Abstract
Objective:
To review whether the incidence of catheter-associated venous thromboses was higher in patients receiving IV dihydroergotamine compared to lidocaine.
Methods:
We retrospectively reviewed all admissions at the University of California, San Francisco Headache Center from February 25, 2008, through October 31, 2014, for age, sex, diagnosis, aura, treatment dose, type of IV line used, days with line, superficial (SVT) or deep venous thrombosis (DVT), and pulmonary embolism (PE).
Results:
A peripherally inserted central catheter (PICC) or midline catheter was placed in 315 of 589 (53%) admissions. Mean age was 38 years with a range of 6 to 79 years; 121 patients (21%) were ≤18 years old. Seventy-four percent (433 of 589) of patients were female. Of 263 dihydroergotamine admissions using a PICC or midline catheter, 19 (7.2%) had either an SVT or DVT or a PE; 2 patients were diagnosed with both DVT and PE. Of 52 lidocaine admissions using a PICC or midline catheter, none had a thrombotic event (p = 0.05, Fisher exact test). Age, sex, aura, total dihydroergotamine dose, and number of days with line were not significant predictors of venous thrombosis.
Conclusions:
IV dihydroergotamine treatment may be associated with an increased risk of catheter-associated venous thrombosis. A low threshold for diagnostic ultrasound investigation is appropriate because anticoagulation therapy was frequently required.
Repetitive dosing of IV dihydroergotamine is used to treat medically refractory primary headache disorders, including chronic migraine and cluster headache. Our 5-day inpatient dihydroergotamine protocol has been published.1 Dihydroergotamine treatment is often administered through a peripheral cannula. However, dihydroergotamine is a venoconstrictor, and in our experience, maintaining vascular access can be difficult with repetitive dosing. In cases when a peripheral IV cannot be replaced, our practice is to insert a peripherally inserted central catheter (PICC) or midline catheter under radiologic guidance to complete treatment. In patients with known difficulty with venous access, this may be done before treatment is initiated.
We observed cases of catheter-associated venous thromboses in patients receiving dihydroergotamine, some requiring anticoagulation therapy, and sought to review whether the incidence was high enough to arouse concern. As a comparison group, we examined patients admitted for treatment with lidocaine infusion, the second most common treatment offered at our center, which is typically administered for 10 days via a PICC or midline catheter.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the University of California, San Francisco Committee for Human Research (protocol No. 13-12648) under a minimal-risk protocol.
Study design and study protocol.
We retrospectively reviewed all adult and pediatric admissions at the University of California, San Francisco Headache Center, a tertiary referral center, from February 25, 2008, through October 31, 2014, and included all patients who were admitted for treatment with either IV dihydroergotamine or lidocaine infusion.
Briefly, dihydroergotamine was given every 8 hours for 5 days, with an initial dose escalation from 0.5 to 0.75 mg and 1 mg thereafter with a goal dose of 11.25 mg in total.1 A weight-adjusted dose was given to pediatric patients ≤50 kg. Antiemetics were given: ondansetron and domperidone before each dihydroergotamine dose and sometimes daily aprepitant.2 Lidocaine therapy was administered as a continuous infusion, typically for 10 days, with dose titration between 1 and 4 mg/min based on clinical response and tolerability. A subset of patients with medication overuse, with opioids or triptans, underwent medication withdrawal with IV aspirin immediately before treatment. Up to 1 g aspirin every 8 hours was given as needed for headache exacerbations (n = 81).3
Clinical charts were examined for documentation of or diagnostic tests for superficial (SVT) or deep venous thrombosis (DVT) or pulmonary embolism (PE). In addition, charts were reviewed for the following variables: age, sex, diagnosis, presence of aura, total cumulative dose (dihydroergotamine) or maximum dose reached (lidocaine), type of IV line used, and number of days with a PICC or midline catheter.
Patients on anticoagulation therapy at baseline were excluded from this analysis.
Data analysis.
A multivariate regression analysis was performed with Stata (version 13, StataCorp, College Station, TX) with the following variables of interest: age (by decade), sex, aura, total dose of dihydroergotamine given, type of line (PICC or midline catheter), and number of days with line.
