Abstract
Women are at increased risk of developing post-traumatic stress disorder (PTSD) following a traumatic event. Recent studies suggest that this may be mediated, in part, by circulating estrogen levels. This study evaluated the hypothesis that individual variation in response to estrogen levels contributes to fear regulation and PTSD risk in women. We evaluated DNA methylation from blood of female participants in the Grady Trauma Project and found that serum estradiol levels associates with DNA methylation across the genome. For genes expressed in blood, we examined the association between each CpG site and PTSD diagnosis using linear models that adjusted for cell proportions and age. After multiple test correction, PTSD associated with methylation of CpG sites in the HDAC4 gene, which encodes histone deacetylase 4, and is involved in long-term memory formation and behavior. DNA methylation of HDAC4 CpG sites were tagged by a nearby single-nucleotide polymorphism (rs7570903), which also associated with HDAC4 expression, fear-potentiated startle and resting-state functional connectivity of the amygdala in traumatized humans. Using auditory Pavlovian fear conditioning in a rodent model, we examined the regulation of Hdac4 in the amygdala of ovariectomized (OVX) female mice. Hdac4 messenger RNA levels were higher in the amygdala 2 h after tone-shock presentations, compared with OVX-homecage control females. In naturally cycling females, tone-shock presentations increased Hdac4 expression relative to homecage controls for metestrous (low estrogen) but not the proestrous (high estrogen) group. Together, these results support an estrogenic influence of HDAC4 regulation and expression that may contribute to PTSD in women.
Introduction
Women are twice as likely to develop post-traumatic stress disorder (PTSD) following a traumatic experience when compared with men.1 Studies suggest that this increased risk among women is not due to higher rates of trauma or to differences in the types of trauma commonly experienced by women.2, 3, 4 Further, the higher rates of PTSD among women cannot be attributed to comorbid psychiatric disorders.3 However, women are more likely to report depression and anxiety symptoms at different phases of their menstrual cycle, particularly during periods characterized by low estrogen levels.5, 6, 7, 8, 9, 10
The physiological effects of estrogen are widespread. Variations in estrogen levels have been linked to differences in the hypothalamic-pituitary-adrenal axis response to stress and variation in multiple neurotransmitter systems.11, 12, 13 Women who had recently experienced trauma reported more trauma-related flashbacks if they were in the mid-luteal phase of their cycle at the time of either the trauma or the assessment.14 Further, women with PTSD report more severe psychological symptoms based on the phase of their menstrual cycle.15 Consistent with this observation, female rodents also show more fear and anxiety-related behaviors in phases of their estrous cycle characterized by low estrogen levels, specifically the metestrous or diestrous phases. 16, 17, 18, 19
Emerging evidence suggests that estrogen may have an impact on the degree to which memories, especially fear-related memories, are formed or retrieved. For example, neuroimaging studies show greater activation of neural networks involved in fear expression when women are assessed during low estrogen periods of their cycle (follicular phase) relative to high estrogen phases,20, 21 and this is supported by focused studies of fear learning. A study by Glover and colleagues demonstrated that, compared with men, women in the high estrogen phase of their cycles (luteal phase) had no difference in their ability to discriminate between danger or safety cues nor to inhibit fear in response to a safety cue. However, women in the low estrogen phase of their cycles (follicular phase) had significantly impaired fear inhibition.22 Studies in both humans and animals support this observation by demonstrating that low estrogen levels associate with deficits in fear extinction recall when compared with phases characterized by higher estrogen.23, 24, 25 Women with PTSD have particular deficits in fear extinction when estrogen levels are low, suggesting that low estrogen at the time of trauma may be a vulnerability factor for PTSD development.26, 27
Estrogen can also moderate risk alleles implicated in PTSD,28 suggesting that the effects of estrogen can vary between individuals. For example, we previously reported that a genetic variant in the PAC1 receptor associates with PTSD in a primarily African American cohort of women but not men.28 The risk allele appears to alter functioning of a putative estrogen response element, potentially through epigenetic mechanisms, including DNA methylation or chromatin modifications. DNA methylation, an epigenetic modification previously associated with PTSD diagnosis,29, 30, 31, 32 is one mechanism that may provide insight into sex differences in stress-related disorders.33, 34 Such sex differences, many of which occur near estrogen receptor regulatory elements, are established during development, although their function may not become apparent until later in life.34, 35
In this study, we evaluated the hypothesis that individual variation in response to estrogen levels contributes to fear regulation and PTSD risk in women through differential epigenetic processes. Using nonbiased approaches, we identified an association between methylation of CpG sites in histone deacetylase 4 (HDAC4) in women with PTSD. HDAC4 is a class IIa HDAC that has been previously implicated in learning and memory; however, its regulation in amygdala-mediated forms of learning has not yet been established.36, 37, 38, 39 Using a well-characterized mouse model of fear learning, we examine the regulation of HDAC4 in the amygdala at the time of fear memory formation and its potential interaction with estrogenic tone. Although it is inappropriate to suggest that rodents develop PTSD, the conservation of neuronal circuitry known to mediate responses to traumatic events from mouse to human and the simplicity of rodent models of fear learning have contributed to the utility of these models. As we also know that the onset of PTSD is intricately tied to a traumatic event or series of associated traumatic events, using rodent models to investigate the neurobiological and molecular mechanisms necessary for traumatic memory formation as a result of exposure to tone-shock presentations are vital towards advancing our understanding of PTSD.
