Abstract
The relation between aflatoxin B1 (AFB1) and cirrhosis in chronic carriers of hepatitis B virus (HBV) remains inconclusive. This case-control study nested in a large community-based cohort aimed to assess the effect of AFB1 exposure on cirrhosis and HCC in chronic HBV carriers. Serum AFB1-albumin adduct levels at study entry were measured in 232 cirrhosis cases, 262 HCC cases and 577 controls. Multivariate-adjusted odds ratios (aORs) and 95% confidence intervals (95% CIs) were estimated using logistic regression. Among all chronic HBV carriers, the time intervals between study entry and diagnosis of HCC, cirrhosis, cirrhotic HCC, and non-cirrhotic HCC were all significantly (p<0.0001) shorter in participants with high serum levels of AFB1-albumin adducts than those with low/undetectable levels. There were significant dose-response relations with serum AFB1-albumin adduct level at study entry for cirrhosis (p-trend=0.0001) and cirrhotic HCC (p-trend<0.0001) newly-diagnosed within 9 years after entry as well as non-cirrhotic HCC (p-trend=0.021) newly-diagnosed within 4 years after entry. The aORs (95% CIs) for high vs. undetectable serum AFB1-albumin adduct levels were 2.45 (1.51–3.98) for cirrhosis (p=0.0003), 5.47 (2.20–13.63) for cirrhotic HCC (p=0.0003), and 5.39 (1.11–26.18) for non-cirrhotic (p=0.0368) HCC, respectively. There remained a significant dose-response relation between serum AFB1-albumin adduct level and HCC risk (p-trend=0.0291) in cirrhosis patients, showing an aOR (95% CI) of 3.04 (1.11–8.30) for high vs. undetectable serum levels (p=0.0299). It is concluded that AFB1 exposure may increase the risk of cirrhosis and HCC in a dose-response manner among chronic HBV carriers.
Keywords: Aflatoxin B1-albumin adducts, cirrhosis, HCC, chronic hepatitis B
Introduction
Liver cancer, primarily hepatocellular carcinoma (HCC), is the sixth most common cancer in the world and the second most common cause of cancer-related death worldwide in 2012.1 The vast majority of HCC occurs in patients affected with chronic liver disease or cirrhosis.2–4 Cirrhosis is a late-stage liver disease established in response to liver injury, featuring encapsulation or replacement of injured tissue by scar tissue. Cirrhosis patients are at a greater risk of developing HCC. In chronic hepatitis B virus (HBV) carriers, patients with cirrhosis had a significantly higher annual incidence rate of HCC than patients without cirrhosis (3.5% vs. 0.4%, p<0.0001).5 Cirrhosis led to more than one million deaths globally in 2010.6 Cirrhosis is therefore considered an emerging major global health burden.
The development of HCC is characterized by a multistage process and it usually takes decades from the initial liver damage to HCC. Exposures to various etiologic factors induce the liver injury and result in sustained cycles of necrosis, inflammation, and proliferation of hepatocytes. Repeated episodes of liver cell destruction and regeneration consequently foster an environment culminating in fibrosis and cirrhosis and establish a premalignant condition. Many other mediating events such as production of reactive oxygen species, genetic mutations, DNA damages and genetic instability ultimately contribute to the development of HCC.7
Cirrhosis and HCC share several common risk factors, including chronic infection with HBV and/or hepatitis C virus (HCV), heavy alcohol consumption, and non-alcoholic steatohepatitis (NASH)/non-alcoholic fatty liver disease (NAFLD).6, 8–11 Another risk factor for HCC is the intake of aflatoxin-contaminated food.12–16 It is estimated that four billion people are at the risk of exposure to aflatoxins globally.17 Aflatoxin B1 (AFB1), the most potent and abundant aflatoxin found in contaminated foods, exerts its carcinogenicity through its metabolic activation to the reactive AFB1 8,9-exo-epoxide by phase 1 cytochrome P450 monooxygenases. The reactive epoxide can interact with DNA to form highly mutagenic DNA lesions such as aflatoxin-N7-guanine adducts.18, 19
We have developed the measurement of AFB1-albumin adducts as a biomarker of biologically effective dose of exposure to AFB1. This biomarker reflects AFB1 exposures of liver over several months20, 21 and is stable during a long-term deep-frozen storage.22 We previously demonstrated significant associations between the serum level of AFB1-albumin adducts and the risk of HCC diagnosed within 3 and 13 years after study entry, respectively.15, 16 Study participants with both HBsAg seropositivity and high serum levels of AFB1-albumin adducts [aOR (95% CI) = 10.4 (5.7–18.8)] had a greater HCC risk than participants with HBsAg seropositivity only [aOR (95% CI) = 7.0 (4.5–11.1)] or high serum levels of AFB1-albumin adducts only [aOR (95% CI) = 1.6 (0.9–3.0)].16
A previous study suggested that aflatoxin exposure may be involved in the development of cirrhosis,23 but there was very limited epidemiological evidence supporting the causal relation between AFB1 exposure and cirrhosis. With no clear causation between AFB1 and cirrhosis, the mutational activity of AFB1 has been considered to be the primary driver of AFB1-induced HCC.7 As both AFB1 exposure and cirrhosis are primary risk factors of HCC, it remains unclear whether AFB1 also contributes to the earlier stage of HCC progression, i.e., cirrhosis.
Using a case-control study nested in a large community-based cohort with the measurement of serum AFB1-albumin adduct levels at study entry, this study on chronic HBV carriers aims to (1) evaluate the time interval between the collection of serum sample and the diagnosis of cirrhosis and HCC in which the associations between the serum AFB1-albumin adduct level and the risk of cirrhosis and HCC were statistically significant; and (2) elucidate the associations between AFB1 exposure and the risk of cirrhosis, cirrhotic HCC and non-cirrhotic HCC, respectively.
Materials and methods
Study population
All cases and controls were selected from the participants of a community-based cancer screening project (CBCSP) cohort. Briefly, The CBCSP cohort was established in 1991–1992 when 23,820 residents aged 30 to 65 years were recruited from seven townships in Taiwan. Each participant provided written informed consent. This study was approved by the Institutional Review Board of the College of Public Health, National Taiwan University, Taipei, Taiwan. Detailed information about the CBCSP cohort has been described previously.12
Study participants
From study entry in 1991–1992 to June 30, 2004, a total of 303 newly-developed cirrhosis cases were identified through regular abdominal ultrasonography examination and computerized linkage to the National Health Insurance database; and 302 newly-developed HCC cases were identified through computerized linkage with National Cancer Registry database until December 31, 2011. Because cirrhosis and HCC are both major outcomes in this study, 71 participants with cirrhosis at study entry were excluded (31 cirrhosis only cases and 40 cirrhotic HCC cases). As the cirrhosis status was ascertained only up to June 30, 2004, analyses of the associations with serum AFB1-albumin adduct levels for cirrhosis, cirrhotic HCC and non-cirrhotic HCC were confined to cases diagnosed before June 30, 2004. Cases and controls were matched on age (±5 years), gender, residence, and date of blood sample collection (±3 months). A total of 232 cirrhosis cases, 114 cirrhotic HCC cases, 66 non-cirrhotic HCC cases, and controls unaffected by cirrhosis and HCC were included in this analysis.
