonate-based protecting groups slightly increased the yield (L12 and L13); however, sulfonyl and benzoyl-protected ligands decreased the yield (L14 and L15). Interestingly, introduction of sterics on the benzoyl group restored the ligand’s activity (L17), which indicates that sterics on the protecting group is important. We next examined a variety of acetyl protecting groups (L18–L28). Gratifyingly, bulkier acetyl protecting groups such as pivaloyl (L24) and 1-adamantanecarbonyl (L28) significantly improved the reaction efficiency providing 70% and 78% yield respectively. With the optimal ligand L28 identified, we performed a second round of optimizations of the reaction parameters and found that a decrease in the amount of O-benzoyl hydroxylmorpholine (2a) to 1.5 equivalents and sliver acetate to 2.0 equivalents improves the yield to 81% (77% isolated yield, 3a in Table 2). Several simple 2-hydroxypyride/pyridone ligands were also investigated (L29–L33), giving the aminated product in 10–51% yield. Interestingly, protecting the N‒H on the best ligand L28 with a methyl group (L34) decreased the yield to 55%, which indicates the N‒H is important in this ligand design for the meta-C‒H amination reaction. The role of the free N‒H in the ligand is unclear at this stage though we hypothesize that mono-protected 3-amino-2-hydroxypyridine ligands might coordinate with Pd(II) through the pyridone motif as a carboxylate surrogate in some steps of this reaction and as a bis-dentate ligand (similar to the mono-protected amino acid ligands)7e in others.
Table 2.

|
Reaction conditions: 1 (0.1 mmol), 2a (31.0 mg, 0.15 mmol), Pd(OAc)2 (2.2 mg, 10 mol%), L28 (10 mol%), NBE-CO2Me (21.4 mg, 1.5 equiv.), AgOAc (33.4 mg, 2.0 equiv.), CH2Cl2 (1.0 mL), 100 °C, Air, 24 h.
Isolated yield.
Pd(OAc)2 (3.4 mg, 15 mol%), L28 (15 mol%), NBE-CO2Me (21.4 mg, 1.5 equiv.), AgOAc (50.1 mg, 3.0 equiv.) were used.
The selectivity of mono- and di-products was determined by 1H NMR.
With the optimized conditions in hand, the generality of the meta-C‒H amination was investigated. A variety of functional groups, such as MeS, MeO, BnBocN, F, Cl, Br, ester and ketone, are well tolerated providing the desired meta-C‒H aminated products in synthetically useful yields (3b–3m). Substrates bearing functional groups at the ortho- and para-positions are also compatible with this amination protocol (3n–q), though the para-substituted and simple aniline substrates provided both the mono- and di-aminated products. 1-Naphthylamine (3r) and multiple substituted amines (3s–3u) are suitable substrates for this transformation. To our delight, heterocyclic amines containing indole, indoline, and indazole scaffolds are tolerated in this reaction, affording the desired meta-aminated products in moderate to high yields (3v–y).
Having evaluated the substrate scope of aniline derivatives that are compatible with this amination reaction, we next turned our focus towards the scope of aminating reagents (Table 3). Using 1a as the model substrate, a range of aminating reagents were investigated. Piperazine, thiomorpholine, thiomorpholine 1,1-dioxide, and 2,6-dimethylmorpholine, all of which are privileged motifs in drug discovery, couple smoothly under the reaction conditions to provide the desired products in good yields (4a–4d). For the medicinally important piperidine moieties, various functional groups were well tolerated on the piperidine backbone including TBS-protected hydroxyl groups, esters, phthalimido (Phth)-protected amino groups, and ketones (4d–4k). It is worth noting that the piperidin-4-one derived aminating reagent (4k) is compatible in this meta-amination reaction giving the desired product in 53% yield. The compatibility of this substrate is important as this motif can be readily transformed to the free amine in one step (see SI for more information). Unfortunately, aminating reagents with pyrrolidine, azepane and acyclic dialkylamine scaffolds were not effective and resulted in poor yields under the reaction conditions.
Table 3.

|
Reaction conditions: 1a (35.6 mg, 0.1 mmol), Aminating reagent (1.5 equiv.), Pd(OAc)2 (15 mol%), L28 (15 mol%), NBE-CO2Me (42.8 mg, 3 equiv.), AgOAc (50.1 mg, 3.0 equiv.), CH2Cl2 (1.0 mL), 100 °C, Air, 24 h.
Isolated yield.
