Abstract
Introduction
Sepsis remains one of the leading causes of infant death worldwide. It is characterized as uncontrolled inflammatory responses due to proven bacterial infection. Despite improvement in supportive care and the availability of effective antibiotics, no specific therapy targeting the dysregulated inflammatory response is available for neonatal sepsis. Milk fat globule epidermal growth factor-factor 8 (MFG-E8) is a secretory glycoprotein abundantly present in human milk. MFG-E8 suppresses the systemic inflammatory responses in adult murine injury models by improving the clearance of dying cells. We hypothesized that exogenous administration of recombinant mouse (rm) MFG-E8 could inhibit the exaggerated inflammatory response and lung injury in a murine model of neonatal sepsis.
Methods
Neonatal sepsis was induced in 5–7 day old male and female C57BL6 mice using an intraperitoneal injection of cecal slurry (CS). At 1h after sepsis induction, a single dose of 40 μg/kg rmMFG-E8 or vehicle was administered via retro-orbital injection. All neonates were returned to their mothers as a group. At 10 h after CS injection, pups were euthanized and blood and lung tissues were collected. Control mice underwent similar procedure with the exception of CS IP injection.
Results
Serum LDH, IL-1β and IL-6 were significantly increased 10 h after CS injection. Treatment with rmMFG-E8 decreased these levels by 30%, 56% and 37%, respectively. Lung morphology was significantly compromised in the vehicle group after CS injection whereas the rmMFG-E8 treated groups demonstrated a 48% improvement in the lung injury score. Lung IL-6 and MIP-2 protein levels were significantly reduced with rmMFG-E8 treatment. Lung neutrophil infiltration as observed by Gr-1 staining and, TUNEL positive cells were also significantly reduced with rmMFG-E8 treatment.
Conclusion
Treatment with rmMFG-E8 attenuated inflammation and lung injury in murine neonatal sepsis. Thus, MFG-E8 could be developed as a possible therapy for neonatal sepsis.
Keywords: MFG-E8, neonatal sepsis, lung injury, cytokines, MIP-2
Introduction
Despite improving medical care, neonatal sepsis remains the third leading cause of infant death worldwide, with a mortality rate of 26% [1]. Up to 40% of infants with sepsis develop respiratory complications and among survivors, as many as 31% of neonates will demonstrate persistent disability [1,2]. Although the most common pathogens associated with neonatal sepsis are Listeria and Group B Steptococcus, gram negative, i.e., Escherichia coli, sepsis is frequently encountered, especially in cases of necrotizing enterocolitis and spontaneous intestinal perforation [3,4]. Although septic neonates are treated aggressively with antibiotics, supportive therapies, and surgery when appropriate, no specific agent targeting the dysregulated inflammatory response is currently available [5,6].
Milk fat globule epidermal growth factor-factor 8 (MFG-E8) is a naturally occurring secretory glycoprotein. It is abundant in human milk, but is also expressed by numerous mammalian cell types [7,8]. Hanayama et al. [9] identified two distinct domains in MFG-E8: an arginine-glycine-aspartate (RGD) motif that binds integrins αVβ3 and αVβ5, and a discoidin domain which binds phosphatidylserine (PS). These domains determine the function of MFG-E8. By binding both integrin and PS, MFG-E8 creates a bridge between phagocytes and apoptotic cells, thereby enhancing phagocytosis [9]. Effective clearance of apoptotic cells limits secondary necrosis and, consequently, attenuates inflammation [10]. Furthermore, studies have shown that MFG-E8 suppresses systemic inflammatory response by reducing excessive production of pro-inflammatory cytokines (i.e. IL-6, IL-1β) through NF-κB activation and translocation to the nucleus [11–13].
Adult animal models have shown that MFG-E8 mediates engulfment of apoptotic cells, lowers levels of pro-inflammatory cytokines, and reduces the overall severity of lung injury [12,14]. However, the role of MFG-E8 in neonatal sepsis is not known. Here we hypothesize that recombinant mouse MFG-E8 (rmMFG-E8) would reduce sepsis-induced lung injury and inflammation in murine neonates. In this study, we treated neonatal mice with rmMFG-E8 after the induction of sepsis and subsequently evaluated the effects of this treatment on the severity of organ injury and inflammation, especially in the lungs.