Data collection, serological testing and follow-up for liver cirrhosis and hepatocellular carcinoma
At the study entry, all participants underwent a health examination including abdominal ultrasonography and serological tests. Blood samples at study entry were tested for hepatitis B e antigen (HBeAg) and HBsAg by immunoassay (Abbott Laboratories), HBV DNA by the COBAS Amplicor HBV monitor test kit (Roche Diagnostics), anti-HCV by enzyme immunoassay using commercial kits (Abbott Laboratories) and alanine aminotransferase (ALT) by serum chemistry autoanalyzer (Model 736, Hitachi Co.) using commercial reagents (Biomerieux).
All participants were personally interviewed by trained public health nurses using a structured questionnaire, and information on sociodemographic characteristics, habits of alcohol consumption and cigarette smoking, and family history of major diseases was collected. During the follow-up period from study entry in 1991–1992 to June 30, 2004, participants seropositive for HBsAg or anti-HCV, with elevated serum levels of ALT, aspartate aminotransferase, or α-fetoprotein (AFP), or with a family history of HCC were regularly followed up by abdominal ultrasonography and serological tests.
Incident liver cirrhosis was identified through abdominal ultrasonography and computerized data linkage to the National Health Insurance database and medical chart reviews until June 30, 2004. Cirrhosis was determined by a quantitative scoring system deriving from (a) morphological appearance of the liver surface (normal, irregular, undulated); (b) liver parenchymal texture (normal, heterogeneous, coarse); (c) intrahepatic vascular structure (normal, obscure, narrowing); and (d) spleen size (normal, enlarged). Newly developed HCC cases were ascertained by regular health examinations including abdominal ultrasonography and AFP testing before June 30, 2004 and computerized data linkage to the National Cancer Registry database until December 31, 2011. Medical chart reviews were also performed to confirm the diagnosis of HCC according to the following criteria: (a) histopathologically confirmed HCC lesion; (b) detection of HCC lesion by two imaging techniques (abdominal ultrasonography, angiogram, or computed tomography; or (c) detection of HCC lesion by one imaging techniques in combination with elevated serum AFP level (≥400 ng/mL).
Measurement of serum AFB1-albumin adduct levels
AFB1-albumin adduct levels were measured in serum samples collected at study entry. Albumin extraction from serum and determination of AFB1-albumin adduct levels by ELISA were performed as previously described.12, 24 In brief, 50 μL of proteinase K-digested albumin extract (200 μg) was applied into 96-well plates coated with 3 ng of AFB1 epoxide-modified human serum albumin. A standard curve was prepared from serial dilution of enzymatically digested AFB1 epoxide-modified human serum albumin. Polyclonal antiserum 7 (1:2×105 dilution) and goat anti-rabbit IgG alkaline phosphatase (1:750 dilution) were used as primary and secondary antisera, respectively. Samples with <15% inhibition were considered undetectable and assigned a value of 1 fmol/mg. All samples were assayed in duplicate with laboratory staff blinded to disease status. Two controls were assayed with the test samples in each batch: a pooled sample of plasma from nonsmoking subjects from the United States and serum from a rat treated with 1.5 mg of AFB1 as negative and positive controls, respectively. The baseline serum samples were analyzed in three different batches during the follow-up period.
The percentages of samples with undetectable levels in control subjects in the three batches were 41.3%, 45.6%, and 49.7%. The median detectable adduct levels among control subjects, 21.5 fmol/mg was used as the cutoff for low (<21.5 fmol/mg) and high (≥21.5 fmol/mg) levels of AFB1-albumin adduct.
Statistical analysis
To describe the demographic and clinical features of the case and control groups, means and standard deviations were used for continuous variables, and percentages were used for categorical variables; t-tests and chi-square tests were used to examine differences in the characteristics between cases and controls for continuous variables and categorical variables, respectively. Univariate logistic regression was used to calculate the crude odds ratios (ORs) and 95% confidence intervals (CIs) for associations between the serum AFB1-albumin adduct level and the risk of cirrhosis or HCC. Variables showing significant associations (p<0.05) with serum AFB1-albumin adduct level in controls and with the outcome specified in univariate logistic regression were further incorporated into the corresponding multivariate logistic regression to estimate the adjusted ORs (aORs) and 95% CIs. To assess the dose-response relation, serum AFB1-albumin adduct level was analyzed as a continuous variable in the logistic regression model. To test for the equality of distribution of time intervals from study entry to the diagnosis of cirrhosis and HCC in participants with different AFB1 exposure levels, Kolmogorov–Smirnov test was performed. t-test was used to test the difference of time intervals from study entry to the diagnosis of cirrhosis and HCC between cases with high and low/undetectable serum levels of AFB1-albumin adducts. SAS 9.4 (Cary, NC) was employed for data management and all statistical analyses.
Results
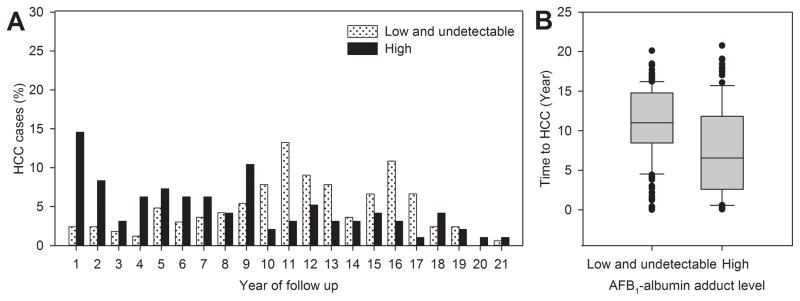
To assess the time-dependent associations between the serum level of AFB1-albumin adducts and the risk of HCC, the frequency distributions of the year at HCC diagnosis after study entry were compared between HCC cases with high and low/undetectable serum levels of AFB1-albumin adducts at study entry as shown in Figure 1. All 262 HCC cases newly diagnosed until December 31, 2011 were included in the analysis. The percentage of HCC cases newly diagnosed within 9 years after study entry was significantly higher (p<0.0001) for cases with high serum levels of AFB1-albumin adducts than those with low/undetectable levels (Figure 1A). The time intervals from study entry to HCC diagnosis were significantly shorter (p<0.0001) for those who had high levels (mean+SD, 7.5±5.6) than those with low/undetectable levels (mean+SD, 10.9±4.5) as shown in Figure 1B.