Pd(OAc)2 (10 mol%), L28 (10 mol%), NBE-CO2Me (21.4 mg, 1.5 equiv.), AgOAc (33.4 mg, 2.0 equiv.) were used.
Phenol substrates bearing the same directing group can also be utilized in this meta-C–H amination reaction under similar conditions, although the conversions are slightly lower when compared to aniline substrates (6a–f, Table 4). A few aminating reagents were tested with phenol substrate 5e, giving the desired products in moderate to good yields (6g–6i).
Table 4.

|
Reaction conditions: 5 (0.1 mmol), 2 (0.15 mmol), Pd(OAc)2 (2.2 mg, 10 mol%), L28 (10 mol%), NBE-CO2Me (21.4 mg, 1.5 equiv.), AgOAc (33.4 mg, 2.0 equiv.), CH2Cl2 (1.0 mL), 100 °C, Air, 24 h.
Isolated yield.
The selectivity of mono- and di-products was determined by 1H NMR.
To demonstrate the utility of this reaction on a laboratory preparative scale, we conducted the reaction on gram scale and 3a was obtained in 74% yield (Scheme 1). Although mild oxidative conditions to remove this directing group have been reported,7e a simple one-step strategy is always desired. We have demonstrated the removal of the Boc-protecting group and the pyridine directing group simultaneously by treating the aminated product 3i with hydrobromic acid at 100 °C. This one-step protocol provides the free amine 7 in 83% yield (see SI for more information). Notably, the 3-fluoro-5-morpholinoaniline 7 is a key intermediate in the synthesis of 8, a BRAF inhibitor.13
Scheme 1.
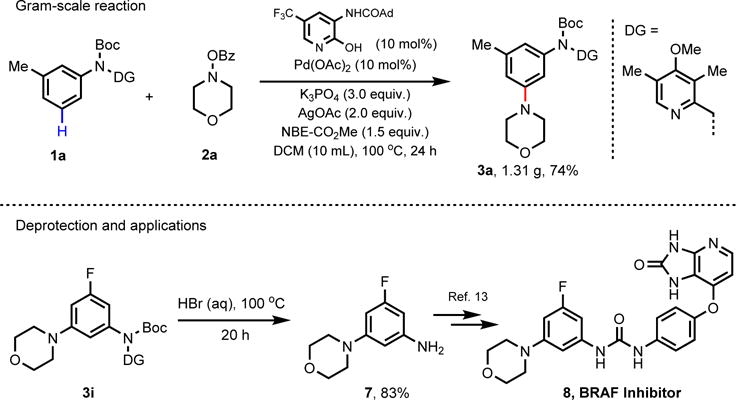
Synthetic application of meta-C‒H amination of anilines
2.2 Meta-C–H alkynylation
Aryl alkynes are privileged structural motifs found in natural products, pharmaceuticals, and materials.14 They also act as valuable precursors participating in many cross-coupling, metathesis, and cycloaddition reactions.15 In the last few decades, the Sonogashira coupling has been extensively studied and represents one of the most important methods to synthesize aryl alkynes in both academic and industrial settings.16 Consequently, installation of alkyne groups via C–H alkynylation has attracted significant interest and has been studied using terminal alkynes,17 alkynyl halides,18 and hypervalent iodine reagents.19 However, meta-C‒H alkynylation has not been reported to date. The lack of precedents using alkynyl coupling partners as electrophiles in the Catellani reaction also attests to the challenge of developing meta-C‒H alkynylation using norbornene as a transient mediator.8 Encouraged by the success of meta-C‒H amination reactions using norbornene as transient mediator, we envisioned that meta-alkynylation could be achieved through a Pd(II)/Pd(IV) process using an alkynyl bromide as the electrophile. To our great delight, 25% yield of the meta-alkynylated product 10a was obtained, accompanied by 14% yield of the ortho-alkynylated product 10a’ in the presence of 10 mol% Pd(OAc)2, 20 mol% ligand L9, alkynyl bromide 9 (2.0 equiv.), LiF (2.0 equiv.) and Ag2CO3 (1.5 equiv.) in 1,2-dichloroethane at 95 °C using NBE-CO2Me as a mediator. Interestingly, the ortho-alkynylated product can also be obtained in this protocol due to the high reactivity of alkynyl bromides. After a thorough investigation of solvents, oxidants, additives and ligands, we found the selectivity of meta- to ortho-products can be improved to >11:1 using TFA-protected ligand L18, allowing the desired meta-alkynylated product to be obtained in 71% isolated yield. The yield can be further improved to 75% by increasing the temperature to 100 °C (see SI for more information).