Materials and methods
Participants and assessments
This study evaluated women recruited as part of the Grady Trauma Project (GTP), a larger study investigating the influence of genetic and environmental factors on the development of PTSD in a predominantly African American, urban population of low socioeconomic status.22, 28, 40, 41, 42 In brief, research participants were approached in the waiting rooms of the primary care clinic or obstetrical-gynecological clinic of a large, urban, public hospital in Atlanta, GA, while either waiting for their medical appointments or while waiting with others who were scheduled for medical appointments. Subjects willing to participate provided written informed consent and participated in a verbal interview and blood draw. Current PTSD diagnosis was assessed using the modified PTSD Symptom Scale, a 17-item self-report scale with excellent internal consistency, high test–retest reliability, and concurrent validity to diagnose PTSD consistent with Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria.43, 44 Estradiol assays were conducted in serum by the Yerkes Biomarkers Core Laboratory at Emory University as described in the Supplementary Information. A subset of subjects underwent a fear conditioning paradigm that has been previously described and neuroimaging to assess functional connectivity.41, 42 Both protocols are described in detail in the Supplementary Information. Study procedures were approved by the Institutional Review Board of Emory University School of Medicine and the Grady Health Systems Research Oversight Committee.
Genetic and epigenetic assays
Detailed genetic methods can be found in the Supplementary Information. Whole blood was collected in EDTA tubes (for DNA extraction) or Tempus tubes (for RNA extraction). DNA methylation was assessed using the HumanMethylation450 BeadChip (Illumina, San Diego, CA, USA) as previously described.30 Beta Mixture Quantile dilation was used to normalize the data set.45 RNA expression was assessed as part of a previous genome-wide study in this cohort.46 The array was log2-transformed and normalized using the Supervised Normalization Method.46 In total, 15 877 probes, including the one interrogating HDAC4 (ILMN_1764396), were sufficiently expressed in blood (that is, had sufficient detection P-values).
Methylation probes (87 388) corresponding to the 15 877 transcripts expressed in blood were included in this analysis. Probe locations relative to a gene were determined from the annotation file for the array. For each CpG site, methylation proportion was modeled as a linear function of estradiol (N=239) or PTSD (N=278), adjusting for age, cellular proportions, positional effects and ancestry (three principal components from genome-wide association studies). For analyses of estradiol and PTSD, a Bonferroni correction was used to adjust for 87 388 tests.
Genotyping was performed using the Omni-Quad 1 M or the Omni Express BeadChips (Illumina) as previously described.47, 48 Genotypes for rs7570903 did not deviate from Hardy–Weinberg proportions, and 118 women had available genotype and methylation data. To test for association, cg22937172, each outcome was regressed on rs7570903 allele count assuming an additive model (0, 1 or 2 copies of risk allele), including sex and ancestry from top genome-wide association study principal components. Similar models were used to test for association between rs7570903 and HDAC4 expression (ILMN_1764396; N=82), startle response (N=95), and functional connectivity (N=38) in women from the broader GTP cohort with available data. For each of these hypothesis-driven tests, P<0.05 was considered significant.
As detailed in the Supplementary Information, Epstein–Barr transformed lymphoblastoid cell lines were selected from the 1000Genomes Project according to the HDAC4 rs7570903 genotype (CC or TT). 49 A t-test was used to compare HDAC4 messenger RNA (mRNA) expression, which was considered to be statistically significant if P<0.05.
Animals and ovariectomy surgical procedures
Adult female C57bl/6J mice, aged 8–10 weeks were obtained from Jackson Labs (Bar Harbor, ME, USA). Female mice were administered i.p. ketamine (75 mg kg−1)/dexdomitor (1 mg kg−1) anesthesia to induce sedation. Upon sedation, mice were ovariectomized (OVX) and implanted with silastic capsules, composed of 2 mm silastic tubing (0.078-by-0.125 inch) containing sesame oil (OVX+Veh) or 1 μg μl−1 Estradiol (OVX+E), a dose based on Jasnow et al.50 As female mice housed together have been observed to have irregular estrous cycling mice used in the naturally cycling experiment were presented with bedding from male mouse cages either 5 days (metestrous) or 3 days (proestrous) before fear conditioning to encourage estrous cycling.51 All procedures involving animals were approved by the Emory University institutional animal care and use committee and conducted under the standards upheld by the National Institutes of Health.
Tone-shock conditioning procedures
OVX female mice were habituated to the training context for 10 min on 2 consecutive days. On the third day, mice were returned to the training context and exposed to five trials of tone-shock pairings consisting of a 30 s, 75 db, 6 khz tone-conditioned stimulus that co-terminated with a 500 ms, 0.6 mA shock-unconditioned stimulus administered through the grid floor. A lower intensity tone-shock conditioning protocol was used for the mice undergoing ovariectomy compared with the naturally cycling group, as we have found that animals undergoing surgical procedures have some level of stress exposure and are thus more sensitive to future tone-shock experiences in the future. Animals were removed from the training context and placed in their cages for 2 h before being rapidly sedated for decapitation and brain collection.
Hdac4 expression
Bilateral punches of the basal lateral nucleus of the amygdala (BLA) were taken using a sliding-freezing microtome and stored at −80. Extracted RNA from the punches was reverse-transcribed to complementary DNA for quantitative PCR analysis to determine Hdac4 expression. (Note that lower case refers to the murine gene, whereas upper case refers to the human gene). Quantitative PCR analysis to examine Hdac4 expression accompanying fear conditioning in the amygdala was conducted using the 2ΔΔ Ct method and normalized to GAPDH Cts as detailed in the Supplementary Information. Group differences were ascertained using these normalized values and conducting t-tests. Analyses were only considered to be statistically significant if P<0.05.
