Abstract
Background
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a key regulator of low-density lipoprotein (LDL) cholesterol and cardiovascular disease (CVD) risk, and is an emerging therapeutic target.
Objective
We compared serum PCSK9 levels in young adults, with and without type 2 diabetes.
Subjects and Methods
Cross-sectional analysis was conducted in a cohort, aged 15 to 26 years, in Cincinnati, OH, from 2005 to 2010. Serum PCSK9 levels were measured in 94 youth with type 2 diabetes, 93 obese control subjects, and 99 lean control subjects. Correlative analyses were conducted to determine significant covariates of PCSK9 by group and sex, and multivariate linear regression models were used to study the independent determinants of PCSK9.
Results
In females, PCSK9 levels were significantly increased in the obese and type 2 diabetes subjects relative to the lean controls (P < 0.01). Moreover, PCSK9 was positively correlated with multiple metabolic parameters in females: body mass index (BMI), systolic blood pressure (SBP), fasting glucose, fasting insulin, and C-reactive protein (CRP) levels (P≤0.02). In males, PCSK9 levels were decreased overall compared to females (P=0.03), and did not differ between the lean, obese, or type 2 diabetes groups.
Conclusions
Obesity and type 2 diabetes were associated with significantly higher levels of PCSK9 in young women, but not in young men. These data suggest that sex could modify the effects of obesity and diabetes on PCSK9 in young adults.
Keywords: PCSK9, LDL-Cholesterol, Young Adult, Type 2 Diabetes, Cardiovascular Disease
Introduction
The prevalence of type 2 diabetes has risen significantly in our society, even in children and adolescents (1). Type 2 diabetes in youth leads to increased risk for cardiovascular disease (CVD) in adulthood, which is the leading cause of mortality in individuals with type 2 diabetes (2). An important contributor to elevated CVD risk in type 2 diabetes is dyslipidemia involving abnormalities in all lipoproteins (3,4). Typically, patients with type 2 diabetes show increased levels of small dense low-density lipoprotein cholesterol (5), decreased levels of high-density lipoprotein (HDL) cholesterol, and increased levels of triglycerides (TG) (6). Numerous large-scale trials have shown that intensive low density lipoprotein (LDL)-lowering therapy in patients with type 2 diabetes results in a significant reduction in CVD risk and improved outcomes (7).
One of the key regulators of circulating LDL cholesterol is proprotein convertase subtilisin/kexin type 9 (PCSK9). PCSK9 promotes the degradation of the LDL receptor (LDLR), resulting in reduced LDL clearance (8–10). Thus, gain-of-function PCSK9 genetic variants lead to higher levels of LDL cholesterol and increased risk of CVD, whereas loss-of function PCSK9 mutations are associated with reductions in both LDL cholesterol and risk of CVD (11,12). As a result, PCSK9 has become a novel target for lipid lowering therapy, and medications that antagonize PCSK9 are now available for clinical use (13).
The effect of diabetes on PCSK9 is unclear. Circulating PCSK9 was found to be higher in individuals with diabetes compared to those without diabetes in some adult studies (14,15), but not in others (16–18). Moreover, the effects of diabetes on PCSK9 levels in young adults has thus far not been studied, despite the fact that adolescents and young adults with obesity and type 2 diabetes are also at increased risk for dyslipidemia (19). Indeed, the earlier onset of type 2 diabetes in this population implies a longer lifetime burden of disease and potential for earlier development of complications (20,21). The purpose of this study was to compare PCSK9 in youth with type 2 diabetes to their lean and obese controls.