Materials and Methods
Experimental animals
Pregnant female C57BL/6 mice were purchased from Charles River Laboratories (Kingston, NY) and were kept in a temperature and light-controlled room. Standard rodent diet was given to the mice. Pregnant females were closely observed to accurately record the date of birth of their litters. Neonatal mice aged 5–7 days were then used for experiments. Pups were kept with their mothers throughout the experiment. The study was approved by the Institutional Animal Care and Use Committee of the Feinstein Institute for Medical Research. Experimental procedures were performed in agreement with the National Institutes of Health Guidelines on the Use of Laboratory Animals.
Animal model of neonatal sepsis
Preparation of the cecal slurry (CS)
To prepare the CS, six adult house-bred mice (3 males and 3 females) aged 11–13 weeks were sacrificed by CO2 inhalation and their cecal contents were collected by laparotomy and cecotomy. The cecal contents were pooled and weighed, then suspended with 5% dextrose in normal saline for a concentration of 70 mg/ml. The CS was then filtered through a 70μm filter to remove large particles. With frequent mixing, the CS was aliquoted into 400 μl portions and immediately frozen in liquid nitrogen and stored at −80°C. A fresh aliquot was used for each experiment and was used within 2 hours of thawing.
Sepsis induction
Neonatal sepsis was induced in male and female C57BL/6 mice, aged 5–7 days, via a cecal slurry (CS) method adapted from Wynn et. al [15] with some modifications. In general, we obtain 6–8 pups from one litter and usually they are evenly distributed in gender. However, it was difficult to correctly identify the gender of the newborn mice at an early age of 5–7 days. Therefore, since they were not separated as either male or female, it was difficult to assess the distribution of the gender in this study. For sepsis induction, neonates were removed as a group from their mothers and were placed on a 37°C heating pad. Mice were anesthetized by inhalation with 2.5% isoflurane. Pups were then gently restrained and CS (0.9 mg/g body weight) was delivered by intraperitoneal (IP) injection. Following recovery from anesthesia, all neonates were again returned to the cage with their mothers as a group. Lung and blood samples were collected 10 h after CS injection and were stored at −80°C until analysis. Control mice in the study underwent similar procedure to the experimental mice with the exception of the IP injection.
Administration of rmMFG-E8
Septic neonates were randomly assigned to treatment or vehicle groups. The treatment group received a retro-orbital injection of 40 μg/kg body weight (BW) recombinant mouse MFG-E8 (rmMFG-E8; Cat. No.: 2805-MF-050; R&D systems, Minneapolis, MN) in a volume of 5 μl/g BW of phosphate-buffered saline (PBS) 1 h after IP CS injection. The vehicle group received an equivalent volume of PBS.
Measurement of lactate dehydrogenase (LDH)
Blood samples were centrifuged at 7,000 g for 10 min, and collected serum was stored at −80°C until analysis. Lactate dehydrogenase (LDH) was then measured using a commercial assay kit (Pointe Scientific, Lincoln Park, MI) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay (ELISA)
Frozen lung tissue samples were crushed into powder, lysed in buffer (10 mM TBS pH 7.5, 1% Triton-X-100, 1 mM ethylenediaminetetraacetic acid, 1 mM aminopolycarboxylic acid), and protease inhibitor cocktail (Thermo Scientific, Rockford, IL) and homogenized with a sonic dismembranator. After measuring the protein concentrations with a DC Protein Assay kit (Bio-Rad Laboratories), a standardized quantity of protein was loaded onto the ELISA plate. Equal volumes of serum samples collected were also loaded onto ELISA plate. Both serum and lung tissue lysates were analyzed by ELISA kits specific for interleukin 1β (IL-1β) and IL-6 (BD Biosciences) and mouse macrophage inflammatory protein-2 (MIP-2) (R&D Systems) according to the manufacturers’ instructions.
Histological evaluation
Lung tissue was placed in 10% formalin prior to embedding in paraffin. Tissue was cut into 5-μm sections and stained with hematoxylin and eosin (H&E) on glass slides. Light microscopy was used to evaluate the degree of lung injury in a blinded fashion. A modified version of the lung injury scoring system from the American Thoracic Society (ATS) published by Matute-Bello et. al. [16] was used to generate a lung injury score. Parameters such as neutrophils in the alveolar space and in the interstitial space, hyaline membranes, proteinaceous debris filling the airspaces, and alveolar septal thickening were examined. Based on the presence of each of the parameters, scores per visual field were assessed from 0 to 2 as none, moderate, and severe injury, respectively. The parameter scores were combined using a weighted formulation modified from that described by Matute-Bello et. al. [16] for a maximum score of 100 per field. The average of the scores per field was calculated as the final lung injury score in each group.