Results
Estradiol levels associate with DNA methylation
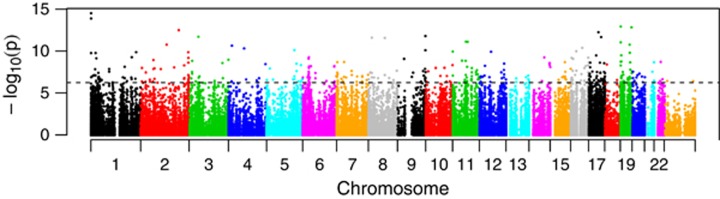
We evaluated 239 women that represent the range of serum estradiol in adult women during childbearing years, pregnancy and menopause (2.85–546 pg ml−1). A genome-wide analysis revealed that 18 254 CpG sites associated with estradiol levels (P<0.05), 390 of which remained associated after a Bonferroni correction for multiple testing (Figure 1), suggesting that methylation of genes across genome are influenced by estradiol-mediated signaling. Evaluation of women that fell within the normal cycling range for adults (30–400 pg ml−1; N=84) were consistent with the results of the overall analysis (r=0.32; P<0.00001).
Figure 1.
Manhattan plot of the association between estradiol levels and DNA methylation. The x axis is the position of each CpG site by chromosome. The y axis is the negative log10 of the P-value for association of each CpG site. The dashed black line indicates experiment-wide significance based on a Bonferroni correction.
DNA methylation differences in women with PTSD
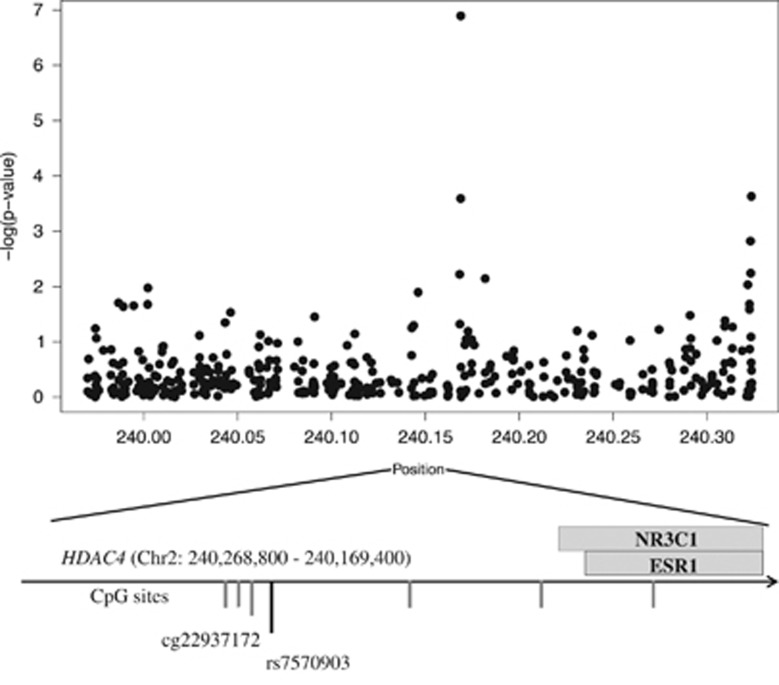
We next examined the association between DNA methylation and PTSD in 278 women from the GTP cohort (39.2% with PTSD). Methylation of 5288 CpG sites nominally associated with PTSD (P<0.05), and there was significant overlap between CpG sites that associate with PTSD and those that associate with estradiol levels (P<0.0001), supporting the link between PTSD and estradiol-mediated signaling. After a Bonferroni correction for multiple tests, only cg22937172 remained associated with PTSD (Figure 2). Methylation of this CpG site in HDAC4 was higher in PTSD cases than controls (t(277)=5.43; P=1.27 × 10−7), and higher methylation of this site was associated with lower estradiol levels (t(248)=−2.21; P=0.028).
Figure 2.
DNA methylation in HDAC4 associates with post-traumatic stress disorder (PTSD) diagnosis. CpG sites are ordered by position on the x axis (gray lines), and the y axis indicates the negative log of the P-value for association of each CpG site with PTSD. The position of the estrogen response element and glucocorticoid response elements relative to cg22937172 are indicated. The direction of transcription is indicated by the arrow.
Further evaluation of the region surrounding cg22937172 revealed that it is near binding sites for the glucocorticoid and estrogen receptors (Figure 2). It also revealed that the probe interrogating this CpG site contained a common single-nucleotide polymorphism (rs7570903; minor allele frequency of 33%), suggesting that methylation of cg22937172 may be influenced by an individual’s genotype. Indeed, the CC genotype of rs7570903 associated with higher cg22937172 methylation (t(117)=3.47; P=0.0017) and lower HDAC4 expression (t(81)= P=0.049). However, rs7570903 genotype did not associate with PTSD (P=0.067), suggesting this relationship cannot be attributed to genotype alone. To further examine genotype-dependent alterations in HDAC4 expression, we examined baseline expression of HDAC4 in lymphoblastoid cell lines from the Thousand Genomes Project and confirmed that cultures from CC cell lines had lower HDAC4 mRNA levels than TT cell lines at baseline (Supplementary Figure S1) in the absence of cortisol or estrogen.