Methods
Participants
Extensive details of this cohort have previously been published (22,23). Briefly, youth ages 11 to 26 years with type 2 diabetes, and obese and lean controls without diabetes, were recruited as part of the Type 2 Cardiovascular Disease Study conducted at Cincinnati Children's Hospital Medical Center between 2005 and 2010. Type 2 diabetes was defined by the American Diabetes Association Criteria (24) with negative islet cell antibody titers (Barbara Davis Center, Denver CO). Obese controls had a BMI ≥ 95th percentile and lean controls had a BMI <85th percentile, as defined by age and sex specific percentiles for BMI. For participants over the age of 20 years, age was set to 19.99 years and BMI percentile was calculated. All obese participants underwent a 2 hour oral glucose tolerance test to exclude the diagnosis of diabetes. Pregnant females were excluded from the study. Prior to enrollment, written informed consent was obtained from subjects ≥18 years old, or the parent or guardian for subjects <18 years old. Written assent was also obtained for subjects <18 years old, according to the guidelines established by the institutional review board at Cincinnati Children's Hospital Medical Center. The study was reviewed and approved by the local institutional review boards at Cincinnati Children's Hospital Medical Center and Boston Children's Hospital.
Data Collection
All participants completed an in person study visit, during which demographics, medication history, anthropometrics, and a fasting (>10 hours) blood sample were obtained. Height and weight were obtained twice and averaged as previously described (22,23). BMI was calculated as weight in kilograms divided by the square of height in meters. Blood pressure was measured manually with a mercury sphygmomanometer (Baum Desktop model with V-Lok cuffs, Copiague, NY) three times and averaged according to the Fourth Report (25).
Laboratory Data Collection
At the time of the study, plasma glucose was measured using a Hitachi model 704 glucose analyzer (Roche Hitachi, Indianapolis, IN, intra-assay and interassay coefficients of variation of 1.2 and 1.6%, respectively). Plasma insulin was measured by radioimmunoassay using an anti-insulin serum raised in guinea pigs, 125I-labeled insulin (Linco, St. Louis, MO), and a double antibody method to separate bound from free tracer. Fasting lipids were measured in a laboratory that is NHLBI/Centers for Disease Control and Prevention standardized, with the LDL cholesterol concentration calculated using the Friedewald equation. C-reactive protein (CRP) was measured using a high sensitivity ELISA. HbA1c was measured in red blood cells using high-performance liquid chromatography methods.
For the current study, 286 participants were randomly selected from the lean, obese and type 2 diabetes groups, and were matched for age and sex between each group using the frequency matching method. Selected study subjects ranged in age from 15 to 26 years. Subjects who reported taking lipid-lowering medications, such as statins, at the time of the study were excluded from the analyses since these medications have been shown to increase PCSK9 levels (26–28). Serum PCSK9 levels were determined in duplicate using a commercially available quantitative sandwich enzyme immunoassay ELISA (Cat. No. Circulex CY-8079; CycLex Co., Ltd., Japan), according to manufacturer's instructions. The intraassay coefficient of variability (CV) was 8.2%. The interassay coefficient of variability was 9.7%.
Statistical Analysis
All analyses were performed with Statistical Analyses Software (SAS, version 9.3). Data are presented as mean and standard deviation, frequency and percent, or median and interquartile range. Variance-stabilizing measures to transform non-normal values were performed as appropriate for parametric analyses. χ2 analyses were performed to determine group differences for categorical variables. Independent t-tests, one-way ANOVA, and ANCOVA were performed to test for mean differences between the groups. Bivariate correlations were calculated between PCSK9 levels and potential covariates. Multivariate linear regression models were constructed using race, sex, and group to elucidate the independent determinants of PCSK9. Group was chosen over BMI because it was a key factor in the study design, and the independent effects of BMI and group on PCSK9 were difficult to discern. Interactions between group and the other potential independent determinants of PCSK9 were also evaluated. P-value <0.05 was considered significant for all analyses.
Results
Table 1 lists the demographic, anthropometric, and laboratory data for all participants, stratified by study group. By design, there were no significant age or sex differences between the three groups. As expected, fasting plasma glucose and insulin levels differed significantly between all three groups (P<0.01). Plasma lipids varied among the groups, with total cholesterol, LDL, and non-HDL cholesterol increasing from lean to obese to type 2 diabetes (P<0.01). In addition, plasma CRP levels were higher in the subjects with obesity and diabetes, compared to lean controls (P<0.01).