Figure 1.
Frequency distribution and box plot of time intervals from study entry to diagnosis of 262 newly-developed HCC cases by serum levels of AFB1-albumin adducts at study entry. (A) Distribution of time intervals from study entry to HCC diagnosis in cases with high or low/undetectable serum AFB1-albumin adduct levels (p<0.0001). (B) Box plot of time intervals (means ± SD) from study entry to HCC diagnosis in cases with high (7.5±5.6) and low/undetectable (10.9±4.5) serum levels of AFB1-albumin adducts (p<0.0001). In the box plot, the filled circles indicate the outliers lying outside the 10th and 90th percentiles.
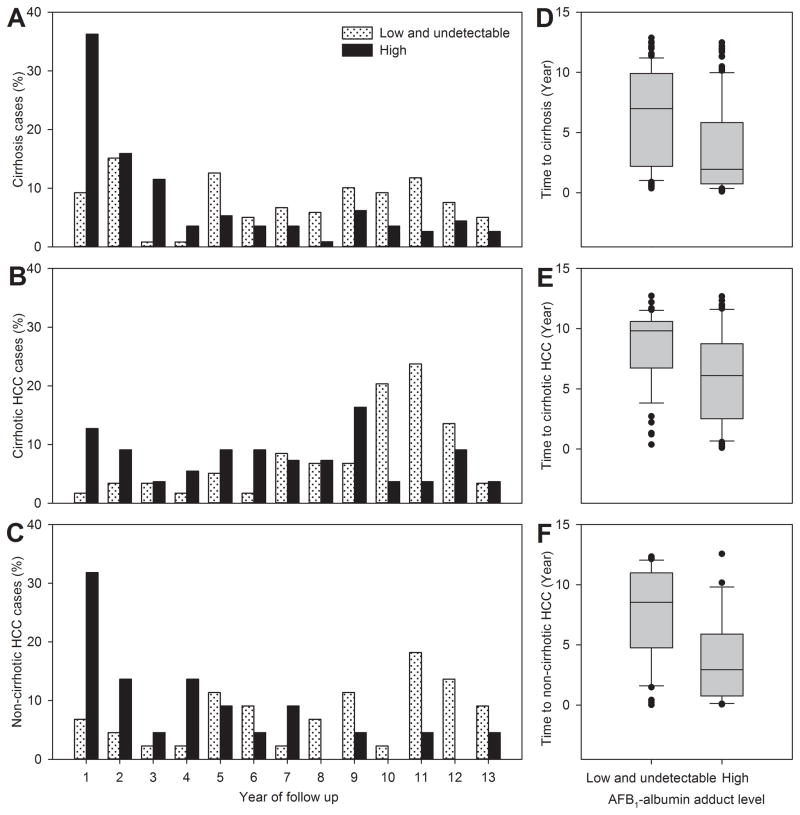
Further analyses of the time intervals from study entry to the diagnosis of newly-developed cirrhosis, cirrhotic HCC and non-cirrhotic HCC by serum level of AFB1-albumin adducts are shown in Figure 2. A total of 232 cirrhosis cases, 114 cirrhotic HCC cases and 66 non-cirrhotic HCC cases newly diagnosed until June 30, 2004 were included in the analyses. Similarly, cases with high serum levels of AFB1-albumin adducts at study entry had a significantly higher percentage of cases diagnosed in earlier years after entry than those with low/undetectable levels for cirrhosis (Figure 2A, p<0.0001), cirrhotic HCC (Figure 2B, p=0.0001), and non-cirrhotic HCC (Figure 2C, p=0.0025). The time intervals from study entry to the diagnosis of cirrhosis, cirrhotic HCC and non-cirrhotic HCC were much shorter for those who had high serum AFB1-albumin adduct levels than those with low/undetectable levels as shown in Figure 2D (mean±SD, 3.6±3.7 vs. 6.5±3.8, p<0.0001), 2E (mean±SD, 6.0±3.7 vs. 8.6±9.8, p<0.0001), and 2F (mean±SD, 3.6±3.5 vs. 7.7±3.8, p<0.0001), respectively. The time intervals from cirrhosis to cirrhotic-HCC were similar for cirrhosis patients with high and low/undetectable serum levels of AFB1-albumin adducts (mean+SD, 2.7±3.2 vs, 2.1±2.6, p=0.27).
Figure 2.
Frequency distribution and box plot of time intervals from study entry to diagnosis of newly-developed cirrhosis, cirrhotic HCC and non-cirrhotic HCC by serum levels of AFB1-albumin adducts at study entry. (A) Distribution of time intervals from study entry to cirrhosis diagnosis in cases (n=232) with high or low/undetectable serum AFB1-albumin adduct levels (p<0.0001). (B) Distribution of time intervals from study entry to diagnosis of cirrhotic HCC in cases (n=114) with high or low/undetectable serum AFB1-albumin adduct levels (p=0.0001). (C) Distribution of time intervals from study entry to diagnosis of non-cirrhotic HCC in cases (n=66) with high or low/undetectable serum AFB1-albumin adduct levels (p=0.0025). (D) Box plot of time intervals (means ± SD) from study entry to diagnosis of cirrhosis in cases with high (3.6±3.7) and low/undetectable (6.5±3.8) serum levels of AFB1-albumin adducts (p<0.0001). (E) Box plot of time intervals (means ± SD) from study entry to diagnosis of cirrhotic HCC in cases with high (6.0±3.7) and low/undetectable (8.6±9.8) serum levels of AFB1-albumin adducts (p<0.0001). (F) Box plot of time intervals (means ± SD) from study entry to diagnosis of non-cirrhotic HCC in cases with high (3.6±3.5) and low/undetectable (7.7±3.8) serum levels of AFB1-albumin adducts (p<0.0001). In the box plot, the filled circles indicate the outliers lying outside the 10th and 90th percentiles.
Table 1 shows the comparisons of the associations with serum AFB1-albumin adduct levels for HCC, cirrhosis, cirrhotic HCC and non-cirrhotic HCC by the year of disease onset since study entry. During a long follow-up period of 20 years, the association between the serum AFB1-albumin adduct level at study entry and the risk of HCC was not statistically significant for all HCC cases (n=262) newly developed from 1991 to 2011. But there was a significant dose-response relation with between the level of serum AFB1-albumin adducts and the risk of HCC newly diagnosed within 9 years (n=112) after study entry (p-trend=0.0007). High serum AFB1-albumin adduct levels were significantly associated with an increased risk of HCC (crude OR = 2.17; 95% CI = 1.26–3.73) compared with the undetectable levels.
Table 1.
