Abstract
Objective:
To determine the prevalence of diffusion-weighted imaging (DWI)–negative acute ischemic stroke (AIS) and to identify clinical characteristics of patients with DWI-negative AIS.
Methods:
We systematically searched PubMed and Ovid/MEDLINE for relevant studies between 1992, the year that the DWI sequence entered clinical practice, and 2016. Studies were included based upon enrollment of consecutive patients presenting with a clinical diagnosis of AIS prior to imaging. Meta-analysis was performed to synthesize study-level data, estimate DWI-negative stroke prevalence, and estimate the odds ratios (ORs) for clinical characteristics associated with DWI-negative stroke.
Results:
Twelve articles including 3,236 AIS patients were included. The meta-analytic synthesis yielded a pooled prevalence of DWI-negative AIS of 6.8%, 95% confidence interval (CI) 4.9–9.3. In the 5 studies that reported proportion data for DWI-negative and DWI-positive AIS based on the ischemic vascular territory (n = 1,023 AIS patients), DWI-negative stroke was strongly associated with posterior circulation ischemia, as determined by clinical diagnosis at hospital discharge or repeat imaging (OR 5.1, 95% CI 2.3–11.6, p < 0.001).
Conclusions:
A small but significant percentage of patients with AIS have a negative DWI scan. Patients with neurologic deficits consistent with posterior circulation ischemia have 5 times the odds of having a negative DWI scan compared to patients with anterior circulation ischemia. AIS remains a clinical diagnosis and urgent reperfusion therapy should be considered even when an initial DWI scan is negative.
Rapid diagnosis is critical for optimizing outcomes in patients with acute ischemic stroke (AIS). Since time is brain,1 a clinician's ability to quickly recognize AIS and administer thrombolytic or endovascular therapy has consistently been shown to improve long-term functional recovery.2,3 Historically, AIS has been a clinical diagnosis based on the sudden onset of focal neurologic deficits. Accordingly, the inclusion criteria for the landmark 1995 National Institute of Neurological Disorders and Stroke study of IV tissue plasminogen activator (tPA) for AIS included a neurologic deficit that is measureable on the NIH Stroke Scale.4 Imaging data, mostly head CT, were used to exclude patients with intracranial hemorrhage or other pathology such as tumor or abscess. However, because CT is not sufficiently sensitive to diagnose ischemia, radiographic findings were not an inclusion criterion for thrombolysis.
Recently, there has been a significant increase in the use of brain MRI in the evaluation of patients with AIS.5 The rationale for MRI, in particular diffusion-weighted imaging (DWI), in the evaluation of AIS is that this modality has substantially higher sensitivity (88%–100%)6,7 than that of head CT for detecting acute ischemia,8–11 with a specificity reported to be as high as 95%–100%.6 In addition, MRI identifies acute intracranial hemorrhage with similar reliability to CT.11–13 In 2010, the American Academy of Neurology (AAN) published an evidence-based guideline on the role of DWI for the diagnosis of AIS, stating that “DWI should be performed for the most accurate diagnosis of acute ischemic stroke.”14 This recommendation represents a paradigm shift in the diagnostic evaluation of patients with AIS. Rather than using head CT solely to rule out a contraindication to thrombolysis, brain MRI is now being used to rule in AIS.
However, there is emerging evidence that DWI fails to identify AIS in a substantial minority of patients. These DWI-negative stroke cases broadly fall into 3 categories. First, multiple reports and observational cohort studies indicate that posterior circulation ischemia is associated with DWI negativity.15–17 Second, small strokes, particularly in the brainstem, may evade detection by DWI.16–18 Third, hyperacute ischemia may be underestimated19,20 or missed by DWI.21,22 These reports of patients with a clinical diagnosis of AIS but normal DWI scans have led to questions about the appropriate balance between clinical and radiologic diagnostic criteria for AIS. Here, we perform a meta-analysis of DWI-negative AIS to determine the prevalence of DWI-negative AIS and to identify clinical characteristics associated with DWI-negative AIS. We hypothesize that posterior circulation ischemia, small volume infarction, and hyperacute DWI (within 6 hours of symptom onset) are associated with DWI-negative AIS. We aim to help clinicians avoid misdiagnosis or delays in diagnosis, since it is essential that thrombolysis or endovascular therapy be administered urgently in eligible patients to optimize outcomes.