Table 1. Clinical characteristics of study participants according to group.
| Patient Characteristic | Lean (n = 99) | Obese (n = 93) | Type 2 diabetes (n = 94) | P-Values |
|---|---|---|---|---|
| Age (years) | 20.6 (2.1) | 21.1 (2.4) | 20.4 (2.2) | 0.10 |
| Sex (n, % female) | 58 (59) | 53 (57) | 56 (60) | 0.94 |
| Race¥ | 0.02 | |||
| Caucasian(%) | 56 | 47 | 37 | |
| African-American (%) | 44 | 53 | 62 | |
| Other (%) | 0 | 0 | 2 | |
| Medications | ||||
| Insulin (%) | 0 | 0 | 40 | |
| Oral hypoglycemic (%) | 0 | 0 | 46 | |
| Estrogen containing (%) | 4 | 2 | 2 | 0.19 |
| Height (cm) | 170.3 ± 7.9 | 170.7 ± 9.3 | 169.8 ± 9.7 | 0.75 |
| Weight (Kg)* | 65 (58, 76) | 87.5 (75, 100) | 107 (83, 126) | <0.0001 |
| BMI (kg/m2)# | 22 (20.7, 24.6) | 30 (27.4, 34.1) | 35.4 (30.9, 42.9) | <0.0001 |
| Systolic BP (mmHg)# | 114 (108, 121) | 118 (111, 124) | 121 (114, 130) | 0.0003 |
| Diastolic BP (mmHg) | 69 ± 7 | 71 ± 6 | 69 ± 13 | 0.07 |
| HbA1c (%) | 8.7 ± 3.1 | |||
| HbA1c (mmol/mol) | 72 ± 10 | |||
| Fasting Glucose (mg/dL)* | 87 (82, 91) | 90 (85, 93) | 134 (98, 246) | <0.0001 |
| Fasting Insulin (mU/mL)# | 11.9 (8.9, 16.0) | 16.6 (12.4, 21.2) | 17.1 (12.3, 25.9) | <0.0001 |
| Total cholesterol (mg/dL)* | 160 (141, 183) | 156 (143, 181) | 177 (157, 216) | <0.0001 |
| LDL cholesterol (mg/dL)# | 89.5 (75, 108) | 88 (77, 109) | 112 (90, 137) | <0.0001 |
| HDL cholesterol (mg/dL)# | 53 (44, 64) | 50 (45, 58) | 42 (37, 51) | <0.0001 |
| Non-HDL cholesterol (mg/dL)# | 108 (89, 129) | 104 (89, 129) | 136 (112, 170) | <0.0001 |
| Triglycerides (mg/dL)# | 80 (57, 105) | 72 (57, 99) | 99 (70, 165) | <0.0001 |
| CRP (mg/dL)# | 0.5 (0.2, 1.6) | 1.2 (0.8, 3.0) | 3.5 (1, 6.8) | <0.0001 |
| PCSK9 (ng/mL)# | 239 (210, 312) | 284 (229, 363) | 288 (226, 391) | 0.002 |
Values are mean ± SD or median (Interquartile Range).
Comparisons between groups using Fisher's Exact Test.
Indicates non-normally distributed data; Kruskal-Wallis Test was used for comparisons between groups.
Indicates data that was log transformed to achieve normality; one way ANOVA was used for comparisons between groups.
The PCSK9 measurements by group and by sex are presented in Figure 1. Overall, PCSK9 concentrations differed significantly between the lean and obese groups (P= 0.01), and between the lean and type 2 diabetes groups (P=0.003; Figure 1A). A similar relationship was present in females, with PCSK9 levels increasing from the lean to obese (P=0.004), and obese to type 2 diabetes groups (P=0.01; Figure 1B). In males, PCSK9 levels were lower than in females (P = 0.03; Figure 1C), and PCSK9 levels did not differ between lean, obese and type 2 males (Figure 1B). Though race differed significantly between the groups (Table 1), it did not have an independent effect on PCSK9 levels, and the relationships between PCSK9 and group, and PCSK9 and sex, remained significant after controlling for race (data not shown).