HDAC4 genotype associates with fear-related traits
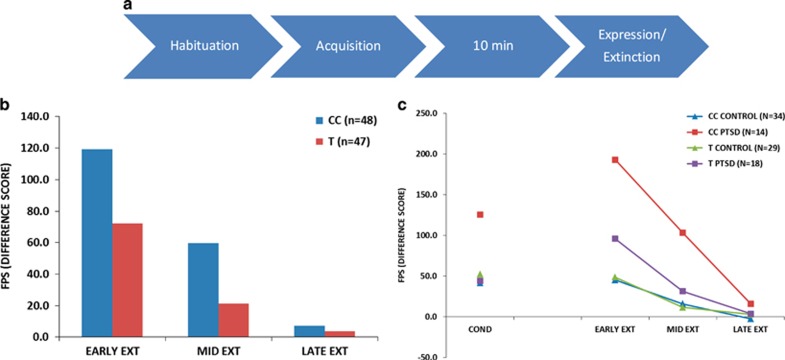
Overexpression of conditioned fear, termed ‘fear load’ is considered an intermediate phenotype of PTSD and related syndromes.52 As HDAC4 is implicated in the regulation of learning and memory,36, 37, 38, 39 we examined whether rs7570903 genotype predicted a subject’s differences in the expression of conditioned fear. GTP subjects with the CC genotype at rs7570903 exhibited enhanced fear expression on a fear-potentiated startle task (P=0.026; Figure 3a). PTSD also interacted with rs7570903 genotype to predict increased fear conditioning and higher fear-potentiated startle during early extinction (P=0.019; Figure 3b), suggesting that those with the CC genotype and PTSD have the greatest fear load.
Figure 3.
Genetic variation in HDAC4 associates with fear expression. (a) Schematic of the human physiology experiment. (b) Average fear-potentiated startle (FPS) difference score across the fear extinction phase for CC and CT/TT genotypes. (c) Average FPS difference score across the conditioning and expression (extinction) session for genotype X post-traumatic stress disorder (PTSD).
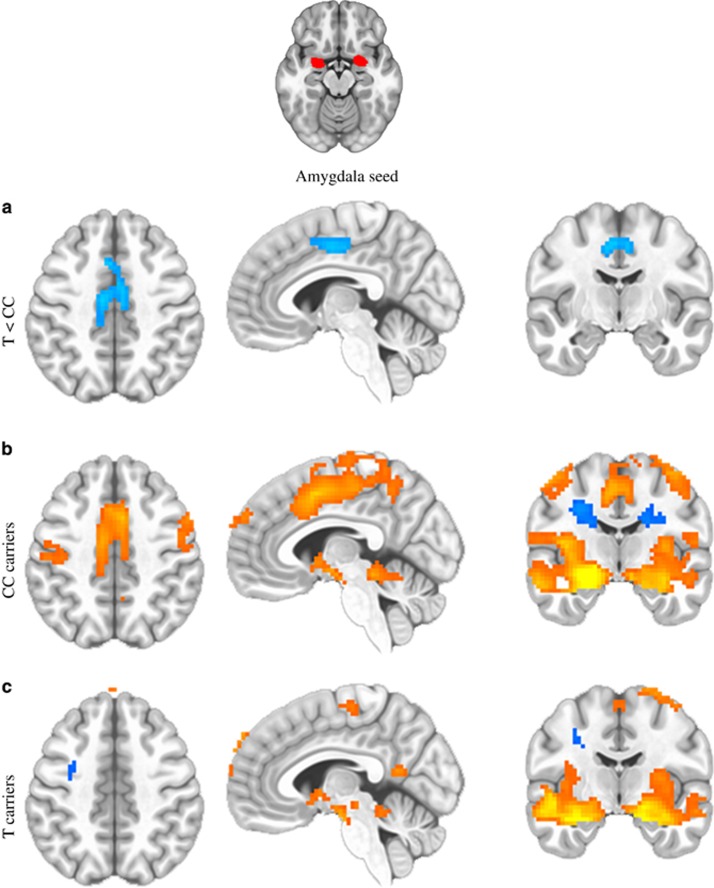
We next examined functional brain connectivity in a highly traumatized cohort of subjects as an additional potential intermediate phenotype related to PTSD symptoms. Consistent with the idea that subjects with the rs7570903 CC genotype have higher fear load, which has been associated with structural connectivity in the cingulum tract,53 we observed that the CC genotype associated with increased resting-state functional connectivity between the amygdala and cingulate cortex, two regions of the brain implicated in fear memory (Figures 4a–c).54, 55, 56, 57, 58, 59 In this analysis, we compared those with CC genotype at rs7570903 to those with one ore more copies of the T allele, examining amygdala functional connectivity data at rest. Following whole-brain correction, subjects with the CC genotype showed increased functional coupling between amygdala and dorsal cingulate cortex, compared with those with one or more copies of the T allele, independent of PTSD diagnosis (P<0.05 after multiple test correction).
Figure 4.
HDAC4 genotype (rs7570903) effects on amygdala functional connectivity at rest. The CC genotype groups show significant increased functional coupling of the amygdala–cingulate circuitry compared with those with the CT/TT genotype. (a) Group differences are shown in the horizontal (left), sagittal (middle) and coronal (right) planes between the groups. (b) Regions associated with amygdala functional activity at resting state in the CC genotype group. (c) Regions associated with amygdala functional activity at resting state in those with the CT/TT genotypes. Orange is indicative of significant positive associations/greater connectivity, and blue is indicative of significant negative associations/lesser connectivity. All signals represent whole-brain corrected voxels at P<0.05.