Associations between the serum aflatoxin B1 (AFB1)-albumin adducts level at study entry and the risk of hepatocellular carcinoma and cirrhosis in chronic hepatitis B virus carriers by the year of disease onset
| AFB1-albumin level (fmol/mg) | Control (%) (n=577) |
Case (%) | Crude OR (95% CI) | p-trenda | |
|---|---|---|---|---|---|
| HCC | |||||
|
| |||||
| Cases diagnosed between 1991–2011 (n=262) | Undetectable | 154 (26.7) | 81 (30.9) | 1.00 (Referent) | 0.13 |
| Low (<21.5) | 196 (34.0) | 85 (32.4) | 0.83 (0.57–1.19) | ||
| High (≥21.5) | 227 (39.3) | 96 (36.6) | 0.80 (0.56–1.15) | ||
|
| |||||
| Cases diagnosed in 9 years of follow-up (n=112) | Undetectable | 154 (26.7) | 20 (17.9) | 1.00 (Referent) | 0.0007 |
| Low (<21.5) | 196 (34.0) | 28 (25.0) | 1.10 (0.60–2.03) | ||
| High (≥21.5) | 227 (39.3) | 64 (57.1) | 2.17 (1.26–3.73)** | ||
|
| |||||
| Cirrhosis | |||||
|
| |||||
| Cases diagnosed between 1991–2004 (n=232) | Undetectable | 154 (26.7) | 48 (20.7) | 1.00 (Referent) | 0.0068 |
| Low (<21.5) | 196 (34.0) | 71 (30.6) | 1.16 (0.76–1.77) | ||
| High (≥21.5) | 227 (39.3) | 113 (48.7) | 1.60 (1.08–2.37)* | ||
|
| |||||
| Cases diagnosed in 9 years of follow-up (n=177) | Undetectable | 154 (26.7) | 29 (16.4) | 1.00 (Referent) | <0.0001 |
| Low (<21.5) | 196 (34.0) | 50 (28.3) | 1.36 (0.82–2.24) | ||
| High (≥21.5) | 227 (39.3) | 98 (55.4) | 2.29 (1.44–3.64)*** | ||
|
| |||||
| Cirrhotic HCC | |||||
|
| |||||
| Cases diagnosed between 1991–2004 (n=114) | Undetectable | 154 (26.7) | 27 (23.7) | 1.00 (Referent) | 0.0745 |
| Low (<21.5) | 196 (34.0) | 32 (28.1) | 0.93 (0.54–1.62) | ||
| High (≥21.5) | 227 (39.3) | 55 (48.3) | 1.38 (0.84–2.29) | ||
|
| |||||
| Cases diagnosed in 9 years of follow-up (n=67) | Undetectable | 154 (26.7) | 6 (9.0) | 1.00 (Referent) | <0.0001 |
| Low (<21.5) | 196 (34.0) | 17 (25.4) | 2.23 (0.86–5.78) | ||
| High (≥21.5) | 227 (39.3) | 44 (65.7) | 4.98 (2.07–11.96)*** | ||
|
| |||||
| Non-cirrhotic HCC | |||||
|
| |||||
| Cases diagnosed between 1991–2004 (n=66) | Undetectable | 154 (26.7) | 23 (34.9) | 1.00 (Referent) | 0.0881 |
| Low (<21.5) | 196 (34.0) | 21 (31.8) | 0.72 (0.38–1.34) | ||
| High (≥21.5) | 227 (39.3) | 22 (33.3) | 0.65 (0.35–1.21) | ||
|
| |||||
| Cases diagnosed in 4 years of follow-up (n=21) | Undetectable | 154 (26.7) | 2 (9.5) | 1.00 (Referent) | 0.0063 |
| Low (<21.5) | 196 (34.0) | 5 (23.8) | 1.96 (0.38–10.26) | ||
| High (≥21.5) | 227 (39.3) | 14 (66.7) | 4.75 (1.06–21.19)* | ||
p<0.05,
p<0.01,
p<0.001.
One-sided p-value for Cochran-Armitage trend test.
Low/high vs. undetectable.
High vs. undetectable/low.
There was a significant dose-response relation between the serum level of AFB1-alubumin adducts at study entry and the risk of cirrhosis newly-diagnosed from 1991 to 2004 (n=232) with the p-trend of 0.0068 and a crude OR (95% CI) of 1.60 (1.08–2.37) for high levels of AFB1-alubumin adducts vs. the undetectable levels. For cirrhosis diagnosed in 9 years of follow-up (n=177), the dose-response relation was more striking (p-trend<0.0001) with a crude OR (95% CI) of 2.29 (1.44–3.64) for high levels of AFB1-alubumin adducts vs. the undetectable levels.
When stratifying 180 HCC cases diagnosed before June 30, 2004 into 114 cirrhotic HCC cases and 66 non-cirrhotic HCC cases, the serum AFB1-albumin adduct levels were not significantly associated with the long-term risk of HCC. However, significant dose-relations with serum AFB1-albumin adduct levels were observed for cirrhotic HCC within 9 years (n=67) of follow-up and non-cirrhotic HCC within 4 years (n=21) of follow-up with the p-trends of <0.0001 and 0.0063, respectively.
Further analyses were carried out to estimate multivariate-adjusted OR of the serum level of AFB1-albumin adducts at study entry for cirrhosis, and cirrhotic HCC developed within 9 years of follow-up, and non-cirrhotic HCC developed within 4 years of follow-up with adjustment for other major risk factors. Table 2 shows the frequency distributions of major risk factors collected at study entry of 577 control participants unaffected with cirrhosis or HCC, 110 cirrhosis only cases, 67 cirrhotic HCC cases, and 21 non-cirrhotic HCC cases. Compared with control participants, newly-diagnosed cases of cirrhosis, and cirrhotic HCC had higher percentages of HBeAg seropositivity and higher serum levels of ALT, HBsAg, and HBV DNA; and non-cirrhotic HCC cases had higher percentages of cigarette smoking, alcohol drinking, high body mass index, family history of HCC, HBeAg seropositivity, and elevated serum levels of ALT and HBV DNA. The percentage of high serum AFB1-alubmin adduct levels was higher in cases of cirrhosis, cirrhotic HCC and non-cirrhotic HCC than controls (49.1%, 65.7%, 66.7% vs. 39.3%).
Table 2.