METHODS
This meta-analysis adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.23
Information sources and study selection.
We created a search strategy to identify studies of DWI-negative AIS. We searched PubMed and Ovid/MEDLINE from January 1, 1992, to April 1, 2016, using the following search terms: “acute ischemic stroke” or “acute ischaemic stroke” and “diffusion-weighted imaging” or “diffusion weighted imaging” or “DWI.” Studies published in non-English-language journals were excluded, as were studies focusing exclusively on TIA and experimental studies involving animals. Two authors (B.L.E., J.A.E.) identified potentially relevant studies from the PubMed and Ovid/MEDLINE searches. Reference lists from all articles and the authors' own files were also searched for relevant publications. Studies were selected for meta-analysis if they included participants with a diagnosis of DWI-negative AIS. Studies that tested the effect of DWI sequence parameters (e.g., spatial resolution, b value) on DWI lesion conspicuity but did not report the number of patients with DWI-negative AIS were excluded. If there was disagreement between the 2 authors performing the literature search about inclusion of a study, agreement was reached during a second consensus review.
Data classification.
For each study, we extracted demographic, clinical, and radiologic data in accordance with the PRISMA criteria23 for preferred reporting in meta-analyses: type of study (i.e., prospective or retrospective), number of adult participants with AIS, mean age, sex distribution, number of DWI-negative AIS cases, criteria for AIS diagnosis, types of neurologic deficits, NIH Stroke Scale (NIHSS) scores, cerebrovascular circulation implicated in pathogenesis of AIS (i.e., posterior vs anterior), and neuroanatomic localization of stroke lesions. Studies were critically appraised to determine if they met the a priori inclusion criterion: enrollment of consecutive patients presenting with AIS as a clinical diagnosis prior to any imaging.
Studies that selected patients based upon presence or absence of treatment (e.g., IV tPA or antithrombotic therapy) were considered for inclusion, because patient selection based on therapy is unlikely to bias the results of a meta-analysis away from the null hypotheses pertaining to cerebrovascular circulation, stroke size, or timing of DWI scan. However, studies that selected patients based upon symptom type, NIHSS score, or cerebrovascular territory were excluded because of the possibility of introducing systematic bias.
For studies that reported DWI results using both clinical and investigational DWI sequences (e.g., DWI sequences with high b values), only data from the clinical DWI sequences were analyzed. We included studies in which the DWI data were obtained within 72 hours of symptom onset. This conservative time threshold reduced the likelihood that an association of DWI negativity with hyperacute DWI data acquisition might confound the relationship between DWI negativity and cerebrovascular circulation or stroke size. Furthermore, this conservative time threshold reduced the likelihood that we would overestimate the prevalence of DWI-negative AIS.
Statistical analysis.
Data were analyzed using Comprehensive Meta-Analysis software, version 2.2 (Biostat, Englewood, NJ). We used a random effects model to estimate a pooled prevalence and odds ratio (OR) with 95% confidence intervals (CI), where weights were calculated using the inverse variance method for random effects. We assessed heterogeneity using I2, reflecting the percentage of observed variation across studies that is due to true differences, and we assessed publication bias by inspecting funnel plot symmetry. To evaluate whether any study had an extreme influence as an outlier, we checked the standardized residuals and the range of results when the meta-analysis was repeated with each individual study excluded.
RESULTS
Literature search.
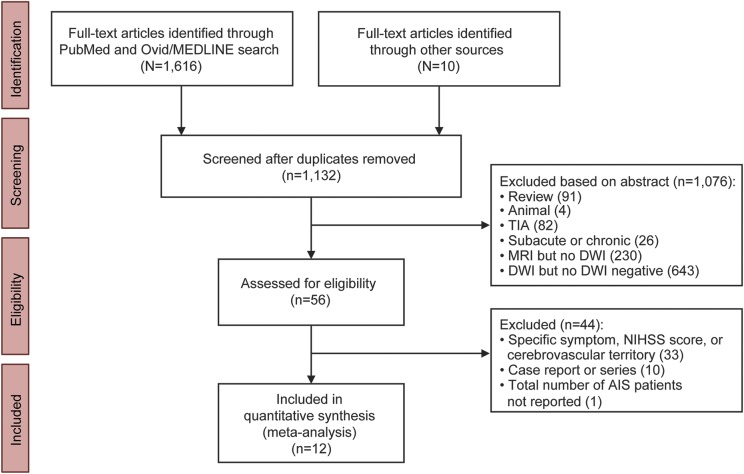
Our literature search yielded 1,132 eligible articles, of which 1,122 were identified via PubMed and Ovid/MEDLINE searches and 10 were identified from our personal reference libraries. Of these 1,132 articles, 45 were identified by both authors performing the literature search as containing patients with DWI-negative AIS. Eleven articles were identified by 1 of the 2 authors during the first literature review, followed by agreement during a second consensus review. The search of PubMed, Ovid/MEDLINE, and personal reference libraries thus yielded a total of 56 studies.