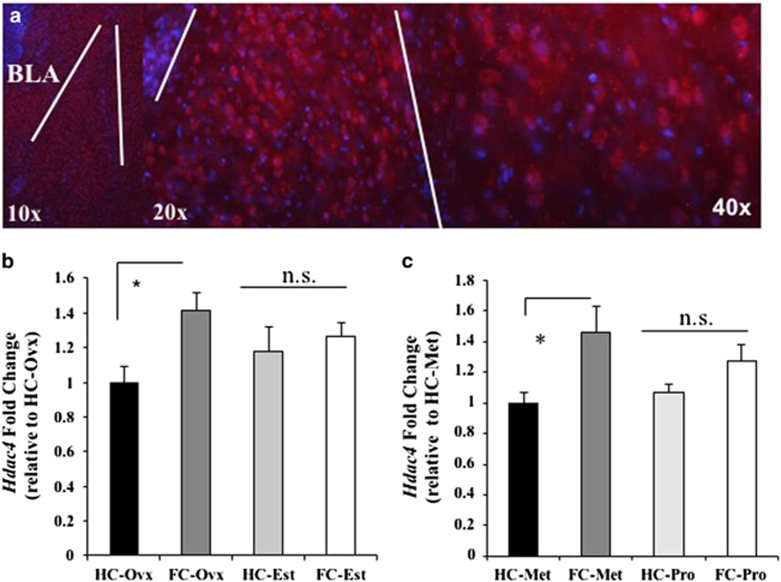
Tone-shock presentations in female mice regulates Hdac4 mRNA in the amygdala
Before examining whether Hdac4 is regulated in the murine brain at the time of fear memory formation, we first confirmed the expression of Hdac4 in the amygdala, an area that is consistently implicated in the formation of fear memories and coordinating responses to threating and traumatic situations (Figure 5a).
Figure 5.
Tone-shock exposure mediated alterations in Hdac4 expression are influenced by estrogen status. (a) Mouse amygdala Hdac4 immunohistochemistry (red=Hdac4; blue=4,6-diamidino-2-phenylindole/Hoechst). (b) Amygdala Hdac4 mRNA expression in ovariectomized mice in the presence (Est) or absence (Ovx) of estrogen 2 h after tone-shock exposure (FC) compared with homecage (HC) controls. Mean Hdac4 foldchange relative to HC-Ovx and normalized to Gapdh foldchange. (c) Amygdala Hdac4 level in naturally cycling female mice in either metestrous (Met) or proestrous (Pro) 2 h after tone-shock exposure (FC) compared with HC controls. Mean Hdac4 foldchange relative to HC-Met and normalized to Gapdh fold change. BLA, basal lateral nucleus of the amygdala; NS, nonsignificant.
As our clinical data suggest that regulation of HDAC4 is altered in females with PTSD, we next sought to examine whhether Hdac4 expression in the BLA is regulated in female mice at the time of tone-shock presentations and whether estrogen moderates any observed regulation. To this end, we conducted two experiments first, in OVX mice in the presence or absence of estrogen replacement and second, in naturally cycling females that experienced tone-shock presentations. In our first experiment, female mice were OVX and maintained with estradiol replacement or vehicle (oil) throughout recovery and behavioral testing. Following recovery, mice underwent tone-shock presentations and were then killed 2 h later (Figure 5b). Examination of Hdac4 mRNA expression using quantitative PCR revealed an increase in Hdac4 mRNA in tone-shock exposed mice maintained on vehicle (FC-Ovx) compared with homecage (HC-Ovx; t(13)=2.99, P=0.015). No difference was observed in the expression of Hdac4 in homecage mice maintained on vehicle (HC-Ovx) or estradiol (HC-Est) (t(12)=1.07, P=0.31), and no difference was found in Hdac4 expression for homecage (HC-Est) or tone-shock exposed (FC-Est) mice maintained on estradiol (t(12)=0.5, P=0.60). These data suggest that, although tone-shock exposure results in an increase in Hdac4 mRNA in the BLA, this regulation may be buffered by estrogen. To further examine the potential influence of estrogen on Hdac4 expression, we examined its regulation accompanying tone-shock exposure in naturally cycling females. Female mice underwent tone-shock exposure and were killed 2 h later, examined for estrous cycle phase and separated into metestrous (low estrogen status) and proestrous (high estrogen status) groups (Figure 5c). Examination of Hdac4 mRNA expression in the BLA 2 h after tone-shock exposure revealed no difference in Hdac4 between metestrous and proestrous homecage control groups (t(21)=0.539, P=0.59). Tone-shock exposure was found to increase Hdac4 expression relative to homecage for metestrous (t(16)=4.99, P=0.0001), whereas no difference was observed in Hdac4 expression for the proestrous tone-shock exposed relative to homecage group (t(22)=1.84, P=0.079). In addition, tone-shock exposure-related expression of Hdac4 between metestrous and proestrous groups was found to be significantly different (t(17)=1.74, P=0.040; Figure 5c; Supplementary Information).
These data suggest that during metestrous when estrogen levels are low, a tone-shock-related regulation of Hdac4 expression is evident, while mice in proestrous do not exhibit this regulation. These data also demonstrate the same pattern of results concerning a potential buffering of Hdac4 expression by estrogen level in both OVX or naturally cycling females. Previous studies have noted that estrogen may be protective against the development of PTSD and may facilitate extinction recall. 24, 60 If Hdac4 regulation in females is required for the formation of aversive memories resulting from tone-shock exposure, then our observation that mice in proestrous fail to exhibit this regulation supports the existence of estrogenic protection against traumatic memory formation accompanying experience with tone-shock exposure.