Baseline characteristics of chronic hepatitis B virus carriers affected and unaffected with cirrhosis and/or hepatocellular carcinoma
| Baseline characteristics | Cirrhosis
|
Non-cirrhotic HCC in 4 years of follow-up (n=21) No. (%) |
||
|---|---|---|---|---|
| Control (n=577) No. (%) |
Cirrhosis only in 9 years of follow-up (n=110) No. (%) |
Cirrhotic HCC in 9 years of follow-up (n=67) No. (%) |
||
| Cigarette smokinga | ||||
| No | 350 (60.9) | 66 (60.0) | 37 (55.2) | 10 (47.6) |
| Yes | 225 (39.1) | 44 (40.0) | 30 (44.8) | 11 (52.4) |
| Alcohol drinkingb | ||||
| No | 491 (85.5) | 97 (88.2) | 56 (83.6) | 13 (65.0) |
| Yes | 83 (14.5) | 13 (11.8) | 11 (16.4) | 7 (35.0) |
| Body mass index (kg/m2)c | ||||
| <27 | 456 (79.0) | 82 (74.6) | 57 (85.1) | 0 (0.0) |
| ≥27 | 121 (21.0) | 28 (25.5) | 10 (14.9) | 21 (100.0) |
| Family history of HCC | ||||
| No | 548 (95.0) | 103 (93.6) | 62 (92.5) | 18 (85.7) |
| Yes | 29 (5.0) | 7 (6.4) | 5 (7.5) | 3 (14.3) |
| Serum ALT level (U/L) | ||||
| <45 | 552 (95.7) | 87 (79.1) | 57 (85.1) | 16 (76.2) |
| ≥45 | 25 (4.3) | 23 (20.9) | 10 (14.9) | 5 (23.8) |
| Anti-HCVd | ||||
| Negative | 532 (92.2) | 99 (90.0) | 61 (92.4) | 19 (95.0) |
| Positive | 45 (7.8) | 11 (10.0) | 5 (7.6) | 1 (5.0) |
| HBeAg seropositivitye | ||||
| No | 489 (86.9) | 63 (58.3) | 35 (55.6) | 14 (73.7) |
| Yes | 74 (13.1) | 45 (41.7) | 28 (44.4) | 5 (26.3) |
| HBsAg level (IU/mL)f | ||||
| <1000 | 323 (62.5) | 20 (21.1) | 15 (40.5) | 5 (62.5) |
| ≥1000 | 194 (37.5) | 75 (79.0) | 22 (59.5) | 3 (37.5) |
| HBV DNA level (copies/mL)g | ||||
| <10000 | 364 (66.8) | 16 (16.7) | 7 (13.2) | 4 (30.8) |
| ≥10000 | 181 (33.2) | 80 (83.3) | 46 (86.8) | 9 (69.2) |
| AFB1-albumin adducts (fmol/mg) | ||||
| Undetectable | 154 (26.7) | 23 (20.9) | 6 (9.0) | 2 (9.5) |
| Low (<21.5) | 196 (34.0) | 33 (30.0) | 17 (25.4) | 5 (23.8) |
| High (≥21.5) | 227 (39.3) | 54 (49.1) | 44 (65.7) | 14 (66.7) |
2 controls were missing for baseline smoking status.
3 controls and 1 non-cirrhotic HCC case were missing for baseline drinking status.
1 cirrhosis only case was missing for baseline BMI data.
1 cirrhotic HCC case, and 1 non-cirrhotic HCC case were missing for baseline anti-HCV serostatus.
14 controls, 2 cirrhosis only cases, 4 cirrhotic HCC cases, and 2 non-cirrhotic HCC cases were missing for baseline serostatus of HBeAg.
60 controls, 15 cirrhosis only cases, 30 cirrhotic HCC cases, and 13 non-cirrhotic HCC cases were missing for baseline serum HBsAg level.
32 controls, 14 cirrhosis cases, 14 cirrhotic HCC cases, and 8 non-cirrhotic HCC cases were missing for baseline serum HBV DNA level.
Table 3 shows the univariate analyses of risk factors for cirrhosis, cirrhotic HCC and non-cirrhotic HCC. Elevated serum levels of ALT and HBV DNA were significantly associated with an increased risk of cirrhosis, cirrhotic HCC and non-cirrhotic HCC. While HBeAg seropositivity and serum HBsAg level were significantly associated with cirrhosis and cirrhotic HCC, but not with non-cirrhotic HCC; alcohol drinking was significantly associated with non-cirrhotic HCC, but not with cirrhosis and cirrhotic HCC.
Table 3.
Univariate analysis of risk factors for cirrhosis, cirrhotic hepatocellular carcinoma (HCC) and non-cirrhotic HCC among chronic hepatitis B virus carriers
| Baseline characteristics | Crude odds ratio (95% Confidence interval) | |||
|---|---|---|---|---|
|
| ||||
| Cases developed cirrhosis/cirrhotic-HCC in 9 years of follow-up | Cases developed non-cirrhotic-HCC in 4 years of follow-up | |||
|
| ||||
| Cirrhosis vs. control | Cirrhotic HCC vs. cirrhosis only | Cirrhotic HCC vs. control | Non-cirrhotic HCC vs. control | |
| Cigarette smoking | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Yes | 1.12 (0.79–1.57) | 1.22 (0.66–2.25) | 1.26 (0.76–2.10) | 1.71 (0.72–4.10) |
| Alcohol drinking | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Yes | 0.93 (0.57–1.51) | 1.47 (0.62–3.49) | 1.16 (0.59–2.31) | 3.19 (1.24–8.22)* |
| Body mass index (kg/m2) | ||||
| <27 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | N.A. |
| ≥27 | 1.03 (0.68–1.55) | 0.51 (0.23–1.14) | 0.66 (0.33–1.33) | N.A. |
| Family history of HCC | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Yes | 1.37 (0.69–2.75) | 1.19 (0.36–3.90) | 1.52 (0.57–4.08) | 3.15 (0.88–11.31) |
| Serum alanine ALT level (U/L) | ||||
| <45 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| ≥45 | 5.06 (2.92–8.78)*** | 0.66 (0.29–1.50) | 3.87 (1.77–8.47)** | 6.90 (2.34–20.34)*** |
| Anti-HCV | ||||
| Negative | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Positive | 1.18 (0.65–2.15) | 0.74 (0.25–2.23) | 0.97 (0.37–2.53) | 0.62 (0.08–4.76) |
| HBeAg seropositivity | ||||
| No | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Yes | 4.92 (3.34–7.27)*** | 1.12 (0.60–2.10) | 5.29 (3.04–9.20)*** | 2.36 (0.83–6.75) |
| HBsAg level (IU/mL) | ||||
| <1000 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| ≥1000 | 4.61 (3.02–7.06)*** | 0.39 (0.17–0.89)* | 2.44 (1.24–4.82)* | 1.00 (0.24–4.23) |
| HBV DNA level (copies/mL) | ||||
| <10000 | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| ≥10000 | 11.02 (6.83–17.78)*** | 1.31 (0.50–3.43) | 13.21 (5.85–29.85)*** | 4.53 (1.38–14.89)* |
| AFB1-albumin adducts (fmol/mg) | ||||
| Undetectable | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) |
| Low (<21.5) | 1.36 (0.82–2.24) | 1.98 (0.68–5.77) | 2.23 (0.86–5.78) | 1.96 (0.38–10.26) |
| High (≥21.5) | 2.29 (1.44–3.64)** | 3.12 (1.17–8.35)* | 4.98 (2.07–11.96)** | 4.75 (1.06–21.19)* |
| (p-value for trend) | <0.0001 | 0.0072 | <0.0001 | 0.0063 |
p<0.05,
p<0.01,
p<0.001.