Figure 1.
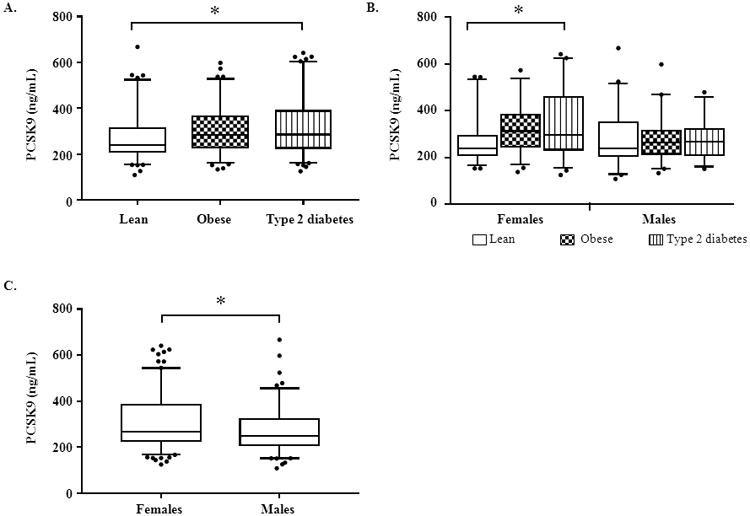
A: Comparison of PCSK9 levels by group. B: Comparison of PCSK9 levels by group within each sex. C: Comparison of PCSK9 levels by sex. The boxes denote the distribution between the 75th and 25th percentiles; the horizontal lines within the boxes denote the median values; the whiskers represent the 5th and 95th percentiles. Closed circles represent individuals below the 5th or above the 95th percentiles. The asterisk indicates a P-value of <0.01 between the groups by ANOVA (A, B) or independent t-test (C).
Table 2 shows the bivariate correlations between PCSK9 levels and other clinical variables by sex. Overall, PCSK9 levels significantly correlated with BMI (P<0.001), systolic blood pressure (SBP) (P=0.002), fasting glucose (P=0.003) fasting insulin (P<0.001), and CRP (P=0.02) in females, but not in males. Correlations between PCSK9 and total cholesterol, LDL, and non-HDL were observed in both males and females (P ≤ 0.008).
Table 2.
Correlation of PCSK9 with clinical variables by sex.
| Females | Males | |||
|---|---|---|---|---|
|
| ||||
| Clinical Variable | Correlation Coefficient* | P-value | Correlation Coefficient* | P-value |
| BMI (kg/m2) | 0.341 | <0.0001 | 0.006 | 0.95 |
| Systolic BP (mmHg) | 0.247 | 0.002 | 0.114 | 0.23 |
| Diastolic BP (mmHg) | 0.052 | 0.53 | 0.158 | 0.10 |
| Fasting Glucose (mg/dL) | 0.235 | 0.003 | 0.129 | 0.17 |
| Fasting Insulin (mU/mL) | 0.300 | 0.0003 | -0.008 | 0.94 |
| Total cholesterol (mg/dL) | 0.293 | 0.0002 | 0.275 | 0.003 |
| LDL cholesterol (mg/dL) | 0.337 | <0.0001 | 0.292 | 0.002 |
| HDL cholesterol (mg/dL) | -0.154 | 0.05 | 0.131 | 0.16 |
| Non-HDL cholesterol (mg/dL) | 0.328 | <0.0001 | 0.246 | 0.008 |
| Triglycerides (mg/dL) | 0.154 | 0.05 | 0.094 | 0.32 |
| CRP (mg/dL) | 0.235 | 0.02 | 0.223 | 0.07 |
Correlations between clinical variables and PCSK9 within sex were determined using Spearman correlation. Significant values denoted in bold.
Discussion
We show in young adults with obesity/type 2 diabetes that serum PCSK9 levels are associated with metabolic dysfunction, but only in females. In young women, circulating PCSK9 is higher in obesity/type 2 diabetes. In contrast, PCSK9 levels in young men are lower than in young women, and are not correlated with metabolic dysfunction.