Immunohistochemistry
Immunohistochemistry for granulocyte-differentiation antigen-1 (Gr-1) was performed to assess neutrophil infiltration in the lungs. Paraffin-fixed lung tissues were dewaxed in xylene and rehydrated in a sequence of varying concentrations of ethanol. The slides were heated at 95°C for 30 min in 0.92% citric acid buffer (Vector Laboratories, Burlingame, CA). Slides were then cooled to room temperature before incubating in 2% H2O2/60% methanol and blocking in normal goat serum/Tris-buffered saline. Slides were then incubated overnight with anti-Gr-1 antibody (BioLegend, San Diego, CA), counterstained with 4′, 6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and observed under a light microscope (Eclipse Ti-S; Nikon, Melville, NY). Gr-1-positive staining cells or neutrophils appearing brown were counted at 200× magnification in 3 visual fields/section. The mean was computed thereafter.
TUNEL assay
Lung tissue sections were de-waxed and rehydrated as stated above, and were immersed in 20 μg/ml proteinase K at room temperature for 20 min. Tissue samples were then stained with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) kit (Roche Diagnostics, Indianapolis, IN), and counterstained with DAPI. Apoptotic cells appeared green under a fluorescence microscope and were counted at 200× magnification in 5 visual fields/section. The average of the number of apoptotic cells/field was calculated.
Statistical analysis
Data (n=5–7/group) were compared using one-way analysis of variance (ANOVA) with Student-Newman-Keuls (SNK) test or t-test where appropriate. Results are expressed as mean ± standard error of the mean (SEM). Significance was defined as P<0.05.
Results
Treatment with rmMFG-E8 mitigated organ injury and inflammation in neonatal sepsis
To assess the degree of organ injury induced by sepsis, serum LDH was measured. At 10 h after CS injection, LDH levels increased by 1.7-fold, compared to the control (Fig 1). In contrast, rmMFG-E8 treatment significantly reduced LDH levels by 30% (Fig 1). Serum IL-1β and IL-6 levels were measured to evaluate the systemic inflammation associated with neonatal sepsis. At 10 h after CS injection, while IL-1β increased by 4-fold, IL-6 was increased by 98-fold in the vehicle group, compared to the control group (Fig 1B–C). Treatment with rmMFG-E8 reduced IL-1β by a significant 56% whereas IL-6 was reduced by 37% (Fig 1B–C).
Figure 1. Effect of rmMFG-E8 on organ injury and inflammation after neonatal sepsis.
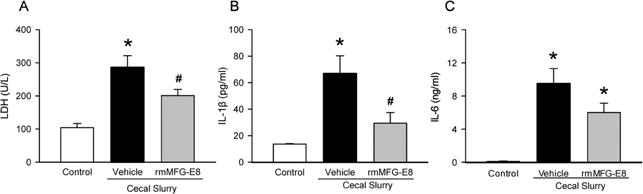
Male and female C57BL/6 pups were subjected to neonatal sepsis by IP CS injection and treated with PBS (Vehicle) or rmMFG-E8 (40 μg/kg body weight) at 1 h after CS. Serum samples collected at 10 h after CS were measured for (A) lactate dehydrogenase (LDH), (B) IL-1β and (C) IL-6. Serum samples from neonatal mice with neither CS injection nor treatments were included as controls (Control) for the experiment. Data shown as mean ± SE and compared by one-way ANOVA and SNK. *P< 0.05 vs. Control; #P< 0.05 vs. Vehicle.
Treatment with rmMFG-E8 attenuated lung injury in neonatal sepsis
To determine lung injury, the lung architecture was examined histologically by using H&E staining at 10 h after CS injection. As shown in Figure 2A, lung injury was evident in vehicle neonates with the presence of neutrophils in the alveolar space and interstitial space, the occurrence of hyaline membranes and proteinaceous debris, and increased alveolar septal thickening, compared to the control group. However, rmMFG-E8 treated neonates depicted improved lung morphology, compared to the vehicle group (Fig 2A). As quantified in Figure 2B, the histological lung injury score increased by 4-fold in the vehicle group compared to the control, whereas the score was significantly decreased by 48% in the rmMFG-E8 treatment group.
Figure 2. Effect of rmMFG-E8 on lung injury after neonatal sepsis.