There was a significant dose-response relation of serum AFB1-albumin adduct level with the risk of cirrhosis (p-trend<0.0001), cirrhotic HCC (p-trend<0.0001), and non-cirrhotic HCC (p-trend=0.0063). The crude ORs (95% CIs) for high serum levels compared to undetectable levels were 2.29 (1.44–3.64), 4.98 (2.07–11.96), and 4.75 (1.06–21.19) for cirrhosis, cirrhotic HCC and non-cirrhotic HCC, respectively.
In the comparison between cirrhosis and cirrhotic HCC cases, serum level of AFB1-albumin adducts was associated with an increased risk of developing cirrhotic HCC showing a dose-response relation [high vs. undetectable: crude OR (95% CI) = 3.12 (1.17–8.35); p-trend=0.0072].
As serum AFB1-albumin adduct level was significantly associated with cigarette smoking, alcohol drinking, and serum ALT level in control participants (Supplementary Table 1), they were considered as potential confounders and included in the multivariate analyses shown in Table 4. After adjusting for age, gender and other significant risk factors, the serum AFB1-albumin adduct level was significantly associated with an increased risk of cirrhosis, cirrhotic HCC and non-cirrhotic HCC in a dose-dependent manner (p-trend=0.0001, <0.0001 and 0.021, respectively). The multivariate-adjusted OR (95% CI) for high vs. undetectable serum levels of AFB1-albumin adducts was 2.45 (1.51–3.98) for cirrhosis, 5.47 (2.20–13.63) for cirrhotic HCC, and 5.39 (1.11–26.18) for non-cirrhotic HCC. In the comparison between cirrhotic HCC cases and cirrhosis only cases, serum level of AFB1-albumin adducts was significantly associated with an increased cirrhotic HCC risk showing a dose-response relation (p-trend=0.0291) with an OR (95% CI) of 3.04 (1.11–8.30) for high vs. undetectable levels.
Table 4.
Multivariate-adjusted odds ratios of developing cirrhosis, cirrhotic hepatocellular carcinoma (HCC) and non-cirrhotic HCC for serum aflatoxin B1 (AFB1)-albumin adducts levels among chronic hepatitis B virus carriers
| AFB1-albumin adducts (fmol/mg) | Multivariate-adjusted odds ratio (95% Confidence interval)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Cirrhosisa vs. controlb | p-value | Cirrhotic HCCa vs. cirrhosis onlyc | p-value | Cirrhotic HCCa vs. controlb | p-value | Non-cirrhotic HCCa vs. controld | p-value | |
| Undetectable | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | 1.00 (Referent) | ||||
| Low (<21.5) | 1.47 (0.87–2.48) | 0.15 | 2.20 (0.73–6.62) | 0.16 | 2.53 (0.95–6.73) | 0.06 | 2.38 (0.44–12.96) | 0.3166 |
| High (≥21.5) | 2.45 (1.51–3.98) | 0.0003 | 3.04 (1.11–8.30) | 0.0299 | 5.47 (2.20–13.63) | 0.0003 | 5.39 (1.11–26.18) | 0.0368 |
| (p-value for trend) | 0.0001 | 0.0291 | <0.0001 | 0.021 | ||||
Cirrhosis and cirrhotic HCC developed in 9 years of follow-up and non-cirrhotic HCC developed in 4 years of follow-up.
Adjusted for other significant risk factors including age, gender, and serum alanine aminotransferase level.
Adjusted for other significant risk factors including age and gender.
Adjusted for other significant risk factors including age, gender, alcohol drinking and serum alanine aminotransferase level.
Discussion
In this nested case-control study on cirrhosis and HCC in chronic HBV carriers, the associations between the serum level of AFB1-albumin adducts at study entry and the newly-developed cirrhosis and HCC were assessed. Cases and unaffected controls were selected from the CBCSP cohort which had been followed for 20 years from 1991 to 2011. In Taiwan, people had been exposed to aflatoxin through consumption of aflatoxin-contaminated foods, particularly peanut products.25, 26 Although the dietary intake of AFB1 of study participants was not measured, serum AFB1-albumin adduct levels were measured to reflect the biologically effective dose of AFB1 exposure in liver over past months.27 With a half-life of 21 days, AFB1-albumin adducts are the most reliable and feasible long-term marker for AFB1 exposure in humans.27, 28 While no significant associations between the serum AFB1-albumin adduct level at study entry and the risk of HCC newly developed in the entire follow-up period of 20 years; a significant dose-response relation with the baseline serum AFB1-albumin adduct level was observed for HCC cases diagnosed within 9 years after study entry. There seemed to be a time-dependent effect of AFB1 on the development of HCC. In other words, AFB1 exposure in recent 9 years may predict the development of HCC in chronic HBV carriers, but not the AFB1 exposure 10 or more years ago. The failure for AFB1 exposure to predict the risk of HCC developed 10 or more years later may be explained by (1) A single measurement of serum AFB1-albumin adduct level may not accurately reflect the cumulative AFB1 exposure over a long follow-up period due to the change in diet intake or other environmental exposure of AFB1. Repeated measurement of AFB1-albumin adduct levels in serial serum samples collected at different follow-up time points will be needed to clarify this limitation. (2) The hepatocarcinogenic effect of AFB1 may be more potent at the late stage of disease progression of chronic hepatitis B, i.e., the existence of cirrhosis. In other words, AFB1 exposure at the early stage of chronic hepatitis B progression may have less impact on the HCC development due to the normal liver function to repair genetic damages induced by AFB1. (3) AFB1 exposure may accelerate the progression of chronic hepatitis B to cirrhosis and lead to the development of cirrhotic HCC.
The hepatocarcinogenic effect of AFB1 is well-documented, but whether AFB1 may contribute to the development of cirrhosis in humans still awaits elucidation. Owing to the difficulties in the recruitment of cirrhosis cases, the research on the effect of AFB1 on cirrhosis is very limited. In this study, we found elevated serum AFB1-albumin adduct levels at study entry were significantly associated with an increased risk of developing cirrhosis and cirrhotic HCC; high serum levels of AFB1-albumin adducts were associated with a greater odds ratio of developing cirrhotic HCC than that of developing cirrhosis; and the serum AFB1-albumin adduct level was associated with the risk of developing HCC in a dose-dependent manner in cirrhosis patients. In addition, the dose-response relation between the serum AFB1-albumin adduct level and the risk of cirrhosis and cirrhotic HCC remained statistically significant in the stratification analyses by other major risk factors including age, gender, HBeAg serostatus and serum levels of ALT, HBsAg and HBV DNA. These findings together suggest that AFB1 exposure may contribute not only to the development of cirrhosis, but also to the development of HCC even after cirrhosis is established.