Several studies in adults have also shown type 2 diabetes to be associated with increased levels of PCSK9 (14,15,29). These studies, coupled with studies in cells and mice showing that insulin induces PCSK9 (30,31), suggest that hyperinsulinemia in obesity/type 2 diabetes may drive PCSK9 levels. Consistent with this, insulin levels have been shown to be positively associated with PCSK9 in a large pediatric population (32). On the other hand, some clinical studies have not found an association between diabetes and PCSK9 (18, 28). The reason for this discordance is not clear. However, we would note that our study, as well as other studies showing a positive association between PCSK9 and diabetes (14,15), included significant numbers of non-Caucasians. Studies that failed to find an association were performed in largely Caucasian (18) or Asian (33) populations. This raises the possibility that the effects of diabetes on PCSK9 may be influenced by the racial composition of the population being studied.
Prior studies have also shown PCSK9 levels to be elevated in females versus males, in both adults and in children (14,32,34,35). Furthermore, some of these studies found associations between PCSK9 and BMI that were either stronger or only present in females (14,34). Interestingly, mice also appear to manifest a sex-dependent association between PCSK9 and body weight, though in this case, the association may be more prominent in males: leptin treatment of obese mice produced similar weight loss in males and females, which was associated with a 90% decrease in plasma PCSK9 in males, but no change in plasma PCSK9 in females (36). Taken together, these data suggest that the effects of obesity on PCSK9 could be modified by sex.
The mechanisms underlying the sex differences observed in PCSK9 levels remain unclear; however, most of the data to date suggest that estrogen suppresses PCSK9. Although estrogen replacement does not significantly alter plasma PCSK9 in post-menopausal females (37,38), high dose estradiol treatment suppresses hepatic PCSK9 expression in rats (39). Moreover, plasma PCSK9 is higher in post-menopausal compared to pre-menopausal women (35), and is inversely correlated with endogenous estrogen levels in healthy women (40). Additionally, studies in cells and mice have raised the possibility that estrogen may alter PCSK9 activity. First, estrogen treatment of cultured human hepatocarcinoma cells increases PCSK9 phosphorylation and impair the ability of PCSK9 to promote LDLR degradation (41). Second, PCSK9 may interfere with the accumulation of the LDL receptor at the plasma membrane only in the absence of estrogen (42). Interestingly, we found that females with obesity and type 2 diabetes had the highest levels of PCSK9, suggesting that these metabolic states could potentially impact the relationship between estrogen and PCSK9.
One limitation of our study is that we did not assess pubertal status in all of the subjects. This is potentially important since sex-dependent changes in PCSK9 have been observed during the peri-pubertal years; in boys, PCSK9 decreased from ages 9 to 16 years, whereas in girls, PCSK9 levels trended upwards (32). However, most of the males in our study were 15 years of age or older, and almost all of the females were postmenarcheal by report, suggesting that the majority of our patients were in the later stages of puberty or were post-pubertal. Thus, it is unlikely that differences in pubertal status confounded our results.
In summary, in a young adult population, we show that PCSK9 is increased in subjects with obesity and type 2 diabetes, but that this increase is limited to females. Women without diabetes have previously been shown to be relatively protected against CVD when compared to men (43,44). In contrast, females with type 2 diabetes have an equivalent rate of CVD as males without diabetes (45). Given recent findings that circulating PCSK9 may predict future CV events (46), it is possible that PCSK9 mediates the increased risk of CVD observed in females with diabetes. Thus, therapies targeting PCSK9 may eventually prove to be of particular benefit to young females with obesity and type 2 diabetes.
Supplementary Material
Acknowledgments
Funding. This study was supported by NIH (NHLBI) R01 HL105591 and, in part, by US Public Health Service Grant #UL1 RR026314 from the National Center for Research Resources, NIH (CTSA grant). Additional funding was provided by NIH (NIDDK) 5K12DK094721-04 (A.E.L.), the Boston Children's Heart Foundation (S.D.dF.), and NIH (NHLBI) R01HL109650 (S.B.B.).