Discussion
The results of this study suggest that estrogen status may increase risk for PTSD in some women, in part through its regulation of HDAC4. To the best of our knowledge, this is the first report of which we are aware to take an unbiased approach towards revealing alterations in DNA methylation that are associated with PTSD diagnosis in women. As summarized in Supplementary Figure S2, DNA methylation differences in HDAC4 associate with PTSD in women, and methylation levels of the PTSD-associated CpG site also associates with rs7570903 in a genotype-dependent manner. The CC genotype at rs7570903, which tags higher methylation levels at the PTSD-associated CpG site, associates with lower HDAC4 expression relative to the TT genotype. Interestingly, the CpG site and single-nucleotide polymorphism lie upstream of estrogen (ESR1) and a glucocorticoid receptor (NR3C1)-binding sites (Figure 2), both of which are consistent with our hypothesis of altered HDAC4 regulation in females with PTSD. Further examination also revealed that females with the CC genotype have heightened fear expression, or fear load, and alterations in resting-state amygdala–dorsal anterior cingulate functional connectivity. A limitation of this approach is that the human study relied primarily on biological measures from peripheral tissue, and these results could be influenced by cellular heterogeneity within whole blood or may not be reflective of processes occurring in the brain. Using mouse models to delve deeper into examining the potential influence of estrogen on HDAC4 regulation at the time of traumatic memory formation revealed a tone-shock exposure-related increase in Hdac4 mRNA in the amygdala in female mice with low estrogen status, either OVX or in metestrous, whereas mice with high estrogen status, OVX with estradiol replacement or in proestrous, were not observed to have tone-shock-related regulation of Hdac4.
PTSD is characterized by an inability to regulate emotional responses that are associated with trauma and has been associated with increased amygdala activity and alterations in precortical regions known to mediate and modulate fear memories. As PTSD emerges following a traumatic and emotionally charged event or series of traumatic events, examination of the neurobiological mechanisms that mediate fear behaviors and memory formation have proven to be productive and have substantial face validity. Indeed, just as PTSD is associated with impaired fear extinction and prolonged fear expression mediated by alterations in precortical regions and amygdala hyperactivity, human and animal studies note the importance of precortical regions in mediating and modulating fear memories by serving to inhibit amygdala responsivity to emotional stimuli.61, 62, 63 Specifically, PTSD has been associated with decreased activation in the ventromedial prefrontal cortex, a region thought to be analogous to the rodent infralimbic cortex, which is typically noted for its importance in fear inhibition processes. In addition, PTSD is associated with increased dorsal anterior cingulate cortex (dACC) activation, a region typically noted for its role in the initial acquisition of fear behaviors and possibly analogous to the rodent prelimbic cortex, which our group has revealed to be critical for the initial formation and consolidation of fear memories,64 consistent with a mechanism for the sustained fear expression.65, 66 Whereas our group has recently reported that females with PTSD have disrupted amygdala–ventromedial prefrontal cortex connectivity,67 our current data reveal that subjects with the CC genotype at rs7570903 have enhanced amygdala–ACC functional connectivity. Consistent with this alteration in amygdala–dACC connectivity in PTSD, subjects with the CC genotype at rs7570903 were also found to have sustained fear expression shortly following acquisition, which may be attributable to the observed altered amygdala–dACC connectivity. As the amygdala–dACC are both brain structures that have been well implicated in fear processing in both rodent and human studies, the observation that subjects with the CC genotype have increasing resting-state connectivity between these regions may coincide with the observed prolonged fear expression in those with the CC genotype and PTSD. Future experiments will be necessary for addressing the downstream consequences of these observed differences in functional resting-state connectivity within these fear-related structures, especially in how they may be mechanistically related to alterations in fear load and expression.
The observation of higher HDAC4 methylation in PTSD cases suggests that the role of HDAC4 in the development of PTSD warranted further examination. Using auditory fear conditioning, a well-characterized murine model of fear memory formation, we found that OVX female mice without estradiol replacement (FC-Ovx) have a tone-shock exposure-related increase in Hdac4 mRNA in the amygdala, whereas no regulation was observed in mice that were maintained on estradiol replacement (FC-Est). Although we did not observe a significant difference between FC-Ovx and FC-Est, the observation of differential tone-shock exposure-related regulation between FC-Ovx and FC-Est suggests a potential estrogenic buffering of Hdac4 in response to tone-shock exposure across mammalian species.
As a class IIA HDAC, HDAC4 is trafficked from the nucleus to cytoplasm in an activity-dependent manner through Ca2+-mediated signaling,38, 68, 69, 70 and estrogen has been demonstrated to dampen the nuclear export,71 suggesting that naturally cycling estrogenic tone may be critical in the regulation of HDAC4. Thus, we next examined the tone-shock exposure-related regulation of Hdac4 in naturally cycling female mice. Similar to our OVX experiment, we observed a tone-shock exposure-related increase in Hdac4 mRNA in mice in a low estrogen phase of estrous (metestrous), which was significantly different from tone-shock exposed mice in a high estrogen phase (proestrous). No tone-shock exposure-related regulation of Hdac4 was observed in proestrous mice compared with homecage. These data reveal for the first time that estrogenic tone at the time of tone-shock exposure influences the regulation of Hdac4 in the amygdala. It is worth noting that, although some studies have noted that estrogen may be protective against the acquisition of fear learning,72 others have noted that estrogen appears to facilitate memory formation both across a variety of preparations including initial acquisition and the acquisition of extinction memories and further studies have demonstrated that estrogen may serve to reduce pain responses.16, 17, 18, 19, 23, 24, 25, 60, 73 The implication that estrogen may alter pain perception is an important one, as our current data do not allow for direct examination of this effect. It is entirely possible that one important route through which fear behaviors may be impacted by estrogen is via alterations in pain perception, thus alterations in shock reactivity would be predicted to have a marked impact on subsequent fear expression.60 An additional caveat is the previous work that has demonstrated that high levels of estrogen can impair the acquisition of fear inhibition,74 which may suggest that both low and high estrogen may result in impaired memory formation and furthers contributes to the overall need for increased research in this area. Thus, given the wide range of potential roles and influences of estrogen, future studies will be necessary to reveal the functional consequences of this novel estrogenic mediation of HDAC4 expression at the time of tone-shock exposure.22, 23, 24, 25, 73
Taken together, these data suggest that regulation of HDAC4 may increase risk for PTSD in women. Genetic and epigenetic variation can cause differences in HDAC4 expression, although estrogen seems to provide an additional level of regulation, supported by our findings in animal models of estrogen- and stress-dependent regulation of Hdac4 within amygdala. In traumatized human subjects, lower HDAC4 expression, predicted by higher methylation at cg22937172 and the CC genotype at rs7570903, associates with increased resting-state functional connectivity between areas of the brain implicated in fear expression as well as heightened fear load, particularly for those with PTSD (Supplementary Figure S2). Future studies should further characterize the role of HDAC4 in mediating sexually dimorphic neuroendocrine function and PTSD risk.