Of these 56 studies, 12 met our a priori inclusion criterion that patients were enrolled based upon a clinical diagnosis of AIS prior to imaging (table 1). These 12 studies included 231 patients with a clinical diagnosis of DWI-negative AIS (table 2). Of the 44 studies that were excluded, 33 were excluded because they only enrolled AIS patients with a specific type of symptom (e.g., dizziness) or stroke syndrome (e.g., middle cerebral artery territory syndrome), 10 were excluded because they were case reports or case series of nonconsecutive AIS patients, and 1 was excluded because the total number of AIS patients who underwent DWI was not reported17 (figure 1).
Table 1.
Demographics, clinical characteristics, and diffusion-weighted imaging (DWI) sequence measures for patients with DWI-negative acute ischemic stroke (AIS) (n = 12 studies; n = 3,236 patients)
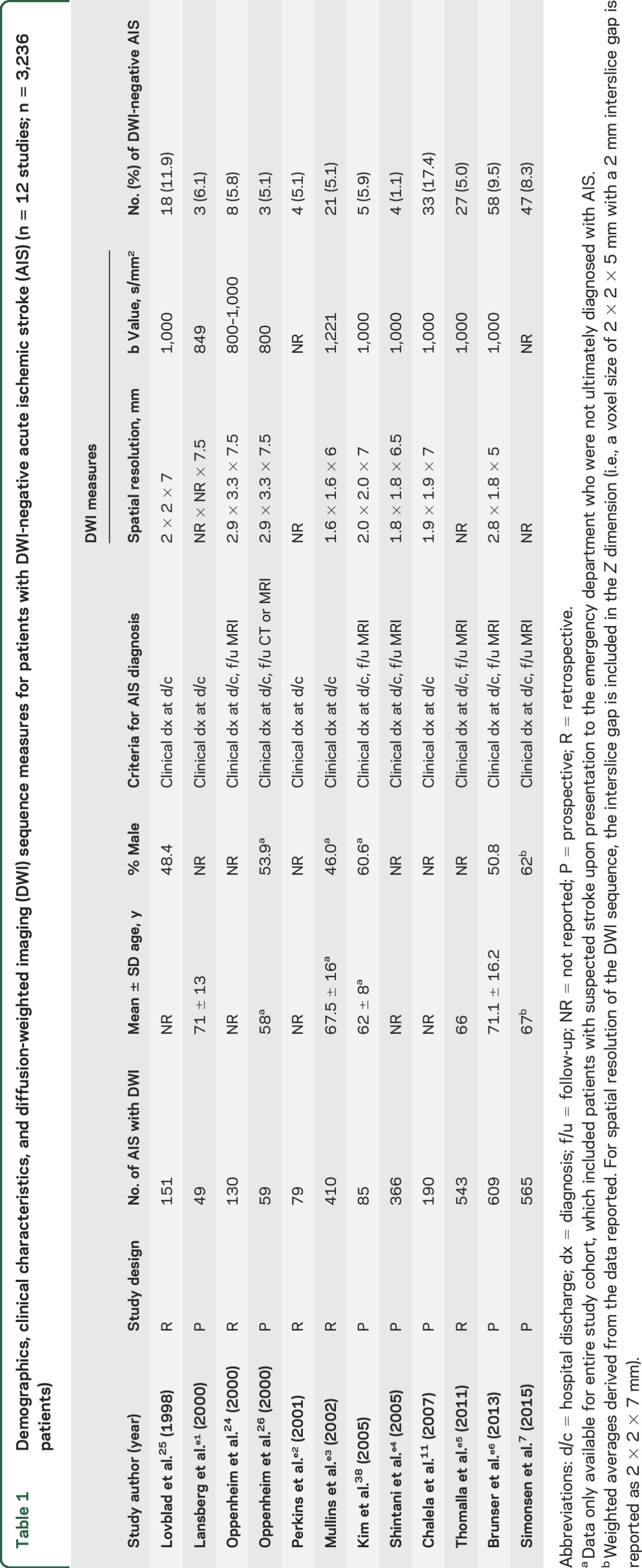
Table 2.