References
- 1.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005 May;146(5):693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Maahs DM, Daniels SR, de Ferranti SD, Dichek HL, Flynn J, Goldstein BI, et al. Cardiovascular disease risk factors in youth with diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2014 Oct 21;130(17):1532–58. doi: 10.1161/CIR.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 3.Eliasson B, Cederholm J, Eeg-Olofsson K, Svensson AM, Zethelius B, Gudbjörnsdottir S. Clinical usefulness of different lipid measures for prediction of coronary heart disease in type 2 diabetes: a report from the Swedish National Diabetes Register. Diabetes Care. 2011 Sep;34(9):2095–100. doi: 10.2337/dc11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen LN, Karter AJ, Waitzfelder BE, Crosson JC, Marrero DG, Mangione CM, et al. Predictors of mortality over 8 years in type 2 diabetic patients: Translating Research Into Action for Diabetes (TRIAD) Diabetes Care. 2012 Jun;35(6):1301–9. doi: 10.2337/dc11-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006 Mar 21;47(6):1093–100. doi: 10.1016/j.jacc.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421. [PubMed] [Google Scholar]
- 7.American Diabetes Association. 8. Cardiovascular Disease and Risk Management. Diabetes Care. 2014 Dec 23;38(Supplement_1):S49–57. doi: 10.2337/dc15-S011. [DOI] [PubMed] [Google Scholar]
- 8.Abifadel M, Varret M, Rabès JP, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003 Jun;34(2):154–6. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 9.Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004 Nov 19;279(47):48865–75. doi: 10.1074/jbc.M409699200. [DOI] [PubMed] [Google Scholar]
- 10.Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen JC, et al. A spectrum of PCSK9 alleles contributes to plasma levels of low-density lipoprotein cholesterol. Am J Hum Genet. 2006 Mar;78(3):410–22. doi: 10.1086/500615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berge KE, Ose L, Leren TP. Missense mutations in the PCSK9 gene are associated with hypocholesterolemia and possibly increased response to statin therapy. Arterioscler Thromb Vasc Biol. 2006 May;26(5):1094–100. doi: 10.1161/01.ATV.0000204337.81286.1c. [DOI] [PubMed] [Google Scholar]
- 12.Cohen JC, Boerwinkle E, Mosley TH, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006 Mar 23;354(12):1264–72. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 13.Shantha GPS, Robinson JG. Emerging innovative therapeutic approaches targeting PCSK9 to lower lipids. Clin Pharmacol Ther. 2015 Oct 22; doi: 10.1002/cpt.281. [DOI] [PubMed] [Google Scholar]
- 14.Lakoski SG, Lagace TA, Cohen JC, Horton JD, Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab Endocrine Society. 2009 Jul 2;94(7):2537–43. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nekaies Y, Baudin B, Kelbousi S, Sakly M, Attia N. Plasma proprotein convertase subtilisin/kexin type 9 is associated with Lp(a) in type 2 diabetic patients. J Diabetes Complications. 2015 Jan;29(8):1165–70. doi: 10.1016/j.jdiacomp.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Vergès B, Duvillard L, Brindisi MC, Gautier E, Krempf M, Costet P, et al. Lack of association between plasma PCSK9 and LDL-apoB100 catabolism in patients with uncontrolled type 2 diabetes. Atherosclerosis. 2011 Nov;219(1):342–8. doi: 10.1016/j.atherosclerosis.2011.07.098. [DOI] [PubMed] [Google Scholar]
