Abstract
Background
Cholecystectomy can be associated with considerable postoperative pain. While the benefits of paravertebral block (PVB) on pain after thoracotomy and mastectomy have been demonstrated, not enough investigations on the effects of PVB on pain after open cholecystectomy have been conducted. We tested the hypothesis that a single-injection thoracic PVB reduces pain scores, decreases opioid consumption, and prolongs analgesic request time after cholecystectomy.
Methods
Of 52 patients recruited, 50 completed the study. They were randomly allocated into two groups: the paravertebral group and the control group. The outcome measures were the severity of pain measured on numeric pain rating scale, total opioid consumption, and first analgesic request time during the first postoperative 24 hours.
Result
The main outcomes recorded during 24 hours after surgery were Numerical Rating Scale (NRS) pain scores (NRS, 0–10), cumulative opioid consumption, and the first analgesic request time. Twenty four hours after surgery, NRS at rest was 4 (3–6) vs 5 (5–7) and at movement 4 (4–7) vs 6 (5–7.5) for the PVB and control groups, respectively. The difference between the groups over the whole observation period was statistically significant (P<0.05). Twenty-four hours after surgery, median (25th–75th percentile) cumulative morphine consumption was 0 (0–2) vs 2.5 (2–4) mg (P<0.0001) and cumulative tramadol consumption was 200 (150–250) mg vs 300 (200–350) mg in the paravertebral and in the control group, respectively (P=0.003). After surgery, the median (25th–75th percentile) first analgesic requirement time was prolonged in the PVB group in statistically significant fashion (P<0.0001).
Conclusion and recommendations
Single-shot thoracic PVB as a component of multi-modal analgesic regimen provided superior analgesia when compared with the control group up to 24 postoperative hours after cholecystectomy, and we recommend this block for post cholecystectomy pain relief.
Keywords: open cholecystectomy, postoperative pain, paravertebral block, landmark technique
Background of the study
Upper abdominal procedures including cholecystectomy are very painful surgical procedures, and optimal pain management is needed to reduce morbidity, mortality, improve patient outcomes, and reduce hospital costs.1
Systemic opioids and epidural anesthesia are commonly employed for the treatment of postoperative pain. But they are highly associated with complications, demanding experienced staff and a high degree of surveillance.2–4
Accumulated evidence reveals that thoracic paravertebral blocks (TPVBs) can provide high-quality analgesia for patients undergoing many types of operations, including patients with thoracoabdominal trauma and chronic pain with minimal complications.1,5,6 The block is easy to perform, has a high success rate, and is widely performed as multimodal pain control strategy for thoracoabdominal surgeries including cholecystectomy.7 Moreover, single-shot TPVB markedly reduced postoperative pain after laparascopic8 and open cholecystectomy.9,10
The efficacy and spread of the block can be affected by technical difficulty of the block and patient-related factors such as obesity, both in the upper and lower thoracic levels, and anatomic factors.11,12 The use of ultrasound may improve the success rate of the block and decrease potential complications.13,14
However, a number of studies depicted the great efficacy of TPVB when performed using anatomic landmark technique after thoracoabdominal surgery for a variety of surgical procedures if it is performed strictly. Studies done on different thoracoabdominal procedures using the blind technique of the thoracic paravertebral nerve block showed a comparable result to the ultrasound-guided ones.1,6,9,15 Postoperative pain management in developing countries like Ethiopia are substandard because of lack of awareness about the problem; shortage of supply of systemic opioids, adjuvants drugs, epidural kits, and ultrasound machines; and lack of skilled anesthetists in ultrasound scanning. Furthermore, open cholecystectomy (no laparoscopic surgery in our case) is one of the most common operations performed in our hospital that may cause severe postoperative pain. Thoracic paravertebral nerve block is routinely performed as part of multimodal analgesia for open cholecystectomy. Unfortunately, its efficacy has never been addressed before. In this study, we assessed the efficacy of unilateral single-shot thoracic paravertebral nerve block using landmark technique for postoperative pain control after open cholecystectomy surgery.
Methods
An institutional-based randomized controlled clinical trial was conducted from February 1 to April 30, 2016. Previously, nonsteroidal anti-inflammatory drugs and sometimes low-dose weak opioids (tramadol) with or without local wound infiltration were the main management modalities to control postoperative pain. Postoperative pain following cholecystectomy may be controlled with systemic opioids, epidural analgesia, nonsteroidal anti-inflammatory drugs, or posterior intercostal nerve block. Each method has its advantages, but it needs experienced staff and a high degree of surveillance because of the potential risk of hypotension, motor blockade, and respiratory depression. All American Society of Anesthesiologists (ASA) class I and II surgical patients who underwent open cholecystectomy surgery were included in the study. On the other hand, patients with any contraindication for regional anesthesia, such as infection of the puncture site, anatomic deformities, coagulopathy, and allergies to any medication included in the local pain management protocol (data obtained from preoperative anesthetic record), common bile duct exploration, and high body mass index (BMI; >35 kg/m2) were excluded from the study.