We next evaluated the scope of this meta-alkynylation reaction under the optimal conditions (see Table 5). Functional groups such as Me, MeO, Benzyl, Ph, F, Cl, and Br, are well tolerated under the reaction conditions affording the meta-alkynylated products 10a–h in moderate to good yields. Indoline and indazole-containing amines are also compatible with this protocol and the desired products 10i and 10j could be obtained in 53% and 46% yield respectively. The scope of alkyl and aryl alkynes is also investigated. Only various bulky silyl-protected alkynyl bromides give meta-alkynylated products in good yields. The simple alkyl and aryl alkynyl bromides led to trace products (See SI).
Table 5.

|
Reaction conditions: 1 (0.1 mmol), 9 (52.2 mg, 0.2 mmol), Pd(OAc)2 (2.2 mg, 10 mol%), L18 (8.2 mg, 30 mol%), NBE-CO2Me (38.0 mg, 2.5 equiv.), Ag2CO3 (41.3 mg, 1.5 equiv.), LiF (5.2 mg, 0.2 mmol), CH2Cl2 (1.0 mL), 100 °C, Air, 24 h.
Isolated yield.
3. Conclusion
In summary, Pd(II)-catalyzed meta-C–H amination and meta-C–H alkynylation have been developed for the first time, indicating that norbornene mediated meta-C–H functionalization is a highly general platform that is compatible with a wide variety of transformations. Both the mono-protected 3-amino-2-hydroxypyridine ligands and the modified norbornene (NBE-CO2Me) are crucial to realize these transformations. High yields and a broad substrate scope have been achieved for both meta-C–H amination and meta-C–H alkynylation reactions using either N-benzoyloxyamines or alkynyl bromides as electrophilic reagents. Future efforts will focus on improving the efficiency of these transformations, as well as exploring new transformations which have not yet been demonstrated in meta-C–H functionalization using this strategy.
4. Experimental Section
4.1 General procedure for amination of anilines
Substrate 1 (0.1 mmol), 2a (0.15 mmol), Pd(OAc)2 (2.2 mg, 10 mol%), L28 (2.2 mg, 10 mol%), AgOAc (33.4 mg, 0.2 mmol), NBE-CO2Me (21.6 mg, 0.15 mmol), K3PO4 (62.8 mg, 0.3 mmol) and CH2Cl2 (1.0 mL) were added to a 2-dram vial. The vial was capped and closed tightly. Then the reaction mixture was stirred at 100 °C for 24 hours. After cooling to room temperature, the mixture was passed through a pad of Celite with CH2Cl2 as the eluent to remove the insoluble precipitate. The resulting solution was concentrated and purified by preparative TLC to afford the desired product 3.
4.2 General procedure for alkynylation of anilines
Substrate 1 (0.1 mmol), alkynylating reagent 9 (52.2 mg, 0.2 mmol), Pd(OAc)2 (2.2 mg, 10 mol%), L18 (8.2 mg, 30 mol%), Ag2CO3 (41.3 mg, 0.15 mmol), NBE-CO2Me (38.0 mg, 0.25 mmol), LiF (5.2 mg, 0.2 mmol) and CH2Cl2 (1.0 mL) were added to a 2-dram vial. The vial was capped and closed tightly. Then the reaction mixture was stirred at 100 °C for 24 hours. After cooling to room temperature, the mixture was passed through a pad of Celite with CH2Cl2 as the eluent to remove the insoluble precipitate. The resulting solution was concentrated and purified by preparative TLC to afford the desired product 10.
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 2R01 GM102265) for financial support. Tyler G. St. Denis is thanked for editorial assistance.
Footnotes
Supporting Information Available. Detailed experimental procedures, characterization of new compounds. This material is available free of charge via the internet at http://pubs.acs.org.
Notes. The authors declare no competing financial interest.