RESULTS
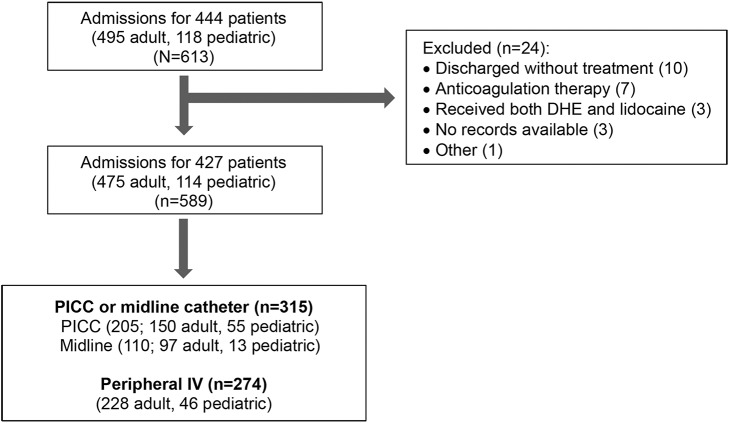
Of 613 admissions during the study period, 24 were excluded for reasons shown in the figure. A subset of patients were admitted repeatedly over the study period, and each admission was considered separately for this analysis. Of the 589 included admissions for 427 patients, a peripheral IV was sufficient for 274 admissions, and a PICC or midline catheter was placed before discharge during 315 admissions (205 PICC, 110 midline catheter).
Figure. Flowchart of admissions during the study period included for analysis and breakdown by line type.
DHE = dihydroergotamine; PICC = peripherally inserted central catheter.
The mean age was 38 years with a range of 6 to 79 years; 121 patients (21%) were ≤18 years of age at the time of admission. Seventy-four percent (433 of 589) of patients were female. Most patients had migraine (n = 472) or new daily persistent headache (n = 73); less commonly, patients had cluster headache (n = 17), short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing (SUNCT)/short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms (SUNA) (n = 14), or other rare headache disorders (n = 13). Twenty-five percent (146 of 589) of patients had aura. Eighty-three patients had medication overuse, most commonly with opioids or triptans. Of these, 80 patients had the overused medication withdrawn with the use of IV aspirin before the respective treatment (75 dihydroergotamine, 5 lidocaine).
Of 263 admissions for dihydroergotamine given through a PICC or midline catheter, 19 (7.2%) had either a DVT or SVT or a PE (table). Of the 3 patients with PE, 2 were also diagnosed with a DVT; 1 patients was not diagnosed with venous thrombosis but became symptomatic 2 days after discharge and was diagnosed with a PE. In contrast, of 52 admissions for lidocaine given through a PICC or midline catheter, no patients were diagnosed with venous thrombosis or PE (p = 0.05, Fisher exact test). Of 270 admissions for dihydroergotamine using a peripheral IV, 1 patient (0.4%) was diagnosed with a superficial saphenous thrombosis in the leg (unrelated to IV); of 4 admissions for lidocaine using a peripheral IV, no patients had a thrombotic event.