Demographics and clinical characteristics of patients with diffusion-weighted imaging (DWI)–negative acute ischemic stroke (AIS) (n = 12 studies; n = 231 patients)
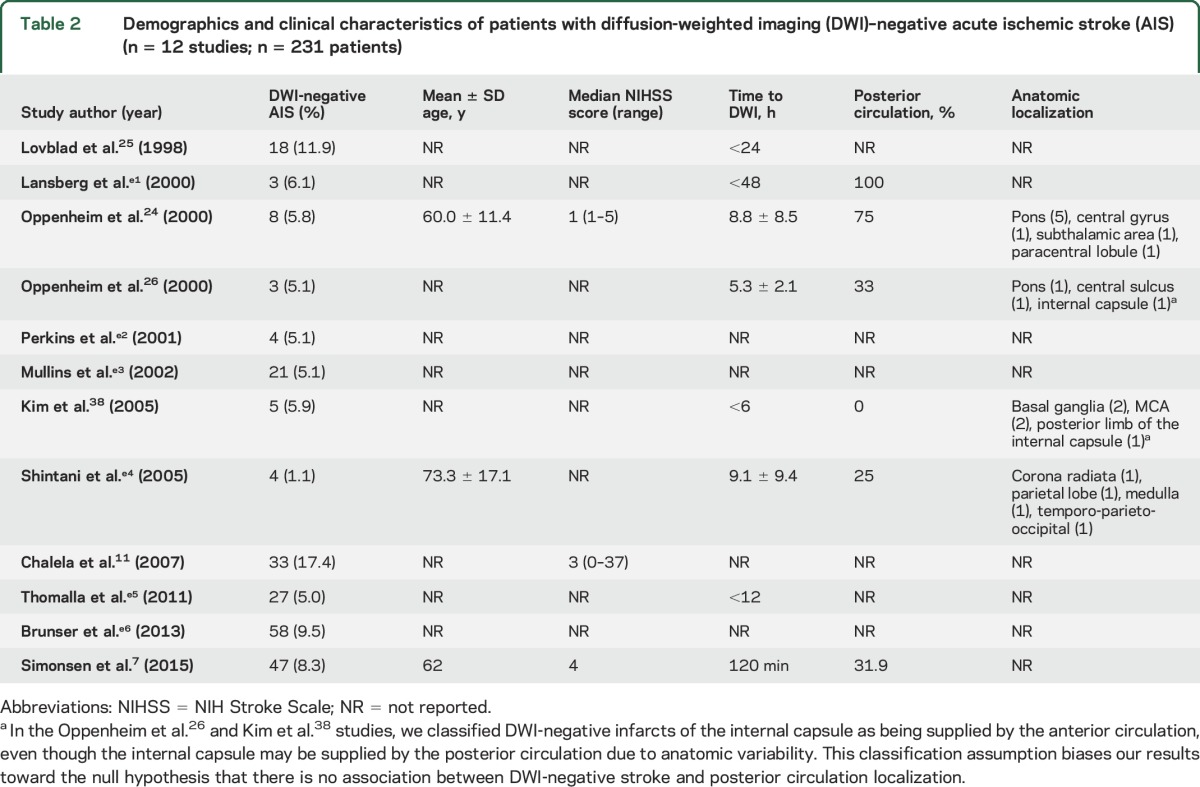
Figure 1. Flow diagram of literature search results, study screening, and study inclusion numbers.
The PubMed and Ovid/MEDLINE searches were performed between January 1, 1992, and April 1, 2016, using the following search terms: “acute ischemic stroke” or “acute ischaemic stroke” and “diffusion-weighted imaging” or “diffusion weighted imaging” or “DWI.” Studies published in non-English-language journals were excluded, as were studies focusing exclusively on TIA and experimental studies involving animals. For articles excluded based on the abstract, some had multiple reasons for exclusion and the primary reason listed by the 2 raters may have differed. Thus, we report average numbers for the 2 reviewers, rounded to the nearest integer. AIS = acute ischemic stroke; DWI = diffusion-weighted imaging; NIHSS = NIH Stroke Scale.