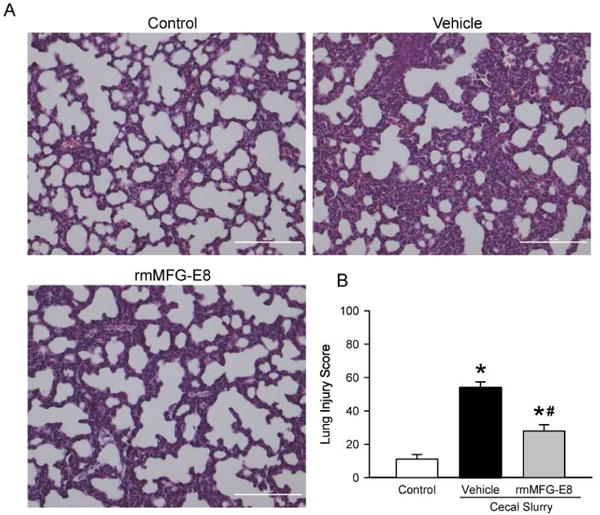
Lung tissues from Control, Vehicle, and rmMFG-E8 treatment groups harvested at 10 h after CS were sectioned, stained with hematoxylin and eosin, and examined under light microscopy. (A) Representative images at 200× magnification. (B) The histological lung injury scores quantified as described in “Materials and Methods”. Data shown as mean ± SE and compared by one-way ANOVA and SNK. *P< 0.05 vs. Control; #P< 0.05 vs. Vehicle.
Treatment with rmMFG-E8 decreased lung inflammation and neutrophil infiltration in neonatal sepsis
We investigated the local inflammatory response induced by neonatal sepsis in the lungs by quantifying lung IL-6 levels. At 10 h after CS injection, lung IL-6 was 7.5-fold higher in the vehicle group than the control and was significantly reduced by 41% in the rmMFG-E8 treatment group (Fig 3A). Moreover, we evaluated neutrophil infiltration in the lungs as an indicator of inflammation by measuring MIP-2 levels (Fig 3B) and GR-1 positive cells (Fig 3C–D). MIP-2 levels increased by 5-fold after CS injection in the vehicle group, compared to the control (Fig 3B). However, MIP-2 levels were significantly reduced by 19% in the rmMFG-E8 treated neonates in comparison with the vehicle group (Fig 3B). Gr-1 antibody staining marked a substantial increase of neutrophils by 3-fold in the vehicle, compared to the control (Fig 3D). In contrast, neutrophil infiltration was significantly reduced by 27% in the rmMFG-E8 treatment group, compared to the vehicle (Fig 3D).
Figure 3. Effect of rm-MFG-E8 on lung inflammation and neutrophil infiltration after neonatal sepsis.
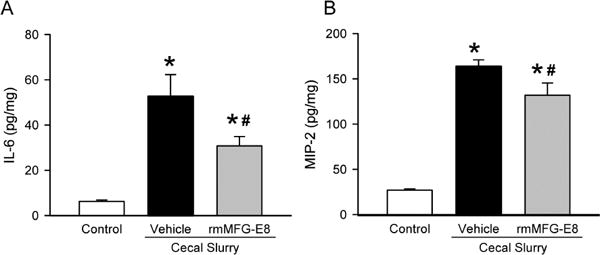
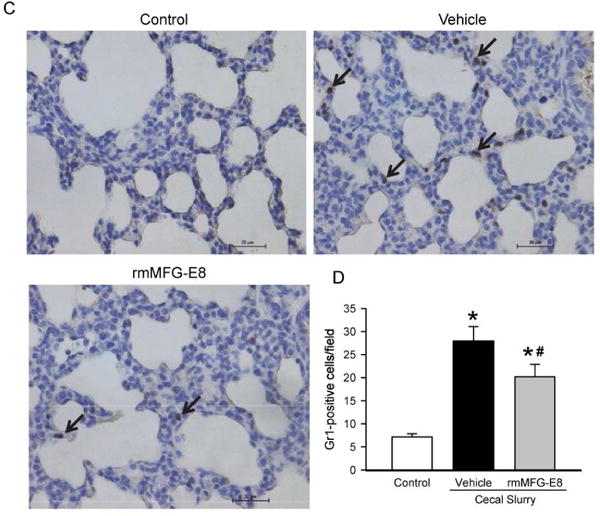
Lung tissue lysates were quantified for (A) IL-6 and (B) IL-1β proteins using enzyme-linked immunosorbent assay. Lung tissue sections were stained with granulocyte-differentiation antigen-1 (Gr-1) and examined under light microscopy. (C) Representative images display brown Gr 1-positive staining cells at 200× magnification. (D) The number of neutrophils or Gr 1-positive staining cells quantified from immunohistochemistry. Data shown as mean ± SE and compared by one-way ANOVA and SNK. *P< 0.05 vs. Control; #P< 0.05 vs. Vehicle.