Dependent variables
The outcome variable for the study was the severity of postoperative pain, which was assessed using numerical pain rating scale with/without coughing (movement), total postoperative analgesics consumption, and first analgesic request time.
Independent variable
Sociodemographic variables (age, weight, height, BMI), duration of surgery and anesthesia, ASA status, Ramsey sedation score, and postoperative hemodynamic changes were the independent variables.
Operational definiation
Numerical pain rating scale is a method of pain assessment where patients are asked to score their pain ratings on a scale of 0–10, corresponding to current, best, and worst pain experienced over the 24 hours. The median value were used to represent patients level of pain.
Pain was manged using tramadol when the numeric pain rating scale score was greater or equal to four (Numerical Rating Scale [NRS] ≥4), morphine was added when NRS ≥7
Severity of the postoperative pain is depicted in the scale in Figure 1.
Figure 1.
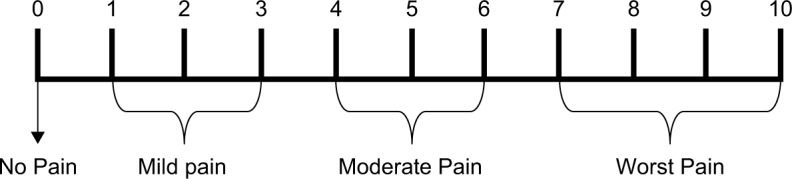
Postoperative pain scale.
Time for first analgesia request is defined as the first time at which patient need tramadol or morphine in the postoperative period.
Total analgesic consumption is defined as type and amounts of analgesic drugs given to the patient within 24 hours postoperatively.
Failed PVB was defined as the no anesthetic dermatome at the level of T6-T8 20 minutes following the administration of the block.
Sample size calculation and sampling techniques
Based on a previous study in Egypt, we took the power as 80% and the level of significance as 5%.16 The incidence of post choleycystectomy pain was about 56% without any intervention.17 Based on a previous study, we took 60% reduction.18
p1 was defined as the incidence of pain in the non- paravertebral group, taken as 56%; p2 was defined as the incidence of pain the paravertebral group, which was taken as 22%; Zα is the standard normal variate of the level of significance, which was 1.96; Zα is the standard normal variate for power or type two error, which was 0.84; r: 1 applying this in the following formula the total simple size would be 52.
P = pooled prevalence = p1+p2/2
Data collection procedures
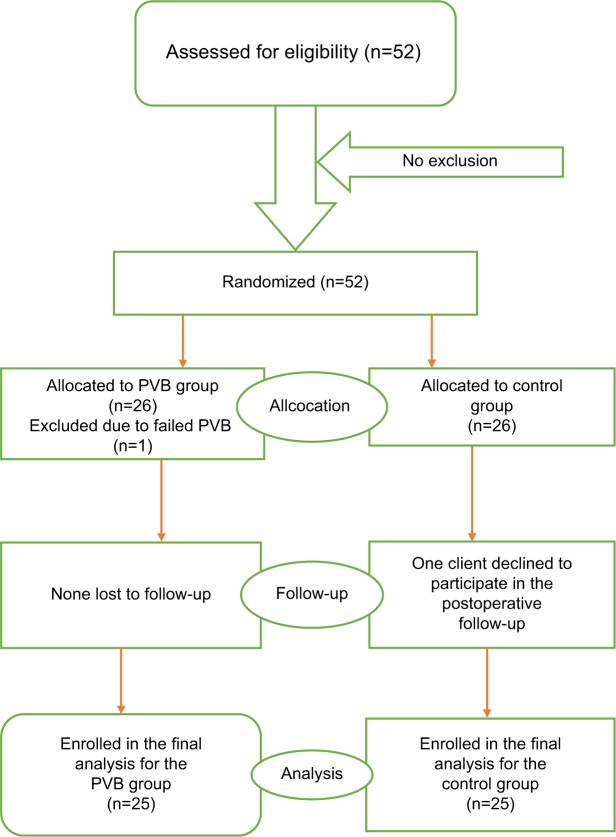
After we obtained informed written consent, randomization (Figure 2) was performed as follows: Letter C and P which were written on a piece of white paper were drawn by the patient from a small bag. If a letter P was drawn, patients were allocated to the paravertebral group, whereas if a letter C was drawn, the patients were allocated to the control group. Intravenous line was instituted and all patients were given crystaloids. Then, all patients were premedicated with paracetamol 20 mg/kg per os and 1 mg/kg and diclofenac intramuscular. For patients in the paraverteberal group, after aseptic preparation of the skin, the site of injection was infiltrated with 2–4 mL of 1% lidocaine, and then using 22 gauge spinal needle TPV block was done by one of the MSC anesthetists. The block was done taking C7, the most prominent cervical vertebral as landmark, counting down seven spinous process, palpating for T7, and then identifying the needle injection site at 2.5 cm lateral and to the right of the spinous process. Patients were observed closely for symptoms and signs of systemic local anesthetic toxicity and other complications related to the block. According to previous studies, we arbitrarily defined block success as loss of pinprick sensation in 3 or more ipsilateral vertebral dermatomes.21 Then, analgesia using the loss of pinprick sensation was checked 15–20 minutes after the institution of the block to elicit the extent of somatic blockade. Failed PVB was defined as inability to identify sensory block in the dermatome between T6 and T8 20 minutes after initiation of block, and these patients were excluded from the study. On the operating table, 0.1 mg/kg dexamethasone intravenous as per our protocol for antiemetic prophylaxis and fentanyl 2 mcg/kg were given for all groups. All patients were induced with 2–3 mg/kg propofol and relaxed with suxamethonium 2 mg/kg intravenous to facilitate tracheal intubation. Intra-operative anesthesia was maintained with halothane, and relaxation with pancronium. Intraoperative analgesia was maintained with 0.1 mg/kg of morphine. All patients were monitored using an electrocardiogram, noninvasive arterial blood pressure monitor, and pulse oximeter. At the end of the surgical procedure, all patients received 1 mcg/kg of fentanyl. Residual neuromuscular blockade was reversed with intravenous neostigmine 0.05 mg/kg and 20 mcg/kg of atropine, after that the trachea was extubated when the patient was awake and recovered fully from anesthesia. Then, patients were transferred to the recovery room and observed for 30 minutes. After 30 minutes of observation postoperatively, presence and severity of pain and analgesic needs was assessed systematically using structured questionnaire by trained data collectors who were blinded to group allocation.