Prevalence of DWI-negative AIS.
The meta-analytic synthesis of the studies listed yielded a pooled prevalence of 6.8%, 95% CI 4.9–9.3, with I2 of 81.
Prevalence estimates obtained by repeating the meta-analysis with each of the 12 studies individually excluded ranged from 6.2%, 95% CI 4.6–8.3 to 7.7%, 95% CI 5.8–10.2.
Clinical characteristics of patients with DWI-negative AIS.
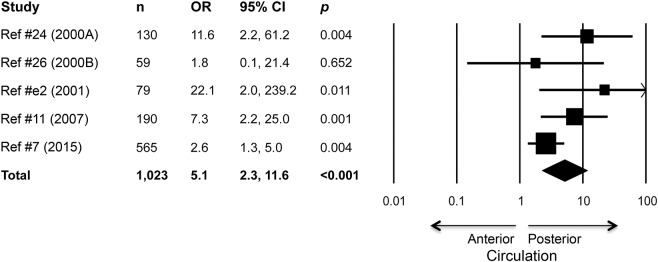
Five of the 12 studies reported the proportions of anterior circulation strokes and posterior circulation strokes that were DWI-negative. The meta-analytic synthesis of these 5 studies showed that with DWI-negative scan as the outcome, the OR for posterior circulation ischemia relative to anterior circulation ischemia was 5.1, 95% CI 2.3–11.6, p < 0.0005 (figure 2). Thus, patients with neurologic deficits consistent with posterior circulation ischemia had 5 times the odds of having a negative DWI scan than patients with anterior circulation ischemia. The I2 was 39, and OR estimates obtained by repeating the meta-analysis with each of the 5 individual studies excluded ranged from 4.3, 95% CI 2.0–9.2, to 8.1, 95% CI 3.4–18.9, all 5 analyses with p < 0.005.
Figure 2. Forest plot shows association between posterior circulation localization and diffusion-weighted imaging (DWI)–negative acute ischemic stroke (n = 1,023 patients who underwent DWI).
Odds ratios (OR) and 95% confidence intervals (CI) for DWI-negative scan are plotted, with the area of square proportional to the study's weight in meta-analysis. ORs and CIs are calculated from frequency data reported in the articles, except for the adjusted OR and 95% CI reported in Chalela et al.11 (2007).
There were insufficient data for a meta-analysis of the potential association between DWI-negative AIS and small stroke volume, as only one study reported the volume of infarction, as detected by follow-up DWI scan.24 In this study, the mean ± SD infarct size was 0.19 ± 0.16 cm3 (range 0.05–0.5 cm3). There were also insufficient data to test for an association between DWI-negative AIS and hyperacute DWI. Only 2 studies reported the proportion of DWI-negative AIS patients who underwent DWI within 6 hours of ictus as compared to after 6 hours.25,26 In the first study, the proportions of DWI negativity within and after 6 hours were 5.9% (2 of 34) and 13.7% (16 of 117), respectively.25 In the second study, the proportions of DWI negativity within and after 6 hours were 14.3% (2 of 14) and 2.2% (1 of 45), respectively.26 Additional studies reported results on an association between DWI negativity and time to DWI, albeit not using a 6-hour threshold. Chalela et al11 found that DWI negativity was strongly associated with DWI acquisition at less than 3 hours, with an OR (95% CI) for DWI negativity of 5.8 (2.3–14.9). Similarly, Simonsen et al7 observed that patients with DWI-negative AIS underwent DWI sooner after symptom onset than patients with DWI-positive AIS: mean 109 minutes vs 120 minutes, respectively (p < 0.05).
DISCUSSION
In this meta-analysis of 3,236 patients from 12 studies that enrolled consecutive patients with a clinical diagnosis of AIS prior to imaging between 1992 and 2016, we found that the pooled prevalence of DWI-negative AIS was 6.8%. Neurologic deficits consistent with posterior circulation ischemia were significantly associated with DWI-negative AIS, as patients with posterior circulation ischemia were 5 times more likely than patients with anterior circulation ischemia to present with a negative DWI scan. These findings suggest that AIS should remain a clinical diagnosis and that clinicians should not exclude patients from urgent IV or endovascular reperfusion therapies based on a negative DWI scan.