Until now, only one epidemiological study reported the detrimental impact of aflatoxin on cirrhosis. A case-control study in Gambia found a significant association between cirrhosis and aflatoxin exposure assessed by lifetime groundnut intake and plasma TP53 R249S mutations.23 Although different biomarkers of aflatoxin exposure were used, our findings are in consistence with this previous report on the effect of aflatoxin on the development of cirrhosis. The consumption of aflatoxin-contaminated peanut meal was reported to induce different degrees of hepatic lesions including formation of fatty cysts, fibrosis, and cirrhosis among children in a clinical study.29 In animal studies, administration of aflatoxin or aflatoxin poisoning lead to liver cell damage,30–32 mononuclear cell infiltration,30, 33, 34 and periportal fibrosis in the liver.30–34 The underlying mechanism of aflatoxin-induced cirrhosis remains unclear. However, a recent study proposed that myofibroblasts may be involved in the fibrosis-associated liver damage induced by AFB1 exposure.35 Molecular mechanisms involved in aflatoxin-induced carcinogenesis including formation of DNA and protein adducts, and also lipid peroxidation have been proposed.36
Furthermore, the baseline serum level of AFB1-albumin adducts was also significantly associated with non-cirrhotic HCC developed within 4 years after study entry. In other words, AFB1 may cause HCC directly without inducing cirrhosis. Based on our findings and previous evidence, it is possible that AFB1 plays a dual role in hepatocarcinogenesis: (1) AFB1 acts as a procarcinogen to induce DNA damages after being activated into AFB1–8,9 epoxide; (2) AFB1 acts as a liver damaging agent to induce the pathogenesis of cirrhosis, rendering the liver cells more vulnerable to AFB1-induced carcinogenesis.
The finding of short time interval from study entry to development of cirrhosis and HCC in chronic HBV carriers with high AFB1 exposure suggests that AFB1 exposure may accelerate the disease progression of chronic hepatitis B. A study examining the immunological parameters of Ghanaians in relation to the AFB1 exposure level demonstrated that high AFB1-albumin adduct levels were associated with impairment in cellular immunity.37 AFB1 exposure could probably cause immune suppression and render the exposed chronic HBV carriers more susceptible to progression to cirrhosis and HCC. However, it would be arbitrary to draw the conclusion by our current data with a single measurement. It is to be noted that in cirrhotic HCC patients, higher AFB1 exposure resulted in a shortened induction period of cirrhotic-HCC due to a more rapid progression to cirrhosis rather than to HCC. It may be possible that AFB1 exposure may not only accelerates the progression of chronic hepatitis B into cirrhosis, but also increase the risk of developing HCC in cirrhosis patients.
To the best of our knowledge, this study is the first follow-up study on the association between the risk of both cirrhosis and HCC and the AFB1 exposure with the adjustment of potential confounders. Among chronic HBV carriers, the serum level of AFB1-albumin adducts was found to be an independent predictor of cirrhosis and HCC developed within 9 years after study entry. In addition, the onset of cirrhosis and HCC was more rapid in chronic HBV carriers with high levels of AFB1 exposure. These findings together not only suggest that AFB1 may increase the risk of cirrhosis but also reveal a complicated role of AFB1 in the etiology of hepatocarcinogenesis.
Supplementary Material
Novelty and impact.
This is the first follow-up study evaluating the impact of AFB1 exposure on both liver cirrhosis and HCC. We found AFB1 exposure increases the risk of developing cirrhosis and HCC, and may accelerate the progression of chronic hepatitis B to cirrhosis and HCC. Our study suggests the complexity in the etiology of AFB1-induced HCC. AFB1 may not only contribute to hepatocarcinogenesis directly, but also increase the risk of liver cirrhosis, thereby rendering liver cells more vulnerable to AFB1-induced carcinogenesis.
Acknowledgments
Analysis of aflatoxin B1-albumin adduct levels was supported by NIH grants R01ES05115 and P30ES009089.
Abbreviations
- AFB1
aflatoxin B1
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto M. Pathology of early hepatocellular carcinoma. Hepatol Res. 2007;37(Suppl 2):S135–8. doi: 10.1111/j.1872-034X.2007.00176.x. [DOI] [PubMed] [Google Scholar]
- 3.Simonetti RG, Camma C, Fiorello F, Politi F, D’Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962–72. doi: 10.1007/BF01297149. [DOI] [PubMed] [Google Scholar]
- 4.Sugimura T. Multistep carcinogenesis: a 1992 perspective. Science. 1992;258:603–7. doi: 10.1126/science.1411570. [DOI] [PubMed] [Google Scholar]
- 5.Do AL, Wong CR, Nguyen LH, Nguyen VG, Trinh H, Nguyen MH. Hepatocellular carcinoma incidence in noncirrhotic patients with chronic hepatitis B and patients with cirrhosis of all etiologies. J Clin Gastroenterol. 2014;48:644–9. doi: 10.1097/MCG.0000000000000015. [DOI] [PubMed] [Google Scholar]
- 6.Mokdad AA, Lopez AD, Shahraz S, Lozano R, Mokdad AH, Stanaway J, Murray CJ, Naghavi M. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6:674–87. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 8.Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19–37. doi: 10.2147/HMER.S16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–55. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 10.Sanyal AJ, Yoon SK, Lencioni R. The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist. 2010;15(Suppl 4):14–22. doi: 10.1634/theoncologist.2010-S4-14. [DOI] [PubMed] [Google Scholar]
- 11.Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838–51. doi: 10.1016/S0140-6736(08)60383-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CJ, Wang LY, Lu SN, Wu MH, You SL, Zhang YJ, Wang LW, Santella RM. Elevated aflatoxin exposure and increased risk of hepatocellular carcinoma. Hepatology. 1996;24:38–42. doi: 10.1002/hep.510240108. [DOI] [PubMed] [Google Scholar]
- 13.Qian GS, Ross RK, Yu MC, Yuan JM, Gao YT, Henderson BE, Wogan GN, Groopman JD. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3:3–10. [PubMed] [Google Scholar]