References
- 1.Accounts for remote C–H activation:; a) Schranck J, Tlili A, Beller M. Angew Chem Int Ed. 2014;53:9426. doi: 10.1002/anie.201405714. [DOI] [PubMed] [Google Scholar]; b) Yang J. Org Biomol Chem. 2015;13:1930. doi: 10.1039/c4ob02171a. [DOI] [PubMed] [Google Scholar]; c) Li J, Ackermann L. Nat Chem. 2015;7:686. doi: 10.1038/nchem.2334. [DOI] [PubMed] [Google Scholar]; d) Li J, Sarkar SD, Ackermann L. Top Organomet Chem. 2016;55:217. [Google Scholar]
- 2.a) Ishiyama T, Takagi J, Ishida K, Miyaura N, Anastasi NR, Hartwig JF. J Am Chem Soc. 2002;124:390. doi: 10.1021/ja0173019. [DOI] [PubMed] [Google Scholar]; b) Cho J-Y, Tse MK, Holmes D, Maleczka RE, Jr, Smith MR., III Science. 2002;295:305. doi: 10.1126/science.1067074. [DOI] [PubMed] [Google Scholar]; c) Maleczka RE, Jr, Shi F, Holmes D, Smith MR., III J Am Chem Soc. 2003;125:7792. doi: 10.1021/ja0349857. [DOI] [PubMed] [Google Scholar]; d) Cheng C, Hartwig JF. Science. 2014;343:853. doi: 10.1126/science.1248042. [DOI] [PubMed] [Google Scholar]; e) Saito Y, Segawa Y, Itami K. J Am Chem Soc. 2015;137:5193. doi: 10.1021/jacs.5b02052. [DOI] [PubMed] [Google Scholar]
- 3.a) Zhang YH, Shi BF, Yu JQ. J Am Chem Soc. 2009;131:5072. doi: 10.1021/ja900327e. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wang YN, Guo XQ, Zhu XH, Zhong R, Cai LH, Hou XF. Chem Commun. 2012;48:10437. doi: 10.1039/c2cc34949c. [DOI] [PubMed] [Google Scholar]
- 4.For select examples of template directed meta-C–H functionalization, see:; a) Leow D, Li G, Mei TS, Yu JQ. Nature. 2012;486:518. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wan L, Dastbaravardeh N, Li G, Yu JQ. J Am Chem Soc. 2013;135:18056. doi: 10.1021/ja410760f. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tang R, Li G, Yu JQ. Nature. 2014;507:215. doi: 10.1038/nature12963. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Yang G, Lindovska P, Zhu D, Kim J, Wang P, Tang RY, Movassaghi M, Yu JQ. J Am Chem Soc. 2014;136:10807. doi: 10.1021/ja505737x. [DOI] [PubMed] [Google Scholar]; e) Chu L, Shang M, Tanaka K, Chen Q, Pissarnitski N, Streckfuss E, Yu JQ. ACS Cent Sci. 2015;1:394. doi: 10.1021/acscentsci.5b00312. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Kuninobu Y, Ida H, Nishi M, Kanai M. Nat Chem. 2015;7:712. doi: 10.1038/nchem.2322. [DOI] [PubMed] [Google Scholar]; g) Li S, Cai L, Ji H, Yang L, Li G. Nat Commun. 2016;7:10443. doi: 10.1038/ncomms10443. [DOI] [PMC free article] [PubMed] [Google Scholar]; For examples of template directed para-C–H functionalization, see:; h) Bag S, Patra T, Modak A, Deb A, Maity S, Dutta U, Dey A, Kancherla R, Maji A, Hazra A, Bera M, Maiti D. J Am Chem Soc. 2015;137:11888. doi: 10.1021/jacs.5b06793. [DOI] [PubMed] [Google Scholar]; i) Patra T, Bag S, Kancherla R, Mondal A, Dey A, Pimparkar S, Agasti S, Modak A, Maiti D. Angew Chem Int Ed. 2016;55:7751. doi: 10.1002/anie.201601999. [DOI] [PubMed] [Google Scholar]