The 2010 AAN Guideline authors acknowledged that “the sensitivity of DWI for the diagnosis of ischemic stroke in a general sample of patients with possible acute stroke is not perfect.” Here, we support and quantify this assessment by providing an unbiased, population-based evaluation of the prevalence of and risk factors for DWI negativity. Our meta-analytic findings also support and expand upon observations from case reports and series that have been accumulating over the last 2 decades,27–30 confirming a correlation between posterior circulation ischemia and DWI-negative AIS. Indeed, our observed OR of 5.1 for an association between posterior circulation ischemia and DWI-negative AIS was robust despite the small number of studies included in this analysis. When each study was individually excluded, the effect size remained large and highly significant. Patients with posterior circulation stroke who present with acute dizziness may have an even higher likelihood of DWI negativity in the first 48 hours.31,32
Our findings should not call into question the utility of DWI as an essential tool in the evaluation of patients with clinically suspected AIS. DWI has repeatedly been shown to be the most sensitive technique for identifying acute ischemia due to its ability to detect rapid shifts in the ratio of extracellular to intracellular water content in the brain.33 Use of DWI in the evaluation of suspected AIS also helps to distinguish stroke from stroke mimics. Although seizure, tumor, traumatic axonal injury, and abscess can present with diffusion restriction on DWI,34 the signal characteristics of these lesions on conventional T1-weighted, T2-weighted, and T2*-weighted MRI sequences typically allow distinction between stroke and nonstroke etiologies.34 Moreover, complicated migraine headache and conversion disorder35 may be identified by the absence of signal change on DWI and other sequences.
Because of a growing appreciation in the field of AIS for the possibility of DWI negativity, various diagnostic approaches have been developed to increase the sensitivity of MRI for detecting acute ischemia. A leading approach has been MRI perfusion-weighted imaging (PWI), which includes gadolinium-based techniques and arterial-spin-labeled techniques. Several studies of concurrent PWI-DWI have suggested that PWI provides enhanced sensitivity for detecting acute ischemia,7,16 and PWI continues to be an active area of investigation. Parallel efforts to increase the sensitivity of MRI for detecting AIS have focused on optimization of the DWI sequence itself. These efforts include increasing the spatial resolution,36 reducing geometric distortion,37 and performing a second coronal DWI acquisition through the posterior fossa. Furthermore, multiple investigators have tested the hypothesis that DWI sensitivity can be optimized by increasing the b value, a value that reflects the strength and duration of the diffusion gradients exerted on water molecules within the brain. While several studies have indicated that higher b values provide increased sensitivity for and increased conspicuity of small infarcts,38,39 this observation has not been consistently replicated.40 At present, neither complementary MRI sequences like PWI nor high-b-value DWI techniques have gained wide acceptance in the clinical evaluation of patients with AIS.
Several limitations should be considered when interpreting the results of this meta-analysis. First, there have been multiple technical improvements in DWI since 1992, the year that DWI entered clinical practice. Given that 8 of the 12 studies included in this meta-analysis were published prior to or in 2005, it is possible that these early studies used DWI sequences that were not as sensitive as today's sequences for detecting AIS. Nevertheless, the imaging measures that are most relevant to DWI sensitivity (e.g., spatial resolution and b value) were similar between the 8 early studies and the 4 recent studies (table 1). Moreover, the study with the highest rate of DWI-negative AIS (17.4%) was published in 2007,11 utilizing a DWI sequence whose parameters were similar to those currently used at many stroke centers. Thus, it remains to be determined whether the prevalence of DWI-negative AIS will decline with future technical improvements in the DWI sequence. Another important limitation is that we included studies that imaged patients up to 72 hours after symptom onset, which likely biased our results toward a lower prevalence of DWI-negative AIS than would have been observed if we limited the analysis to patients scanned earlier. Thus, it is possible that within 6 hours, a time window of greater clinical relevance for therapeutic decision-making, the rate of DWI negativity is even higher than 6.8%. Currently, the role of MRI in guiding hyperacute treatment decisions remains an area of active research.
It is essential that clinicians recognize current limitations in the diagnostic sensitivity of DWI for detecting acute ischemia, so as not to miss opportunities to treat AIS patients with thrombolysis and endovascular thrombectomy. Clinicians using DWI to diagnose stroke need to be aware of the possibility of DWI-negative AIS, especially in patients with neurologic deficits suggestive of posterior circulation ischemia. Future studies of DWI-negative patients should include follow-up imaging to determine whether final infarct volume is correlated with DWI negativity.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Martha Stone for assistance with literature searches.