- 17.Kappelle PJWH, Lambert G, Dullaart RPF. Plasma proprotein convertase subtilisin-kexin type 9 does not change during 24h insulin infusion in healthy subjects and type 2 diabetic patients. Atherosclerosis. 2011 Feb;214(2):432–5. doi: 10.1016/j.atherosclerosis.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 18.Brouwers MCGJ, Troutt JS, van Greevenbroek MMJ, Ferreira I, Feskens EJ, van der Kallen CJH, et al. Plasma proprotein convertase subtilisin kexin type 9 is not altered in subjects with impaired glucose metabolism and type 2 diabetes mellitus, but its relationship with non-HDL cholesterol and apolipoprotein B may be modified by type 2 diabetes mellitus. Atherosclerosis. 2011 Jul;217(1):263–7. doi: 10.1016/j.atherosclerosis.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Kershnar AK, Daniels SR, Imperatore G, Palla SL, Petitti DB, Pettitt DJ, et al. Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr. 2006 Sep;149(3):314–9. doi: 10.1016/j.jpeds.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 20.Halpern A, Mancini MC, Magalhães MEC, Fisberg M, Radominski R, Bertolami MC, et al. Metabolic syndrome, dyslipidemia, hypertension and type 2 diabetes in youth: from diagnosis to treatment. Diabetol Metab Syndr. 2010 Jan;2:55. doi: 10.1186/1758-5996-2-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005 May;28(5):1219–21. doi: 10.2337/diacare.28.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation. 2009 Jun 9;119(22):2913–9. doi: 10.1161/CIRCULATIONAHA.108.830380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah AS, Khoury PR, Dolan LM, Ippisch HM, Urbina EM, Daniels SR, et al. The effects of obesity and type 2 diabetes mellitus on cardiac structure and function in adolescents and young adults. Diabetologia. 2011 Apr;54(4):722–30. doi: 10.1007/s00125-010-1974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Diabetes Association. Standards of medical care in diabetes—2015. Diabetes Care. 2015 Dec 23;38(Supplement_1):S1–2. doi: 10.2337/dc14-2142. [DOI] [PubMed] [Google Scholar]
- 25.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004 Aug;114(2 Suppl 4th Report):555–76. [PubMed] [Google Scholar]
- 26.Davignon J, Dubuc G. Statins and ezetimibe modulate plasma proprotein convertase subtilisin kexin-9 (PCSK9) levels. Trans Am Clin Climatol Assoc. 2009 Jan;120:163–73. [PMC free article] [PubMed] [Google Scholar]
- 27.Mayne J, Dewpura T, Raymond A, Cousins M, Chaplin A, Lahey KA, et al. Plasma PCSK9 levels are significantly modified by statins and fibrates in humans. Lipids Health Dis. 2008 Jan;7:22. doi: 10.1186/1476-511X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, et al. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2004 Aug;24(8):1454–9. doi: 10.1161/01.ATV.0000134621.14315.43. [DOI] [PubMed] [Google Scholar]
- 29.Cariou B, Le Bras M, Langhi C, Le May C, Guyomarc'h-Delasalle B, Krempf M, et al. Association between plasma PCSK9 and gamma-glutamyl transferase levels in diabetic patients. Atherosclerosis. 2010 Aug;211(2):700–2. doi: 10.1016/j.atherosclerosis.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux AL, et al. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem. 2006 Mar 10;281(10):6211–8. doi: 10.1074/jbc.M508582200. [DOI] [PubMed] [Google Scholar]
- 31.Miao J, Manthena PV, Haas ME, Ling AV, Shin DJ, Graham MJ, et al. Role of Insulin in the Regulation of Proprotein Convertase Subtilisin/Kexin Type 9. Arterioscler Thromb Vasc Biol. 2015 Jul 1;35(7):1589–96. doi: 10.1161/ATVBAHA.115.305688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baass A, Dubuc G, Tremblay M, Delvin EE, O'Loughlin J, Levy E, et al. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin Chem. 2009 Sep;55(9):1637–45. doi: 10.1373/clinchem.2009.126987. [DOI] [PubMed] [Google Scholar]