- 5.For examples of Ru(II) catalyzed meta-C–H functionalization via ortho-cyclometallation, see:; a) Saidi O, Marafie J, Ledger AEW, Liu PM, Mahon MF, Kociok-Köhn G, Whittlesey MK, Frost CG. J Am Chem Soc. 2011;133:19298. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]; b) Hofmann N, Ackermann L. J Am Chem Soc. 2013;135:5877. doi: 10.1021/ja401466y. [DOI] [PubMed] [Google Scholar]; c) Li J, Warratz S, Zell D, De Sarkar S, Ishikawa EE, Ackermann L. J Am Chem Soc. 2015;137:13894. doi: 10.1021/jacs.5b08435. [DOI] [PubMed] [Google Scholar]; d) Teskey CJ, Lui AYW, Greaney MF. Angew Chem Int Ed. 2015;54:11677. doi: 10.1002/anie.201504390. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Paterson AJ, StJohn-Campbell S, Mahon MF, Press NJ, Frost CG. Chem Comm. 2015;51:12807. doi: 10.1039/c5cc03951g. [DOI] [PubMed] [Google Scholar]; f) Fan Z, Ni J, Zhang A. J Am Chem Soc. 2016;138:8470. doi: 10.1021/jacs.6b03402. [DOI] [PubMed] [Google Scholar]; For example of Ru(II) catalyzed para-C–H functionalization, see:; g) Liu W, Ackermann L. Org Lett. 2013;15:3484. doi: 10.1021/ol401535k. [DOI] [PubMed] [Google Scholar]
- 6.For selected examples using copper and aryl iodoniums to achieve meta-C–H arylation, see:; a) Phipps RJ, Gaunt MJ. Science. 2009;323:1593. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]; b) Duong HA, Gilligan RE, Cooke ML, Phipps RJ, Gaunt MJ. Angew Chem Int Ed. 2010;50:463. doi: 10.1002/anie.201004704. [DOI] [PubMed] [Google Scholar]; c) Yang Y, Li R, Zhao Y, Zhao D, Shi Z. J Am Chem Soc. 2016;138:8734. doi: 10.1021/jacs.6b05777. [DOI] [PubMed] [Google Scholar]; For an example of using CO2 as a traceless directing group, see:; d) Luo J, Preciado S, Larrosa I. J Am Chem Soc. 2013;136:4109. doi: 10.1021/ja500457s. [DOI] [PubMed] [Google Scholar]; For an example using deprotonation, see:; e) Martinez-Martinez AJ, Kennedy AR, Mulvey RE, O’Hara CT. Science. 2014;346:834. doi: 10.1126/science.1259662. [DOI] [PubMed] [Google Scholar]
- 7.a) Wang XC, Gong W, Fang LZ, Zhu RY, Li S, Engle KM, Yu JQ. Nature. 2015;519:334. doi: 10.1038/nature14214. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dong Z, Wang J, Dong G. J Am Chem Soc. 2015;137:5887. doi: 10.1021/jacs.5b02809. [DOI] [PubMed] [Google Scholar]; c) Shen PX, Wang XC, Wang P, Zhu RY, Yu JQ. J Am Chem Soc. 2015;137:11574. doi: 10.1021/jacs.5b08914. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Han J, Zhang L, Zhu Y, Zheng Y, Chen X, Huang ZB, Shi DQ, Zhao Y. Chem Comm. 2016;52:6903. doi: 10.1039/c6cc02384c. [DOI] [PubMed] [Google Scholar]; e) Wang P, Farmer ME, Huo X, Jain P, Shen PX, Ishoey M, Bradner JE, Wisniewski SR, Eastgate ME, Yu JQ. J Am Chem Soc. 2016;138:9269. doi: 10.1021/jacs.6b04966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.For reviews on norbornene mediated ortho-C–H functionalizations, see:; a) Catellani M. Top Organomet Chem. 2005;14:21. [Google Scholar]; b) Martins A, Mariampillai B, Lautens M. Top Curr Chem. 2010;292:1. doi: 10.1007/128_2009_13. [DOI] [PubMed] [Google Scholar]; c) Ye J, Lautens M. Nat Chem. 2015;7:863. doi: 10.1038/nchem.2372. [DOI] [PubMed] [Google Scholar]; d) Della Ca’ N, Fontana M, Motti E, Catellani M. Acc Chem Res. 2016;49:1389. doi: 10.1021/acs.accounts.6b00165. [DOI] [PubMed] [Google Scholar]; For selected examples:; e) Catellani M, Frignani F, Rangoni A. Angew Chem Int Ed. 1997;36:119. [Google