GLOSSARY
- AAN
American Academy of Neurology
- AIS
acute ischemic stroke
- CI
confidence interval
- DWI
diffusion-weighted imaging
- NIHSS
NIH Stroke Scale
- OR
odds ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PWI
perfusion-weighted imaging
- tPA
tissue plasminogen activator
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Design and/or conceptualization of the study: J.A.E. Analysis and/or interpretation of the data: B.L.E, S.H., J.A.E. Statistical analysis: S.H. Drafting and/or revising the manuscript: B.L.E., S.H., J.A.E.
STUDY FUNDING
NIH National Institute of Neurological Disorders and Stroke (K23NS094538).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Saver JL. Time is brain: quantified. Stroke 2006;37:263–266. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Fonarow GC, Smith EE, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA 2013;309:2480–2488. [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995;333:1581–1587. [DOI] [PubMed] [Google Scholar]
- 5.Burke JF, Kerber KA, Iwashyna TJ, Morgenstern LB. Wide variation and rising utilization of stroke magnetic resonance imaging: data from 11 states. Ann Neurol 2012;71:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jauch EC, Saver JL, Adams HP Jr, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 7.Simonsen CZ, Madsen MH, Schmitz ML, Mikkelsen IK, Fisher M, Andersen G. Sensitivity of diffusion- and perfusion-weighted imaging for diagnosing acute ischemic stroke is 97.5%. Stroke 2015;46:98–101. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen AG, Buonanno FS, Gonzalez RG, et al. Hyperacute stroke: evaluation with combined multisection diffusion-weighted and hemodynamically weighted echo-planar MR imaging. Radiology 1996;199:391–401. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez RG, Schaefer PW, Buonanno FS, et al. Diffusion-weighted MR imaging: diagnostic accuracy in patients imaged within 6 hours of stroke symptom onset. Radiology 1999;210:155–162. [DOI] [PubMed] [Google Scholar]
- 10.Fiebach JB, Schellinger PD, Jansen O, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke 2002;33:2206–2210. [DOI] [PubMed] [Google Scholar]
- 11.Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet 2007;369:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fiebach JB, Schellinger PD, Gass A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: a multicenter study on the validity of stroke imaging. Stroke 2004;35:502–506. [DOI] [PubMed] [Google Scholar]
- 13.Kidwell CS, Chalela JA, Saver JL, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004;292:1823–1830. [DOI] [PubMed] [Google Scholar]
- 14.Schellinger PD, Bryan RN, Caplan LR, et al. ; Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2010;75:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke 2009;40:3504–3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ay H, Buonanno FS, Rordorf G, et al. Normal diffusion-weighted MRI during stroke-like deficits. Neurology 1999;52:1784–1792. [DOI] [PubMed] [Google Scholar]
- 17.Sylaja PN, Coutts SB, Krol A, Hill MD, Demchuk AM, Group VS. When to expect negative diffusion-weighted images in stroke and transient ischemic attack. Stroke 2008;39:1898–1900. [DOI] [PubMed] [Google Scholar]
- 18.Doubal FN, Dennis MS, Wardlaw JM. Characteristics of patients with minor ischaemic strokes and negative MRI: a cross-sectional study. J Neurol Neurosurg Psychiatry 2011;82:540–542. [DOI] [PubMed] [Google Scholar]
- 19.Kawano H, Hirano T, Nakajima M, Inatomi Y, Yonehara T. Diffusion-weighted magnetic resonance imaging may underestimate acute ischemic lesions: cautions on neglecting a computed tomography-diffusion-weighted imaging discrepancy. Stroke 2013;44:1056–1061. [DOI] [PubMed] [Google Scholar]
- 20.Kim EY, Ryoo JW, Roh HG, et al. Reversed discrepancy between CT and diffusion-weighted MR imaging in acute ischemic stroke. AJNR Am J Neuroradiol 2006;27:1990–1995. [PMC free article] [PubMed] [Google Scholar]