- 14.Ross RK, Yuan JM, Yu MC, Wogan GN, Qian GS, Tu JT, Groopman JD, Gao YT, Henderson BE. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet. 1992;339:943–6. doi: 10.1016/0140-6736(92)91528-g. [DOI] [PubMed] [Google Scholar]
- 15.Wang LY, Hatch M, Chen CJ, Levin B, You SL, Lu SN, Wu MH, Wu WP, Wang LW, Wang Q, Huang GT, Yang PM, Lee HS, Santella RM. Aflatoxin exposure and risk of hepatocellular carcinoma in Taiwan. Int J Cancer. 1996;67:620–5. doi: 10.1002/(SICI)1097-0215(19960904)67:5<620::AID-IJC5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 16.Wu HC, Wang Q, Yang HI, Ahsan H, Tsai WY, Wang LY, Chen SY, Chen CJ, Santella RM. Aflatoxin B1 exposure, hepatitis B virus infection, and hepatocellular carcinoma in Taiwan. Cancer Epidemiol Biomarkers Prev. 2009;18:846–53. doi: 10.1158/1055-9965.EPI-08-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams JH, Phillips TD, Jolly PE, Stiles JK, Jolly CM, Aggarwal D. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. Am J Clin Nutr. 2004;80:1106–22. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 18.Raney VM, Harris TM, Stone MP. DNA conformation mediates aflatoxin B1-DNA binding and the formation of guanine N7 adducts by aflatoxin B1 8,9-exo-epoxide. Chem Res Toxicol. 1993;6:64–8. doi: 10.1021/tx00031a010. [DOI] [PubMed] [Google Scholar]
- 19.Wild CP, Turner PC. The toxicology of aflatoxins as a basis for public health decisions. Mutagenesis. 2002;17:471–81. doi: 10.1093/mutage/17.6.471. [DOI] [PubMed] [Google Scholar]
- 20.Groopman JD, Zhu JQ, Donahue PR, Pikul A, Zhang LS, Chen JS, Wogan GN. Molecular dosimetry of urinary aflatoxin-DNA adducts in people living in Guangxi Autonomous Region, People’s Republic of China. Cancer Res. 1992;52:45–52. [PubMed] [Google Scholar]
- 21.Wild CP, Hudson GJ, Sabbioni G, Chapot B, Hall AJ, Wogan GN, Whittle H, Montesano R, Groopman JD. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol Biomarkers Prev. 1992;1:229–34. [PubMed] [Google Scholar]
- 22.Scholl PF, Groopman JD. Long-term stability of human aflatoxin B1 albumin adducts assessed by isotope dilution mass spectrometry and high-performance liquid chromatography-fluorescence. Cancer Epidemiol Biomarkers Prev. 2008;17:1436–9. doi: 10.1158/1055-9965.EPI-07-2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuniholm MH, Lesi OA, Mendy M, Akano AO, Sam O, Hall AJ, Whittle H, Bah E, Goedert JJ, Hainaut P, Kirk GD. Aflatoxin exposure and viral hepatitis in the etiology of liver cirrhosis in the Gambia, West Africa. Environ Health Perspect. 2008;116:1553–7. doi: 10.1289/ehp.11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wild CP, Jiang YZ, Sabbioni G, Chapot B, Montesano R. Evaluation of methods for quantitation of aflatoxin-albumin adducts and their application to human exposure assessment. Cancer Res. 1990;50:245–51. [PubMed] [Google Scholar]
- 25.Chen Y-C, Liao C-D, Lin H-Y, Chiueh L-C, Shih DY-C. Survey of aflatoxin contamination in peanut products in Taiwan from 1997 to 2011. J Food Drug Anal. 2013;21:247–52. [Google Scholar]
- 26.Chen M-T, Hsu Y-H, Wang T-S, Chien S-W. Mycotoxin monitoring for commercial foodstuffs in Taiwan. J Food Drug Anal. 2016;24:147–56. doi: 10.1016/j.jfda.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makarananda K, Pengpan U, Srisakulthong M, Yoovathaworn K, Sriwatanakul K. Monitoring of aflatoxin exposure by biomarkers. J Toxicol Sci. 1998;23(Suppl 2):155–9. doi: 10.2131/jts.23.supplementii_155. [DOI] [PubMed] [Google Scholar]
- 28.Skipper PL, Tannenbaum SR. Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis. 1990;11:507–18. doi: 10.1093/carcin/11.4.507. [DOI] [PubMed] [Google Scholar]
- 29.Amla I, Kamala CS, Gopalakrishna GS, Jayaraj AP, Sreenivasamurthy V, Parpia HA. Cirrhosis in children from peanut meal contaminated by aflatoxin. Am J Clin Nutr. 1971;24:609–14. doi: 10.1093/ajcn/24.6.609. [DOI] [PubMed] [Google Scholar]
- 30.Karaman M, Ozen H, Tuzcu M, Cigremis Y, Onder F, Ozcan K. Pathological, biochemical and haematological investigations on the protective effect of alpha-lipoic acid in experimental aflatoxin toxicosis in chicks. Br Poult Sci. 2010;51:132–41. doi: 10.1080/00071660903401839. [DOI] [PubMed] [Google Scholar]
- 31.Seffner W, Schiller F, Lippold U, Dieter HH, Hoffmann A. Experimental induction of liver fibrosis in young guinea pigs by combined application of copper sulphate and aflatoxin B1. Toxicol Lett. 1997;92:161–72. doi: 10.1016/s0378-4274(97)00052-0. [DOI] [PubMed] [Google Scholar]
- 32.Wogan GN. Chemical nature and biological effects of the aflatoxins. Bacteriol Rev. 1966;30:460–70. doi: 10.1128/br.30.2.460-470.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortatatli M, Oguz H, Hatipoglu F, Karaman M. Evaluation of pathological changes in broilers during chronic aflatoxin (50 and 100 ppb) and clinoptilolite exposure. Res Vet Sci. 2005;78:61–8. doi: 10.1016/j.rvsc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Wouters AT, Casagrande RA, Wouters F, Watanabe TT, Boabaid FM, Cruz CE, Driemeier D. An outbreak of aflatoxin poisoning in dogs associated with aflatoxin B1-contaminated maize products. J Vet Diagn Invest. 2013;25:282–7. doi: 10.1177/1040638713477409. [DOI] [PubMed] [Google Scholar]
- 35.Arana S, Alves VA, Sabino M, Tabata YA, Nonogaki S, Zaidan-Dagli ML, Hernandez-Blazquez FJ. Immunohistochemical evidence for myofibroblast-like cells associated with liver injury induced by aflatoxin B1 in rainbow trout (Oncorhynchus mykiss) J Comp Pathol. 2014;150:258–65. doi: 10.1016/j.jcpa.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 36.Moudgil V, Redhu D, Dhanda S, Singh J. A review of molecular mechanisms in the development of hepatocellular carcinoma by aflatoxin and hepatitis B and C viruses. J Environ Pathol Toxicol Oncol. 2013;32:165–75. doi: 10.1615/jenvironpatholtoxicoloncol.2013007166. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y, Jolly PE, Ellis WO, Wang JS, Phillips TD, Williams JH. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. Int Immunol. 2005;17:807–14. doi: 10.1093/intimm/dxh262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.