Scholar]; f) Lautens M, Piguel S. Angew Chem Int Ed. 2000;39:1045. doi: 10.1002/(sici)1521-3773(20000317)39:6<1045::aid-anie1045>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; g) Faccini F, Motti E, Catellani M. J Am Chem Soc. 2004;126:78. doi: 10.1021/ja039043g. [DOI] [PubMed] [Google Scholar]; h) Bressy C, Alberico D, Lautens M. J Am Chem Soc. 2005;127:13148. doi: 10.1021/ja054472v. [DOI] [PubMed] [Google Scholar]; i) Mariampillai B, Alliot J, Li M, Lautens M. J Am Chem Soc. 2007;129:15372. doi: 10.1021/ja075599i. [DOI] [PubMed] [Google Scholar]; j) Rudolph A, Rackelmann N, Lautens M. Angew Chem Int Ed. 2007;46:1485. doi: 10.1002/anie.200603888. [DOI] [PubMed] [Google Scholar]; k) Dong Z, Dong G. J Am Chem Soc. 2013;135:18350. doi: 10.1021/ja410823e. [DOI] [PubMed] [Google Scholar]; l) Dong Z, Wang J, Ren Z, Dong G. Angew Chem, Int Ed. 2015;54:12664. doi: 10.1002/anie.201506397. [DOI] [PubMed] [Google Scholar]; m) Huang Y, Zhu R, Zhao K, Gu Z. Angew Chem, Int Ed. 2015;54:12669. doi: 10.1002/anie.201506446. [DOI] [PubMed] [Google Scholar]; n) Shi H, Babinski DJ, Ritter T. J Am Chem Soc. 2015;137:3775. doi: 10.1021/jacs.5b01082. [DOI] [PubMed] [Google Scholar]; o) Sun F, Li M, He C, Wang B, Li B, Sui X, Gu Z. J Am Chem Soc. 2016;138:7456. doi: 10.1021/jacs.6b02495. [DOI] [PubMed] [Google Scholar]; For other system using norbornene as a transient mediator:; p) Jiao L, Bach T. J Am Chem Soc. 2011;133:12990. doi: 10.1021/ja2055066. [DOI] [PubMed] [Google Scholar]; q) Jiao L, Herdtweck E, Bach T. J Am Chem Soc. 2012;134:14563. doi: 10.1021/ja3058138. [DOI] [PubMed] [Google Scholar]
- 9.a) Hili R, Yudin AK. Nat Chem Biol. 2006;2:284. doi: 10.1038/nchembio0606-284. [DOI] [PubMed] [Google Scholar]; b) Lawrence SA. Amines: Synthesis Properties and Applications. Cambridge University Press; Cambridge: 2004. pp. 265–305. [Google Scholar]; c) Ricci A, editor. Amino Group Chemistry. From Synthesis to the Life Sciences. Wiley-VCH; Weinheim: 2007. [Google Scholar]
- 10.a) Davies HML, Manning JR. Nature. 2008;451:417. doi: 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zalatan DN, Du Bois J. Top Curr Chem. 2010;292:347. doi: 10.1007/128_2009_19. [DOI] [PubMed] [Google Scholar]; c) Cho SH, Kim JY, Kwak J, Chang S. Chem Soc Rev. 2011;40:5068. doi: 10.1039/c1cs15082k. [DOI] [PubMed] [Google Scholar]; d) Jeffrey JL, Sarpong R. Chem Sci. 2013;4:4092. [Google Scholar]; e) Louillat ML, Patureau FW. Chem Soc Rev. 2014;43:901. doi: 10.1039/c3cs60318k. [DOI] [PubMed] [Google Scholar]; f) Zatolochnaya OV, Gevorgyan V. Nat Chem. 2014;6:661. doi: 10.1038/nchem.2018. [DOI] [PubMed] [Google Scholar]; g) Thirunavukkarasu VS, Kozhushkov SI, Ackermann L. Chem Commun. 2014;50:29. doi: 10.1039/c3cc47028h. [DOI] [PubMed] [Google Scholar]; h) Jiao J, Murakami K, Itami K. ACS Catal. 2016;6:610. [Google Scholar]; i) Kim H, Chang S. ACS Catal. 2016;6:234. [Google Scholar]