Treatment with rmMFG-E8 reduced TUNEL positive cells in the lungs in neonatal sepsis
To analyze the effects of rmMFG-E8 treatment on the lungs, TUNEL assay was performed. While there were no TUNEL positive cells detected in the control group as shown in Figure 4A, the number of TUNEL positive cells increased by an average of 10 cells/field in the vehicle group (Fig 4B). However, TUNEL-positive cells in the rmMFG-E8 treated group were significantly reduced by 71% compared to the vehicle group (Fig 4B).
Figure 4. Effect of rmMFG-E8 on lung apoptosis after neonatal sepsis.
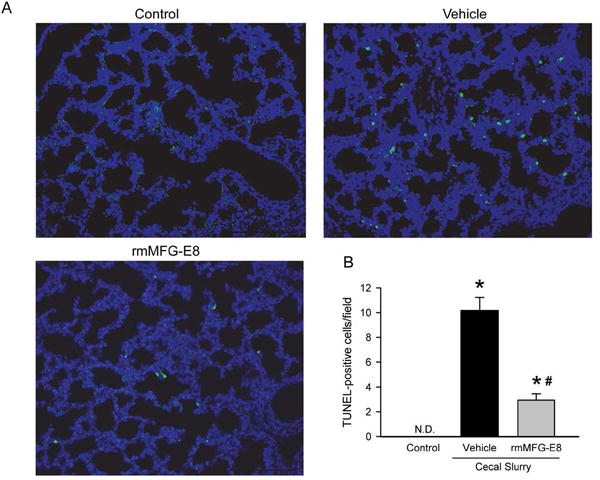
Lung tissue sections were stained with terminal deoxynucleotidyl transferase nick end-labeling (TUNEL) and examined under fluorescent microscopy. (A) Representative images display green TUNEL-positive cells and blue nuclei (DAPI staining) at 200× magnification. (B) The number of TUNEL-positive cells averaged over 10 visual fields/section. Data shown as mean ± SE and compared by one-way ANOVA and SNK. *P< 0.05 vs. Control; #P< 0.05 vs. Vehicle.
Discussion
Neonatal sepsis is a highly inflammatory condition culminating in septic shock and multiple organ dysfunction due to uncontrolled activation of the inflammatory system in response to pathogens. Newborns are at increased risk for severe bacterial infection due to their underdeveloped immune system [17–19]. The initiation of an effective immune response requires innate immune cells, i.e., macrophages and dendritic cells. Cytokines produced by these cells are essential for the host defense in response to pathogens. However, cytokines play a vital role in the development of sepsis in neonates [20–22]. A pathogenic stimulus triggers pro-inflammatory cytokine secretion from the phagocytes which in turn, promote the release of inflammatory mediators from the neutrophils and T cells [23,24]. Neutrophils release pre-formed inflammatory mediators stored in the granules such as elastase and myeloperoxidase which results in excessive inflammation leading to tissue damage and organ dysfunction [25]. Therefore, targeting the exaggerated inflammatory response could be beneficial in preventing neonatal sepsis.
In the current study using an established murine model of neonatal sepsis, we showed that treatment with rmMFG-E8 after induction of sepsis attenuated organ injury and inflammation, reduced lung neutrophil infiltration and TUNEL positive cells in the lung during neonatal mice. Studies have shown that pro-inflammatory cytokines such as interleukin-6 (IL-6) and Interleukin-1β are elevated in the peripheral blood plasma of neonates during early onset of sepsis [25–28]. In this regard, serum IL-1β and IL-6 were markedly increased 10 h after CS injection. In contrast, significant reductions of both cytokines were observed after rmMFG-E8 treatment. LDH, an indicator of organ injury, was also elevated after CS injection which was significantly decreased with rmMFG-E8 treatment. In addition to systemic effects, neonatal sepsis caused severe lung damage as evidenced by alterations in the histological appearance of the lung architecture. Treatment with rmMFG-E8 significantly attenuated sepsis-induced lung injury in the newborn mice. Neonatal sepsis associated increase in lung IL-6 levels were significantly reduced by rmMFG-E8 treatment. Neonatal sepsis also caused neutrophil infiltration in the lungs as observed by MIP-2 levels and Gr-1 positive cells which was attenuated by rmMFG-E8 treatment. Treatment with rmMFG-E8 also significantly reduced neonatal sepsis induced TUNEL positive cells in the lung. In general, we obtained 50 μl blood from a neonatal mouse at 10 h after cecal slurry injection. The serum obtained thereafter was used for the LDH assay and cytokine analyses. Due to the scarcity of the serum samples, serologic measurements of organ injury other than LDH were not assessed. In addition since the aim of the study was to determine the effect of rmMFG-E8 on lung injury, other organs such as the liver and kidneys were not investigated. In adult injury models of gut and liver ischemia reperfusion injury, we have previously shown that rmMFG-E8 significantly decreased liver enzymes (AST and ALT) and creatinine at 4 h after reperfusion [14,29]. Therefore, it is possible that the systemic effect observed with rmMFG-E8 treatment in the current study was due to the effect on multiple systems not just the lungs. However, additional experiments are warranted to determine whether liver/kidneys are involved in this systemic effect. Nevertheless, these data collectively indicated that treatment with rmMFG-E8 protects neonates against exaggerated inflammatory response and lung injury in neonatal sepsis.