Table.
Patients with DVT, SVT, or PE by treatment and line type
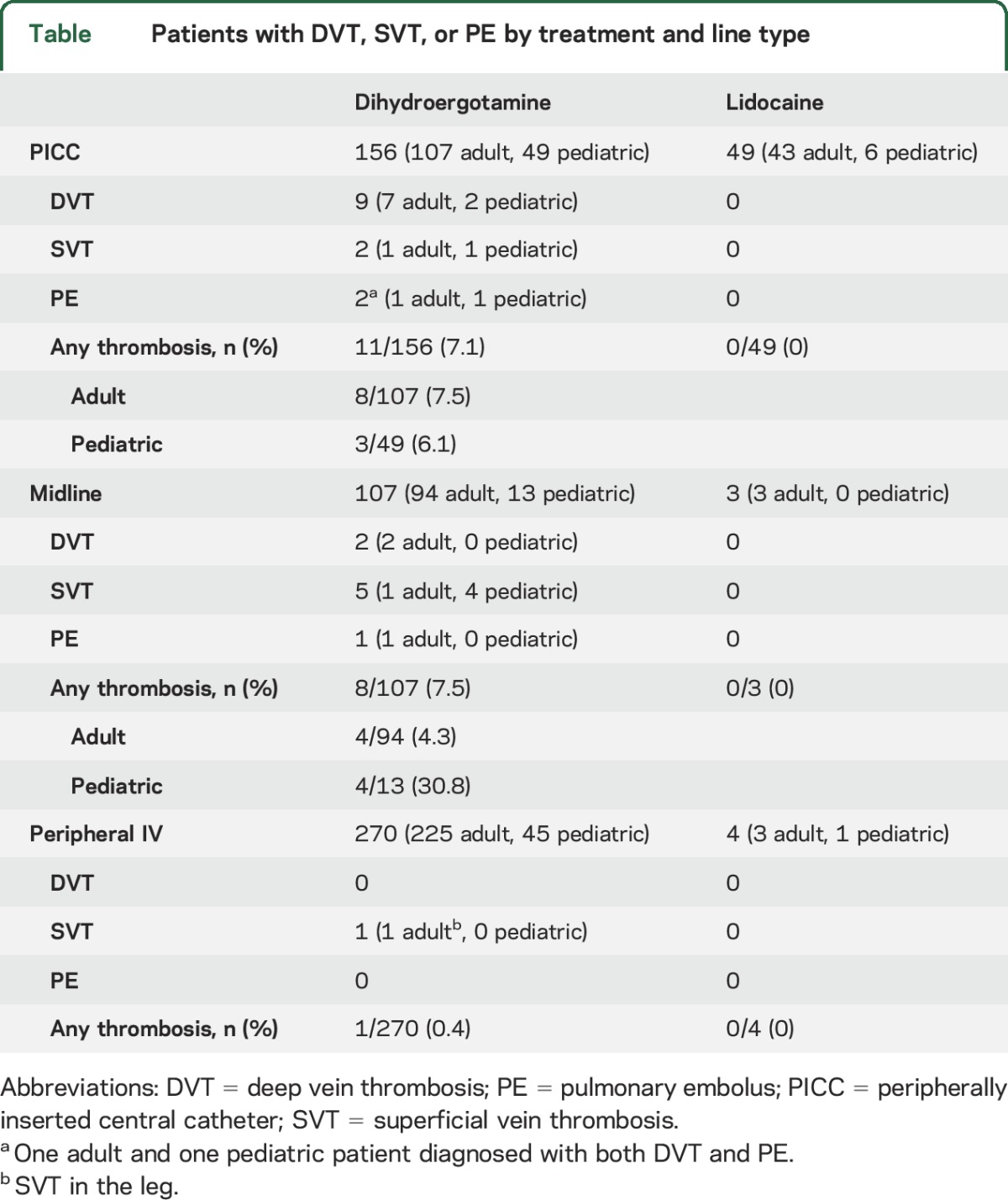
Among the 19 patients with venous thrombosis or PE taking dihydroergotamine, 6 (all adult) had received IV aspirin for medication withdrawal just before dihydroergotamine treatment.
With regard to treatment, all 10 adults with DVT or PE presented with extensive thrombosis, and anticoagulation therapy was recommended by the hematology service for all patients for a minimum of 1 month. Neither adult with SVT required anticoagulation therapy. One pediatric patient with both DVT and PE required anticoagulation treatment; the other 6 pediatric patients were not anticoagulated.
Age, sex, aura, total dihydroergotamine dose, and number of days with line (p = 0.18 for number of days with line) were not significant predictors of venous thrombosis.
DISCUSSION
Here, we report the incidence of venous thrombotic events during elective admissions to a tertiary headache center for treatment. This analysis was prompted by the clinical observation of catheter-associated thromboses extensive enough to require anticoagulation therapy. We found a substantial number of line-associated venous thromboses or even PEs with dihydroergotamine but not lidocaine treatment; furthermore, these events occurred exclusively in patients who had either a PICC or midline catheter, not in patients with a peripheral IV. The cases of PE were presumably a consequence of venous thrombosis because 2 of the 3 cases were diagnosed concurrently with venous thrombosis and all 3 had either a PICC or midline catheter for dihydroergotamine. The difference in thrombosis rates between dihydroergotamine and lidocaine was borderline statistically significant (p = 0.05); our study may have been slightly underpowered to reach significance. We believe the possibility of increased thrombotic risk with dihydroergotamine treatment remains and is important to report so that further research can be performed and so that clinicians are aware of this possibility.