- 11.a) Barlaam B, Ducray R, Lambert-van der Brempt C, Ple P, Bardelle C, Brooks N, Coleman T, Cross D, Kettle JG, Read J. Bioorg Med Chem Lett. 2011;21:2207. doi: 10.1016/j.bmcl.2011.03.009. [DOI] [PubMed] [Google Scholar]; b) Turski L, Huth A, Sheardown M, McDonald F, Neuhaus R, Schneider HH, Dirnagl U, Wiegand F, Jacobsen P, Ottow E. Proc Natl Acad Sci USA. 1998;95:10960. doi: 10.1073/pnas.95.18.10960. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Witherington J, Abberley L, Briggs MA, Collis K, Dean DK, Gaiba A, King NP, Kraus H, Shuker N, Steadman JGA, Takle AK, Sanger G, Wadsworth G, Butler S, McKay F, Muir A, Winborn K, Heightman TD. Bioorg Med Chem Lett. 2008;18:2203. doi: 10.1016/j.bmcl.2007.12.021. [DOI] [PubMed] [Google Scholar]; d) López-Rodríguez ML, Morcillo MJ, Fernández E, Rosado ML, Orensanz L, Beneytez ME, Manzanares J, Fuentes JA, Schaper KJ. Bioorg Med Chem Lett. 1999;9:1679. doi: 10.1016/s0960-894x(99)00254-1. [DOI] [PubMed] [Google Scholar]; e) Dugan BJ, Gingrich DE, Mesaros EF, Milkiewicz KL, Curry MA, Zulli AL, Dobrzanski P, Serdikoff C, Jan M, Angeles TS, Albom MS, Mason JL, Aimone LD, Meyer SL, Huang Z, Wells-Knecht KJ, Ator MA, Ruggeri BA, Dorsey BD. J Med Chem. 2012;55:5243. doi: 10.1021/jm300248q. [DOI] [PubMed] [Google Scholar]; f) Bromidge SM, Brown AM, Clarke SE, Dodgson K, Gager T, Grassam HL, Jeffrey PM, Joiner GF, King FD, Middlemiss DN, Moss SF, Newman H, Riley G, Routledge C, Wyman P. J Med Chem. 1999;42:202. doi: 10.1021/jm980532e. [DOI] [PubMed] [Google Scholar]
- 12.a) Yoo EJ, Ma S, Mei TS, Chan KSL, Yu JQ. J Am Chem Soc. 2011;133:7652. doi: 10.1021/ja202563w. [DOI] [PubMed] [Google Scholar]; b) Zhu D, Yang G, He J, Chu L, Chen G, Gong W, Chen K, Eastgate MD, Yu JQ. Angew Chem Int Ed. 2015;54:2497. doi: 10.1002/anie.201408651. [DOI] [PubMed] [Google Scholar]; c) He J, Shigenari T, Yu JQ. Angew Chem Int Ed. 2015;54:6545. doi: 10.1002/anie.201502075. [DOI] [PubMed] [Google Scholar]
- 13.Niculescu-Duvaz D, Gaulon C, Dijkstra HP, NiculescuDuvaz I, Zambon A, Menard D, Suijkerbuijk BM, Nourry A, Davies L, Manne H, Friedlos F, Ogilvie L, Hedley D, Whittaker S, Kirk R, Gill A, Taylor RD, Raynaud FI, Moreno-Farre J, Marais R, Springer CJ. J Med Chem. 2009;52:2255. doi: 10.1021/jm801509w. [DOI] [PubMed] [Google Scholar]
- 14.Diederich F, Stang PJ, Tykwinski RR, editors. Acetylene Chemistry: Chemistry, Biology and Material Science. Wiley-VCH; Weinheim, Germany: 2005. [Google Scholar]
- 15.a) Fürstner A, Davies PW. Chem Commun. 2005:2307. doi: 10.1039/b419143a. [DOI] [PubMed] [Google Scholar]; b) Kolb HC, Finn MG, Sharpless KB. Angew Chem, Int Ed. 2001;40:2004. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]; c) Finn MG, Fokin VV. Chem Soc Rev. 2010;39:1231. doi: 10.1039/c003740k. [DOI] [PubMed] [Google Scholar]; d) Brand JP, Waser J. Chem Soc Rev. 2012;41:4165. doi: 10.1039/c2cs35034c. [DOI] [PubMed] [Google Scholar]; e) Chinchilla R, Najera C. Chem Rev. 2014;114:1783. doi: 10.1021/cr400133p. [DOI] [PubMed] [Google Scholar]
- 16.a) Sonogashira K. J Organomet Chem. 2002;653:46. [Google Scholar]; b) Negishi E, Anastasia L. Chem Rev. 2003;103:1979. doi: 10.1021/cr020377i. [DOI] [PubMed] [Google Scholar]; c) King AO, Yasuda N. Top Organomet Chem. 2004;6:205. [Google Scholar]; d) Doucet H, Hierso JC. Angew Chem, Int Ed. 2007;46:834. doi: 10.1002/anie.200602761. [DOI] [PubMed] [Google Scholar]; e) Plenio H. Angew Chem, Int Ed. 2008;47:6954. doi: 10.1002/anie.200802270. [DOI] [PubMed] [Google Scholar]; f) Chinchilla R, Najera C. Chem Soc Rev. 2011;40:5084. doi: 10.1039/c1cs15071e. [DOI] [PubMed] [Google Scholar]