This is the first study demonstrating the protective effect of rmMFG-E8 in neonatal sepsis. MFG-E8 is a secretory molecule which is abundant in human milk and mainly produced by the spleen [30]. It is expressed by numerous mammalian cell types including macrophages and dendritic cells [7,8]. The most striking function of MFG-E8 is its ability to act as a tether between phagocytes and apoptotic cells in facilitating clearance of apoptotic cells or phagocytosis. In the current study, treatment with rmMFG-E8 significantly reduced TUNEL positive cells in the lungs. It is possible that the decrease in TUNEL positive cells observed in the presence of rmMFG-E8 is due to the clearance of apoptotic cells rather than actual decrease in apoptosis in the lungs. Efficient clearance of apoptotic cells can inhibit the release of inflammatory and toxic mediators from apoptotic cells and reduce the exaggerated inflammatory response. MFG-E8 mediated apoptotic cell phagocytosis inhibit the mitogen activated protein kinase and NF-kB pathways [31]. In addition there is direct evidence that MFG-E8 binds to integrin receptors, αVβ3 or αVβ5 and inhibits the production of IL-1β in macrophages. In the current study, serum IL-1β levels were significantly reduced in neonatal mice treated with rmMFG-E8. Future studies are warranted to pinpoint the exact mechanism of rmMFG-E8 in neonatal sepsis.
Since the bacterial flora in the neonates qualitatively and quantitatively differ from that of adults, the use of the adult cecal slurry is relevant to mimic sepsis in the newborn mice. Neonatal sepsis is often caused by bacterial pathogens transmitted from the mother to infant before or during delivery. The organisms causing neonatal sepsis are colonizers of the maternal genitourinary tract leading to contamination of the amniotic fluid, placenta, cervix or the vaginal canal. Therefore, the infant acquires the pathogen either in utero or intrapartum. Although Listeria from contaminated foods and vaginal colonization of Group B streptococcus are the most common pathogens they acquire, gram negative or E coli sepsis is the common cause of mortality in the neonates [32]. Thus the transfer of bacterial flora from the mother is the common source for infection in the neonates.
There are several limitations that have to be addressed in our study. First, we only assessed organ injury and inflammation parameters at 10 h after CS injection. This time point was chosen based on our pilot experiment in which while we observed 100% mortality within 20 h using 0.9 mg/g CS, no mortality was seen until >10 h after CS injection. Therefore, as a proof of concept study, we chose a single time point, i.e., 10 h, to determine whether rmMFG-E8 treatment causes any protection in neonatal sepsis. Second, only a single dose of rmMFG-E8, 40 μg/kg, was used in the study. This dose was chosen based on our previous experiments in other adult animal organ injury models where we observed significant decreases in organ injury and inflammatory response in doses as low as 20 μg/kg [13,14]. We have also previously shown that rmMFG-E8 at a dose of 0.4 μg/20 g mouse showed significantly decreased TNF-α levels in the serum and splenic tissues in an adult endotoxemia model of sepsis [33]. In another study of adult cecal ligation and puncture model of sepsis using recombinant human MFG-E8, we observed significant protection with doses as low as 20 μg/kg. With higher doses further reductions in serum injury markers and cytokine levels were seen [13]. The current study showed significant decrease in lung injury score and decreases in circulating and lung cytokine levels indicating significant protection of the lungs with the current dose of rmMFG-E8 treatment. It is possible that higher doses of rmMFG-E8 can exert higher degrees of protection in the lungs in neonatal sepsis. Additional experiments with various doses and time points are warranted to assess optimal dosage and therapeutic window for its beneficial effects.