- 21.Kim JT, Park MS, Kim MK, Cho KH. Minor stroke with total mismatch after acute MCA occlusion. J Neuroimaging 2011;21:399–402. [DOI] [PubMed] [Google Scholar]
- 22.Rosso C, Drier A, Lacroix D, et al. Diffusion-weighted MRI in acute stroke within the first 6 hours: 1.5 or 3.0 Tesla? Neurology 2010;74:1946–1953. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oppenheim C, Stanescu R, Dormont D, et al. False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol 2000;21:1434–1440. [PMC free article] [PubMed] [Google Scholar]
- 25.Lovblad KO, Laubach HJ, Baird AE, et al. Clinical experience with diffusion-weighted MR in patients with acute stroke. AJNR Am J Neuroradiol 1998;19:1061–1066. [PMC free article] [PubMed] [Google Scholar]
- 26.Oppenheim C, Logak M, Dormont D, et al. Diagnosis of acute ischaemic stroke with fluid-attenuated inversion recovery and diffusion-weighted sequences. Neuroradiology 2000;42:602–607. [DOI] [PubMed] [Google Scholar]
- 27.Lefkowitz D, LaBenz M, Nudo SR, Steg RE, Bertoni JM. Hyperacute ischemic stroke missed by diffusion-weighted imaging. AJNR Am J Neuroradiol 1999;20:1871–1875. [PMC free article] [PubMed] [Google Scholar]
- 28.Frey LC, Sung GY, Tanabe J. Early false-negative diffusion-weighted imaging in brainstem infarction. J Stroke Cerebrovasc Dis 2002;11:51–53. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Goldstein S, Scheuer ML, Branstetter BF. Acute stroke syndrome with fixed neurological deficit and false-negative diffusion-weighted imaging. J Neuroimaging 2003;13:158–161. [PubMed] [Google Scholar]
- 30.Lettau M, Laible M. 3-T high-b-value diffusion-weighted MR imaging of hyperacute ischemic stroke in the vertebrobasilar territory. J Neuroradiology 2012;39:243–253. [DOI] [PubMed] [Google Scholar]
- 31.Saber Tehrani AS, Kattah JC, Mantokoudis G, et al. Small strokes causing severe vertigo: frequency of false-negative MRIs and nonlacunar mechanisms. Neurology 2014;83:169–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi JH, Kim HW, Choi KD, et al. Isolated vestibular syndrome in posterior circulation stroke: frequency and involved structures. Neurol Clin Pract 2014;4:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mintorovitch J, Yang GY, Shimizu H, Kucharczyk J, Chan PH, Weinstein PR. Diffusion-weighted magnetic resonance imaging of acute focal cerebral ischemia: comparison of signal intensity with changes in brain water and Na+,K(+)-ATPase activity. J Cereb Blood Flow Metab 1994;14:332–336. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer PW, Grant PE, Gonzalez RG. Diffusion-weighted MR imaging of the brain. Radiology 2000;217:331–345. [DOI] [PubMed] [Google Scholar]
- 35.Toth C. Hemisensory syndrome is associated with a low diagnostic yield and a nearly uniform benign prognosis. J Neurol Neurosurg Psychiatry 2003;74:1113–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura H, Yamada K, Kizu O, et al. Effect of thin-section diffusion-weighted MR imaging on stroke diagnosis. AJNR Am J Neuroradiol 2005;26:560–565. [PMC free article] [PubMed] [Google Scholar]
- 37.Andre JB, Zaharchuk G, Fischbein NJ, et al. Clinical assessment of standard and generalized autocalibrating partially parallel acquisition diffusion imaging: effects of reduction factor and spatial resolution. AJNR Am J Neuroradiol 2012;33:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim HJ, Choi CG, Lee DH, Lee JH, Kim SJ, Suh DC. High-b-value diffusion-weighted MR imaging of hyperacute ischemic stroke at 1.5T. AJNR Am J Neuroradiol 2005;26:208–215. [PMC free article] [PubMed] [Google Scholar]
- 39.Ract I, Ferre JC, Ronziere T, Leray E, Carsin-Nicol B, Gauvrit JY. Improving detection of ischemic lesions at 3 Tesla with optimized diffusion-weighted magnetic resonance imaging. J Neuroradiol 2014;41:45–51. [DOI] [PubMed] [Google Scholar]
- 40.Chen PE, Simon JE, Hill MD, et al. Acute ischemic stroke: accuracy of diffusion-weighted MR imaging–effects of b value and cerebrospinal fluid suppression. Radiology 2006;238:232–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.