The expected rate of venous thrombosis is difficult to estimate in this population. Published rates of symptomatic PICC-associated upper-extremity DVT range from 1.6% to 3.5%4–6 but are typically reported in patients with conditions that may predispose to venous thrombosis such as malignancy or infectious or inflammatory diseases. Indeed, PICC or midline catheters are typically used as a result of the need for extended IV treatment and are rarely placed in medically healthy patients. We chose to examine patients treated with IV lidocaine as a comparator group because they are a comparable patient population. Lidocaine therapy is offered to patients who do not have an adequate response to or have a medical contraindication (coronary, cerebrovascular, or peripheral vascular disease) to dihydroergotamine treatment or to patients with rare headache disorders that are preferentially treated with lidocaine such as SUNCT/SUNA.7 We would not expect any of these scenarios or lidocaine treatment itself to reduce the likelihood of thrombosis. Similarly, although we did not record preventive medications used at the time of admission, none are known to affect coagulability; thus, we would not expect their use to cause a difference between groups. If the catheter itself were responsible for the increased thrombotic risk, by applying the rate of catheter-associated thrombosis in the dihydroergotamine group (7.2%), one would expect 3 or 4 events to have occurred in the 52 patients who received lidocaine through a PICC or midline catheter; zero occurred. Thus, the rate of catheter-associated thrombosis in patients receiving dihydroergotamine seems to be higher than expected compared to both published rates from patient populations with prothrombotic comorbidities and the best available control group.
The lack of thrombotic events in patients receiving dihydroergotamine through a peripheral IV is of interest. This may suggest that dihydroergotamine has a relatively mild influence such that the risk of thrombosis is elevated only in combination with a PICC or midline catheter. Dihydroergotamine is a potent venoconstrictor8,9 via 5-hydroxytryptamine (serotonin) receptor agonism. Dihydroergotamine dissociates slowly from its receptor, resulting in more sustained action compared to triptans.10 Perhaps marked, sustained venoconstriction around a longer foreign surface encourages thrombus propagation. Initiation of thrombus might be more likely with the insertion of a larger catheter. Alternatively, perhaps the relatively high dose of dihydroergotamine used in our protocol acts as a venous irritant and increases the likelihood of thrombophlebitis; one patient receiving dihydroergotamine through a peripheral IV was diagnosed with an SVT in the leg. Lastly, it is possible that high doses of dihydroergotamine might have a prothrombotic effect.11
Of note, 6 of 19 (32%) patients taking dihydroergotamine who were diagnosed with venous thrombosis or PE had received IV aspirin for medication withdrawal just before dihydroergotamine treatment. Thus, aspirin cannot be assumed to be protective against thrombosis in this patient population.
Before the advent of low-molecular-weight heparins, unfractionated heparin with dihydroergotamine was used for DVT prophylaxis in surgical patients. The aim of dihydroergotamine was to induce venoconstriction, thereby reducing venous capacitance and stasis in the lower extremities. This literature differs from our population in that postoperative patients are at high risk for DVT and we found an increased risk of catheter-associated venous thromboses in the upper extremity rather than lower-extremity DVT.
A limitation of this study is that oral contraceptive use was not recorded. We would not, however, expect oral contraceptive use to be more prothrombotic than the comorbidities present in the quoted literature (malignancy, infectious and inflammatory diseases) and therefore believe this is unlikely to account for the higher rates of catheter-associated thrombosis seen in our study population. Other limitations include the lack of hypercoagulability workup or exclusion of comorbid conditions that may predispose to thrombosis. On the other hand, this mimics clinical practice in that hypercoagulability workups are rarely sent before a thrombotic event and are not typically recommended after a catheter-associated DVT. Likewise, it is not known what comorbidities are sufficiently prothrombotic to warrant avoidance of a PICC or midline catheter in a patient. Finally, this study is retrospective, and findings should be replicated with a prospective study.
Catheter-associated thromboses are often not treated beyond removal of the offending line. However, because of the extensive nature of the thromboses, typically extending from the catheter to the axillary or subclavian vein, 10 of 12 adults with thrombotic outcomes were treated with anticoagulation therapy on the recommendation of the hematology service. SVT was more common among pediatric patients, and anticoagulation therapy was required in only one patient with DVT and PE. Even if no treatment is ultimately required, diagnosis remains important because the recommendation for treatment or repeat ultrasound imaging depends on the location and extent of thrombosis.
Dihydroergotamine is an effective and important treatment for this highly disabled population, and we would certainly not allow this observation to deter us from its use. However, the observation is important because anticoagulation therapy was frequently necessary, and venous thrombosis was sometimes associated with PE, which can lead to significant morbidity or even mortality. It seems prudent to use a peripheral IV whenever possible for the administration of repetitive dihydroergotamine dosing, to be cognizant of the potential for catheter-associated thrombosis, and to have a low threshold for diagnostic investigation with a vascular ultrasound.