Third, we have not conducted survival studies of rmMFG-E8 administration in this murine model of neonatal sepsis. We have previously shown that rmMFG-E8 at the doses as low as 20 μg/kg improved survival in an adult model of polymicrobial sepsis [12]. In the current study, our pilot study showed 100% mortality within 20 h using CS dose of 0.9 mg/g. Therefore the CS dose needs to be adjusted to achieve an LD50 model prior to engaging in additional experiments. Further studies with varying doses of CS are needed in the future to determine the long term efficacy of rmMFG-E8 in ameliorating neonatal sepsis.
Another limitation of the study is that the controls in the study did not receive IP injection of the control solution, i.e., 5% dextrose. Even though it is highly unlikely that administration of 5% dextrose produce any inflammation or organ injury, it is difficult to rule out what effect does the trauma of IP injection do to the neonatal mouse in this study. Another limitation is that we have not measured circulating or tissue levels of MFG-E8 in the control or 10 h after neonatal sepsis.
However, previously we have shown that MFG-E8 levels are reduced in the serum and splenic tissues in adult sepsis [12,34]. Although the measurement of splenic levels of MFG-E8 in the current study would have been valuable, we believe that therapeutically-inducible supranormal levels of MFG-E8 would still be beneficial in neonatal sepsis. We also used retro-orbital injection route as the delivery method for rmMFG-E8. This route was chosen based on the clinical premises that the neonates suffering from sepsis would have a venous line created for continuous delivery of fluids and drugs. Additional studies with minimally invasive routes such as subcutaneous or intramuscular delivery could be useful for developing MFG-E8 as a therapeutic strategy for neonatal sepsis. Finally, studies using alternate models such as delivery of known colony forming units of specific bacterial aliquots, i.e. E coli, as opposed to CS injection are warranted for confirming the effect of rmMFG-E8 on neonatal sepsis.
Acknowledgments
This study was supported by National Institutes of Health grant, R35GM118337 to P.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was published in part as an abstract for the 39th Annual Conference on Shock, Jun 11–14, 2016, Austin, TX
References
- 1.Weiss SL, Fitzgerald JC, Pappachan J, Wheeler D, Jaramillo-Bustamante JC, Salloo A, et al. Global epidemiology of pediatric severe sepsis: the sepsis prevalence, outcomes, and therapies study. Am J Respir Crit Care Med. 2015;191:1147–57. doi: 10.1164/rccm.201412-2323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 3.Lim JC, Golden JM, Ford HR. Pathogenesis of neonatal necrotizing enterocolitis. Pediatr Surg Int. 2015;31:509–18. doi: 10.1007/s00383-015-3697-9. [DOI] [PubMed] [Google Scholar]
- 4.Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases NCfI, Respiratory Diseases CfDC, Prevention Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 5.Oeser C, Lutsar I, Metsvaht T, Turner MA, Heath PT, Sharland M. Clinical trials in neonatal sepsis. J Antimicrob Chemother. 2013;68:2733–45. doi: 10.1093/jac/dkt297. [DOI] [PubMed] [Google Scholar]
- 6.Sivanandan S, Soraisham AS, Swarnam K. Choice and duration of antimicrobial therapy for neonatal sepsis and meningitis. Int J Pediatr. 2011;2011:712150. doi: 10.1155/2011/712150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aoki N, Ishii T, Ohira S, Yamaguchi Y, Negi M, Adachi T, et al. Stage specific expression of milk fat globule membrane glycoproteins in mouse mammary gland: comparison of MFG-E8, butyrophilin, and CD36 with a major milk protein, beta-casein. Biochim Biophys Acta. 1997;1334:182–90. doi: 10.1016/s0304-4165(96)00091-8. [DOI] [PubMed] [Google Scholar]
- 8.Peterson JA, Hamosh M, Scallan CD, Ceriani RL, Henderson TR, Mehta NR, et al. Milk fat globule glycoproteins in human milk and in gastric aspirates of mother’s milk-fed preterm infants. Pediatr Res. 1998;44:499–506. doi: 10.1203/00006450-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–7. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 10.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–5. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 11.Deroide N, Li X, Lerouet D, Van Vre E, Baker L, Harrison J, et al. MFGE8 inhibits inflammasome-induced IL-1beta production and limits postischemic cerebral injury. J Clin Invest. 2013;123:1176–81. doi: 10.1172/JCI65167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miksa M, Wu R, Dong W, Komura H, Amin D, Ji Y, et al. Immature dendritic cell-derived exosomes rescue septic animals via milk fat globule epidermal growth factor-factor VIII [corrected] J Immunol. 2009;183:5983–90. doi: 10.4049/jimmunol.0802994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah KG, Wu R, Jacob A, Molmenti EP, Nicastro J, Coppa GF, et al. Recombinant human milk fat globule-EGF factor 8 produces dose-dependent benefits in sepsis. Intensive Care Med. 2012;38:128–36. doi: 10.1007/s00134-011-2353-7. [DOI] [PubMed] [Google Scholar]