Acknowledgments
We thank Rachel Penrod-Martin, Makoto Taniguchi and Christopher Cowan (Integrative Neurobiology Laboratory, McLean Hospital) for their helpful insights and discussions during the formation of this manuscript. The authors also acknowledge the participants of the study and the GTP staff for their assistance with participant recruitment and data collection. This research was supported by the National Institutes of Health Grants MH071537 and MH096764 (KJR), and MH085806 (AKS). This work was also supported by NARSAD YI award #19233 (AKS) the Howard Hughes Medical Institute (KJR), the Behrens-Weise foundation (EBB) and in part by ORIP/OD P51OD011132 (formerly NCRR P51RR000165) Yerkes Base Grant funds.
Footnotes
Supplementary Information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp)
The authors declare no conflict of interest.
Supplementary Material
References
- Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Arch Gen Psychiatry 1998; 55: 626–632. [DOI] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull 2007; 133: 183–204. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52: 1048–1060. [DOI] [PubMed] [Google Scholar]
- Breslau N, Chilcoat HD, Kessler RC, Peterson EL, Lucia VC. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol Med 1999; 29: 813–821. [DOI] [PubMed] [Google Scholar]
- Dean C, Kendell RE. The symptomatology of puerperal illnesses. Br J Psychiatry 1981; 139: 128–133. [DOI] [PubMed] [Google Scholar]
- Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. ANS Adv Nurs Sci 2005; 28: 364–375. [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Rubinow DR. Sex hormones and mood in the perimenopause. Ann NY Acad Sci 2009; 1179: 70–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA. Anxiety symptoms and anxiety disorders: how are they related to premenstrual disorders? J Clin Psychiatry 1997; 58(Suppl 3): 62–67, discussion 68-69. [PubMed] [Google Scholar]
- Pigott TA. Anxiety disorders in women. Psychiatr Clin North Am 2003; 26: 621–672 vi–vii. [DOI] [PubMed] [Google Scholar]
- Hendrick V, Altshuler LL, Burt VK. Course of psychiatric disorders across the menstrual cycle. Har Rev Psychiatry 1996; 4: 200–207. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosom Med 1999; 61: 154–162. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry 1998; 44: 839–850. [DOI] [PubMed] [Google Scholar]
- Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D et al. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry 2006; 60: 704–713. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham KL, Silove D, Creamer M, O'Donnell M, McFarlane AC. The association between menstrual cycle and traumatic memories. J Affect Disord 2011; 131: 398–401. [DOI] [PubMed] [Google Scholar]
- Nillni YI, Pineles SL, Patton SC, Rouse MH, Sawyer AT, Rasmusson AM. Menstrual cycle effects on psychological symptoms in women with PTSD. J Trauma Stress 2015; 28: 1–7. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacology Biochem Behav 2000; 67: 587–596. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol Behav 2001; 74: 435–440. [DOI] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology 1996; 21: 609–620. [DOI] [PubMed] [Google Scholar]
- Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology 2009; 34: 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci 2005; 25: 9309–9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci 2010; 30: 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B et al. Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci 2013; 38: 341–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 2009; 164: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol Psychiatry 2011; 70: 920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience 2010; 168: 652–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry 2012; 72: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebron-Milad K, Graham BM, Milad MR. Low estradiol levels: a vulnerability factor for the development of posttraumatic stress disorder. Biol Psychiatry 2012; 72: 6–7. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature 2011; 470: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Conneely KN, Kilaru V, Mercer KB, Weiss TE, Bradley B et al. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am J Med Genet B Neuropsychiatr Genet 2011; 156B: 700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci USA 2013; 110: 8302–8307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipahi L, Wildman DE, Aiello AE, Koenen KC, Galea S, Abbas A et al. Longitudinal epigenetic variation of DNA methyltransferase genes is associated with vulnerability to post-traumatic stress disorder. Psychol Med 2014; 44: 3165–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R et al. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc Natl Acad Sci USA 2010; 107: 9470–9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Sipahi L, Li J, Koenen KC. Sex differences in DNA methylation may contribute to risk of PTSD and depression: a review of existing evidence. Depress Anxiety 2013; 30: 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Nugent BM, McCarthy MM. Developmental and hormone-induced epigenetic changes to estrogen and progesterone receptor genes in brain are dynamic across the life span. Endocrinology 2010; 151: 4871–4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent BM, McCarthy MM. Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology 2011; 93: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WH, Cheng LC, Pan FY, Xue B, Wang DY, Chen Z et al. Intracellular trafficking of histone deacetylase 4 regulates long-term memory formation. Anat Rec 2011; 294: 1025–1034. [DOI] [PubMed] [Google Scholar]