- 17.For C–H alkynylation with terminal alkynes, see:; a) Wei Y, Zhao H, Kan J, Su W, Hong M. J Am Chem Soc. 2010;132:2522. doi: 10.1021/ja910461e. [DOI] [PubMed] [Google Scholar]; b) Matsuyama N, Kitahara M, Hirano K, Satoh T, Miura M. Org Lett. 2010;12:2358. doi: 10.1021/ol100699g. [DOI] [PubMed] [Google Scholar]; c) de Haro T, Nevado C. J Am Chem Soc. 2010;132:1512. doi: 10.1021/ja909726h. [DOI] [PubMed] [Google Scholar]; d) Yang L, Zhao L, Li CJ. Chem Commun. 2010;46:4184. doi: 10.1039/c0cc00014k. [DOI] [PubMed] [Google Scholar]; e) Kim SH, Yoon J, Chang S. Org Lett. 2011;13:1474. doi: 10.1021/ol200154s. [DOI] [PubMed] [Google Scholar]; f) Jie X, Shang Y, Hu P, Su W. Angew Chem, Int Ed. 2013;52:3630. doi: 10.1002/anie.201210013. [DOI] [PubMed] [Google Scholar]; g) Shang M, Wang HL, Sun SZ, Dai HX, Yu JQ. J Am Chem Soc. 2014;136:11590. doi: 10.1021/ja507704b. [DOI] [PubMed] [Google Scholar]; h) Zhou J, Shi J, Qi Z, Li X, Xu HE, Yi W. ACS Catal. 2015;5:6999. [Google Scholar]
- 18.For C–H alkynylation with alkynyl halides as alkynylated reagents, see:; a) Kobayashi K, Arisawa M, Yamaguchi M. J Am Chem Soc. 2002;124:8528. doi: 10.1021/ja026108r. [DOI] [PubMed] [Google Scholar]; b) Seregin IV, Ryabova V, Gevorgyan V. J Am Chem Soc. 2007;129:7742. doi: 10.1021/ja072718l. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Tobisu M, Ano Y, Chatani N. Org Lett. 2009;11:3250. doi: 10.1021/ol901049r. [DOI] [PubMed] [Google Scholar]; d) Matsuyama N, Hirano K, Satoh T, Miura M. Org Lett. 2009;11:4156. doi: 10.1021/ol901684h. [DOI] [PubMed] [Google Scholar]; e) Dudnik AS, Gevorgyan V. Angew Chem, Int Ed. 2010;49:2096. doi: 10.1002/anie.200906755. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Ano Y, Tobisu M, Chatani N. J Am Chem Soc. 2011;133:12984. doi: 10.1021/ja206002m. [DOI] [PubMed] [Google Scholar]; g) Ano Y, Tobisu M, Chatani N. Org Lett. 2012;14:354. doi: 10.1021/ol203100u. [DOI] [PubMed] [Google Scholar]; h) Ano Y, Tobisu M, Chatani N. Synlett. 2012;23:2763. [Google Scholar]; i) He J, Wasa M, Chan KSL, Yu JQ. J Am Chem Soc. 2013;135:3387. doi: 10.1021/ja400648w. [DOI] [PubMed] [Google Scholar]
- 19.For C–H alkynylation with hypervalent iodine reagents as alkynylated reagents, see:; a) Brand JP, Charpentier J, Waser J. Angew Chem, Int Ed. 2009;48:9346. doi: 10.1002/anie.200905419. [DOI] [PubMed] [Google Scholar]; b) Brand JP, Waser J. Angew Chem, Int Ed. 2010;49:7304. doi: 10.1002/anie.201003179. [DOI] [PubMed] [Google Scholar]; c) Feng C, Loh TP. Angew Chem, Int Ed. 2014;53:2722. doi: 10.1002/anie.201309198. [DOI] [PubMed] [Google Scholar]; d) Xie F, Qi Z, Yu S, Li X. J Am Chem Soc. 2014;136:4780. doi: 10.1021/ja501910e. [DOI] [PubMed] [Google Scholar]; e) Wang H, Xie F, Qi Z, Li X. Org Lett. 2015;17:920. doi: 10.1021/acs.orglett.5b00027. [DOI] [PubMed] [Google Scholar]; f) Waser J. Top Curr Chem. 2015;373:187. doi: 10.1007/128_2015_660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