- 14.Cui T, Miksa M, Wu R, Komura H, Zhou M, Dong W, et al. Milk fat globule epidermal growth factor 8 attenuates acute lung injury in mice after intestinal ischemia and reperfusion. Am J Respir Crit Care Med. 2010;181:238–46. doi: 10.1164/rccm.200804-625OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, et al. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock. 2007;28:675–83. doi: 10.1097/SHK.0b013e3180556d09. [DOI] [PubMed] [Google Scholar]
- 16.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, et al. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44:725–38. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuenca AG, Wynn JL, Moldawer LL, Levy O. Role of innate immunity in neonatal infection. Am J Perinatol. 2013;30:105–12. doi: 10.1055/s-0032-1333412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112:1750–8. doi: 10.1182/blood-2008-01-130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–91. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santana Reyes C, Garcia-Munoz F, Reyes D, Gonzalez G, Dominguez C, Domenech E. Role of cytokines (interleukin-1beta, 6, 8, tumour necrosis factor-alpha, and soluble receptor of interleukin-2) and C-reactive protein in the diagnosis of neonatal sepsis. Acta Paediatr. 2003;92:221–7. doi: 10.1111/j.1651-2227.2003.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 21.Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaeghel G, et al. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005;94:767–73. doi: 10.1093/bja/aei143. [DOI] [PubMed] [Google Scholar]
- 22.Weinberg GA, D’Angio CT. The search for new diagnostic tests for neonatal sepsis. J Pediatr. 2009;155:763–4. doi: 10.1016/j.jpeds.2009.06.034. author reply 4. [DOI] [PubMed] [Google Scholar]
- 23.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–24. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 24.Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013;93:329–42. doi: 10.1189/jlb.0912437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugitharini V, Prema A, Berla Thangam E. Inflammatory mediators of systemic inflammation in neonatal sepsis. Inflamm Res. 2013;62:1025–34. doi: 10.1007/s00011-013-0661-9. [DOI] [PubMed] [Google Scholar]
- 26.Machado JR, Soave DF, da Silva MV, de Menezes LB, Etchebehere RM, Monteiro ML, et al. Neonatal sepsis and inflammatory mediators. Mediators Inflamm. 2014;2014:269681. doi: 10.1155/2014/269681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J, Kim KD, Yang X, Auh S, Fu YX, Tang H. Hyper innate responses in neonates lead to increased morbidity and mortality after infection. Proc Natl Acad Sci U S A. 2008;105:7528–33. doi: 10.1073/pnas.0800152105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin H, Olander B, Norman M. Reactive hyperemia and interleukin 6, interleukin 8, and tumor necrosis factor-alpha in the diagnosis of early-onset neonatal sepsis. Pediatrics. 2001;108:E61. doi: 10.1542/peds.108.4.e61. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda A, Jacob A, Wu R, Zhou M, Aziz M, Wang P. Milk fat globule–EGF factor VIII ameliorates liver injury after hepatic ischemia-reperfusion. J Surg Res. 2013;180:e37–46. doi: 10.1016/j.jss.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–50. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 31.Miksa M, Amin D, Wu R, Jacob A, Zhou M, Dong W, et al. Maturation-induced down-regulation of MFG-E8 impairs apoptotic cell clearance and enhances endotoxin response. Int J Mol Med. 2008;22:743–8. [PMC free article] [PubMed] [Google Scholar]
- 32.Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27:21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aziz M, Jacob A, Matsuda A, Wu R, Zhou M, Dong W, et al. Pre-treatment of recombinant mouse MFG-E8 downregulates LPS-induced TNF-alpha production in macrophages via STAT3-mediated SOCS3 activation. PLoS One. 2011;6:e27685. doi: 10.1371/journal.pone.0027685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komura H, Miksa M, Wu R, Goyert SM, Wang P. Milk fat globule epidermal growth factor-factor VIII is down-regulated in sepsis via the lipopolysaccharide-CD14 pathway. J Immunol. 2009;182:581–7. doi: 10.4049/jimmunol.182.1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]