- Kim MS, Akhtar MW, Adachi M, Mahgoub M, Bassel-Duby R, Kavalali ET et al. An essential role for histone deacetylase 4 in synaptic plasticity and memory formation. J Neurosci 2012; 32: 10879–10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando R 3rd, Gounko N, Pieraut S, Liao L, Yates J 3rd, Maximov A. HDAC4 governs a transcriptional program essential for synaptic plasticity and memory. Cell 2012; 151: 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons HL, Schwartz S, Given FM, Scott MJ. The histone deacetylase HDAC4 regulates long-term memory in Drosophila. PLoS One 2013; 8: e83903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry 2009; 31: 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety 2010; 27: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Vervliet B, Jovanovic T, Boshoven W, Myers KM, Davis M et al. Timing of extinction relative to acquisition: a parametric analysis of fear extinction in humans. Behav Neurosci 2008; 122: 1016–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress 1993; 6: 459–473. [Google Scholar]
- Coffey SF, Gudmundsdottir B, Beck JG, Palyo SA, Miller L. Screening for PTSD in motor vehicle accident survivors using the PSS-SR and IES*. J Trauma Stress 2006; 19: 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teschendorff AE, Marabita F, Lechner M, Bartlett T, Tegner J, Gomez-Cabrero D et al. A beta-mixture quantile normalization method for correcting probe design bias in Illumina Infinium 450 k DNA methylation data. Bioinformatics 2013; 29: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham BH, Nelson PS, Storey JD. Supervised normalization of microarrays. Bioinformatics 2010; 26: 1308–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AK, Kilaru V, Klengel T, Mercer KB, Bradley B, Conneely KN et al. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to brain. Am J Med Genet B Neuropsychiatr Genet 2015; 168B: 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YV, Smith AK, Conneely KN, Chang Q, Li W, Lazarus A et al. Epigenomic association analysis identifies smoking-related DNA methylation sites in African Americans. Hum Genet 2013; 132: 1027–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1000 Genomes Project Consortium, Abecasis GR, Auton A, Brooks LD, DePristo MA, Durbin RM et al. An integrated map of genetic variation from 1,092 human genomes. Nature 2012; 491: 56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav 2006; 49: 197–205. [DOI] [PubMed] [Google Scholar]
- Whitten WK. Occurrence of anoestrus in mice caged in groups. J Endocrinol 1959; 18: 102–107. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Glover EM, Stevens JS, Fani N, Galatzer-Levy IR, Bradley B et al. Fear load: The psychophysiological over-expression of fear as an intermediate phenotype associated with trauma reactions. Int J Psychophysiol 2015; 98(2 Pt 2): 270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, King TZ, Brewster R, Srivastava A, Stevens JS, Glover EM et al. Fear-potentiated startle during extinction is associated with white matter microstructure and functional connectivity. Cortex 2015; 64: 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Kemp A, Das P, Hughes G, Peduto A et al. Amygdala and ventral anterior cingulate activation predicts treatment response to cognitive behaviour therapy for post-traumatic stress disorder. Psychol Med 2008; 38: 555–561. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biol Psychiatry 2007; 62: 1191–1194. [DOI] [PubMed] [Google Scholar]
- Pissiota A, Frans O, Michelgard A, Appel L, Langstrom B, Flaten MA et al. Amygdala and anterior cingulate cortex activation during affective startle modulation: a PET study of fear. Eur J Neurosci 2003; 18: 1325–1331. [DOI] [PubMed] [Google Scholar]
- Schultz DH, Balderston NL, Helmstetter FJ. Resting-state connectivity of the amygdala is altered following Pavlovian fear conditioning. Front Hum Neurosci 2012; 6: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumpers F, Morgan B, Terburg D, Stein DJ, van Honk J. Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Soc Cogn Affect Neurosci 2014; 10: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsson EO, Nader K. Involvement of the anterior cingulate cortex in formation, consolidation, and reconsolidation of recent and remote contextual fear memory. Learn Mem 2012; 19: 449–452. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA. Estrogen and/or progesterone administered systemically or to the amygdala can have anxiety-, fear-, and pain-reducing effects in ovariectomized rats. Behav Neurosci 2004; 118: 306–313. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci 2006; 1071: 67–79. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47: 769–776. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001; 50: 932–942. [DOI] [PubMed] [Google Scholar]
- Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl Psychiatry 2012; 2: e205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 2007; 62: 446–454. [DOI] [PubMed] [Google Scholar]
- Stevens JS, Jovanovic T, Fani N, Ely TD, Glover EM, Bradley B et al. Disrupted amygdala-prefrontal functional connectivity in civilian women with posttraumatic stress disorder. J Psychiatr Res 2013; 47: 1469–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumm F, Mauceri D, Freitag HE, Bading H. Nuclear calcium signaling regulates nuclear export of a subset of class IIa histone deacetylases following synaptic activity. J Biol Chem 2013; 288: 8074–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla S, Vanhoutte P, Arnold FJ, Huang CL, Bading H. Neuronal activity-dependent nucleocytoplasmic shuttling of HDAC4 and HDAC5. J Neurochem 2003; 85: 151–159. [DOI] [PubMed] [Google Scholar]
- Liu Y, Randall WR, Schneider MF. Activity-dependent and -independent nuclear fluxes of HDAC4 mediated by different kinases in adult skeletal muscle. J Cell Biol 2005; 168: 887–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Narayanan R, Dalton JT, McKinsey TA, Levin ER. Estrogen regulates histone deacetylases to prevent cardiac hypertrophy. Mol Biol Cell 2013; 24: 3805–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biol Psychiatry 2012; 72: 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry 2013; 73: 371–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Bowser ME, Davis M. Estrogen disrupts the inhibition of fear in female rats, possibly through the antagonistic effects of estrogen receptor alpha (ERalpha) and ERbeta. J Neurosci 2007; 27: 9729–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.