- 33.Yang SH, Li S, Zhang Y, Xu RX, Guo YL, Zhu CG, et al. Positive correlation of plasma PCSK9 levels with HbA1c in patients with type 2 diabetes. Diabetes Metab Res Rev. 2015 Sep 17; doi: 10.1002/dmrr.2712. [DOI] [PubMed] [Google Scholar]
- 34.Cui Q, Ju X, Yang T, Zhang M, Tang W, Chen Q, et al. Serum PCSK9 is associated with multiple metabolic factors in a large Han Chinese population. Atherosclerosis. 2010 Dec;213(2):632–6. doi: 10.1016/j.atherosclerosis.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh M, Gälman C, Rudling M, Angelin B. Influence of physiological changes in endogenous estrogen on circulating PCSK9 and LDL cholesterol. J Lipid Res. 2015 Feb;56(2):463–9. doi: 10.1194/jlr.M055780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levenson AE, Haas ME, Miao J, Brown RJ, de Ferranti SD, Muniyappa R, et al. Effect of Leptin Replacement on PCSK9 in ob/ob Mice and Female Lipodystrophic Patients. Endocrinology. 2016 Jan 29;:en20151624. doi: 10.1210/en.2015-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo W, Fu J, Chen X, Gao B, Fu Z, Fan H, et al. The effects of estrogen on serum level and hepatocyte expression of PCSK9. Metabolism. 2015 Apr;64(4):554–60. doi: 10.1016/j.metabol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Ooi TC, Raymond A, Cousins M, Favreau C, Taljaard M, Gavin C, et al. Relationship between testosterone, estradiol and circulating PCSK9: Cross-sectional and interventional studies in humans. Clin Chim Acta. 2015 Apr 7;446:97–104. doi: 10.1016/j.cca.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 39.Persson L, Gälman C, Angelin B, Rudling M. Importance of proprotein convertase subtilisin/kexin type 9 in the hormonal and dietary regulation of rat liver low-density lipoprotein receptors. Endocrinology. 2009 Mar;150(3):1140–6. doi: 10.1210/en.2008-1281. [DOI] [PubMed] [Google Scholar]
- 40.Persson L, Henriksson P, Westerlund E, Hovatta O, Angelin B, Rudling M. Endogenous estrogens lower plasma PCSK9 and LDL cholesterol but not Lp(a) or bile acid synthesis in women. Arterioscler Thromb Vasc Biol. 2012 Mar;32(3):810–4. doi: 10.1161/ATVBAHA.111.242461. [DOI] [PubMed] [Google Scholar]
- 41.Starr AE, Lemieux V, Noad J, Moore JI, Dewpura T, Raymond A, et al. β-Estradiol results in a proprotein convertase subtilisin/kexin type 9-dependent increase in low-density lipoprotein receptor levels in human hepatic HuH7 cells. FEBS J. 2015 Jul;282(14):2682–96. doi: 10.1111/febs.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roubtsova A, Chamberland A, Marcinkiewicz J, Essalmani R, Fazel A, Bergeron JJ, et al. PCSK9 deficiency unmasks a sex- and tissue-specific subcellular distribution of the LDL and VLDL receptors in mice. J Lipid Res. 2015 Nov;56(11):2133–42. doi: 10.1194/jlr.M061952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986 Feb;111(2):383–90. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 44.Stampfer MJ, Colditz GA, Willett WC. Menopause and heart disease. A review. Ann N Y Acad Sci. 1990 Jan;592:193–203. doi: 10.1111/j.1749-6632.1990.tb30329.x. discussion 257–62. [DOI] [PubMed] [Google Scholar]
- 45.Kannel WB. Lipids, diabetes, and coronary heart disease: insights from the Framingham Study. Am Heart J. 1985 Nov;110(5):1100–7. doi: 10.1016/0002-8703(85)90224-8. [DOI] [PubMed] [Google Scholar]
- 46.Leander K, Mälarstig A, Van't Hooft FM, Hyde C, Hellénius ML, Troutt JS, et al. Circulating PCSK9 Predicts Future Risk of Cardiovascular Events Independently of Established Risk Factors. 2016 Mar 29;133(13):1230–9. doi: 10.1161/CIRCULATIONAHA.115.018531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