GLOSSARY
- DVT
deep venous thrombosis
- PE
pulmonary embolism
- PICC
peripherally inserted central catheter
- SUNA
short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms
- SUNCT
short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing
- SVT
superficial venous thrombosis
AUTHOR CONTRIBUTIONS
A.R. Tso: study design and conceptualization, data analysis, manuscript drafting, and revision. I.R. Patniyot and A.A. Gelfand: data analysis, manuscript drafting and revision. P.J. Goadsby: manuscript drafting and revision.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
A. Tso and I. Patniyot report no disclosures relevant to the manuscript. A. Gelfand has received research support from eNeura and Allergan, travel expenses from Teva, and consulting fees from Zosano and Eli Lilly. Her spouse has received research support from Genentech, MedDay, and Quest Diagnostics and has received personal compensation for medical-legal consulting and consulting fees from Genentech. P. Goadsby reports grants and personal fees from Allergan, Amgen, and Eli-Lilly and Co, as well as personal fees from Akita Biomedical, Alder Biopharmaceuticals, Autonomic Technologies Inc, Avanir Pharma, Cipla Ltd, Colucid Pharmaceuticals, Ltd, Dr Reddy's Laboratories, eNeura, Electrocore LLC, Novartis, Pfizer Inc, Promius Pharma, Quest Diagnostics, Scion, Teva Pharmaceuticals, Trigemina Inc, Scion, MedicoLegal work, Journal Watch, Up-to-Date, and Oxford University Press. In addition, Dr. Goadsby has a patent pending for magnetic stimulation for headache assigned to eNeura. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Nagy AJ, Gandhi S, Bhola R, Goadsby PJ. Intravenous dihydroergotamine for inpatient management of refractory primary headaches. Neurology 2011;77:1827–1832. [DOI] [PubMed] [Google Scholar]
- 2.Chou DE, Tso AR, Goadsby PJ. Aprepitant for the management of nausea with inpatient IV dihydroergotamine. Neurology 2016;87:1613–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weatherall MW, Telzerow AJ, Cittadini E, Kaube H, Goadsby PJ. Intravenous aspirin (lysine acetylsalicylate) in the inpatient management of headache. Neurology 2010;75:1098–1103. [DOI] [PubMed] [Google Scholar]
- 4.Chopra V, Ratz D, Kuhn L, Lopus T, Lee A, Krein S. Peripherally inserted central catheter-related deep vein thrombosis: contemporary patterns and predictors. J Thromb Haemost 2014;12:847–854. [DOI] [PubMed] [Google Scholar]
- 5.Liem TK, Yanit KE, Moseley SE, et al. Peripherally inserted central catheter usage patterns and associated symptomatic upper extremity venous thrombosis. J Vasc Surg 2012;55:761–767. [DOI] [PubMed] [Google Scholar]
- 6.Smitherman AB, Alexander T, Connelly M, et al. The incidence of catheter-associated venous thrombosis in noncritically ill children. Hosp Pediatr 2015;5:59–66. [DOI] [PubMed] [Google Scholar]
- 7.Matharu MS, Cohen AS, Goadsby PJ. SUNCT syndrome responsive to intravenous lidocaine. Cephalalgia 2004;24:985–992. [DOI] [PubMed] [Google Scholar]
- 8.Aellig WH. Direct effects of vasoactive substances on superficial human veins in vivo. Int Angiol 1985;4:235–242. [PubMed] [Google Scholar]
- 9.Labruijere S, Chan KY, de Vries R, et al. Dihydroergotamine and sumatriptan in isolated human coronary artery, middle meningeal artery and saphenous vein. Cephalalgia 2015;35:182–189. [DOI] [PubMed] [Google Scholar]
- 10.MaassenVanDenBrink A, Reekers M, Bax WA, Ferrari MD, Saxena PR. Coronary side-effect potential of current and prospective antimigraine drugs. Circulation 1998;98:25–30. [DOI] [PubMed] [Google Scholar]
- 11.Iaquinto G, Ambrosone L, Rotiroti D, Cianciulli GM, Rambaldi M. A case of portal thrombosis arisen after treatment with dihydroergotamine. Ital J Gastroenterol 1991;23:219–221. [PubMed] [Google Scholar]