Figure 2.
CONSORT patient flow diagram.
Abbreviation: PVB, paravertebral block.
These assessments were performed at 30 minutes and 1, 2, 6, and 24 hours postoperatively. Moderate pain was managed using tramadol, and morphine was added for severe pain (NRS ≥7). Ward and recovery nurses were informed and trained about the pain management strategies, and they were involved in the postoperative pain assessment, management, and documentation process. Two BSc qualified anesthetists were selected to collect data, and a one-day training was given on patient randomization, data collection, and postoperative pain management. The intraoperative data collector prepared the drugs, assigned the group, and coded them. He/she also recorded the intraoperative events on the intraoperative data collection tool. At the end of operation, in the recovery room, the coded separated postoperative data collection tool was given to the postoperative data collector who was blinded to the group allocation. In the postoperative period, all patients were given diclofenac 50–75 mg TID and 1 g paracetamol QID as soon as they swallowed tablets.
Data management and analysis
Data were coded, cleaned, entered, and cross-checked using SPSS version 20 statistical package (IBM Corp., Armonk, NY, USA). The data were tested for normality using the Shapiro–Wilk normality test. Normally distributed data were analyzed using Student’s t-test. Postoperative opioid consumption and time for first analgesia request, between groups comparisons at each time point were made using Mann–Whitney U-test. For NRS data collected more than once during the study period, the one-way repeated-measures analysis of variance on ranks were used with time of measurement as the repeated factor and group as the nonrepeated factor. If there was a statistical difference (P<0.05) between the two groups by repeated-measures analysis of variance on rank differences, the Mann–Whitney U-test was used to assess differences between the groups. The comparisons of categorical parameters were analyzed using chi-square test or Fisher’s exact test as required. Data are presented as mean ± SD for normally distributed, median (25th–75th percentile) for nonnormally distributed, and categorical data are presented as numbers and frequencies (percentages). P-values <0.05 are considered statistically significant.
Ethical Considerations
Ethical clearance was obtained from University of Gondar School of Medicine ethical committee. Written informed consent was taken from each study participant after a brief explanation and full disclosure of the benefit and risk they will get from participation. Those patients who were complaining of pain during the data collection period were given standard analgesic drugs. Confidentiality was ensured by removing identifiers and locking the questionnaires after data collection.
Results
Sociodemographic characteristics of patients
Fifty two patients were prospectively enrolled in the current study. Two participants were excluded from the study, one from the PVB group because of failed block diagnosed before induction of general anesthesia by pinprick test that revealed three unblocked adjacent dermatomes, and the other one from the control because of refusal to participate in the postoperative time. Only 50 patients were included in the final analysis. Of these patients, 25 were cases who took 15–20 mL of 0.5% bupivacaine and 25 were controls, who were without the block but managed with systemic opioid analgesics. The mean age was (mean± SD) 43.04±11.74 years (range, 22–70). The demographic characteristics (age, sex, weight, height BMI, and ASA physical status), the duration of surgery, and duration of anesthesia were comparable between the two groups (Table 1). In this study, 84% of the PVB group and 80% of the control group were females and 16% of the PVB group and 20% of the control group were males. Assessment of ASA physical status showed that 26% of the PVB patients and 40% of the controls were ASA I and 24% of the PVB patients and 10% of the controls were ASA II (Table 1).
Table 1.
Sociodemographic and intraoperative variables in both groups that underwent open cholecystectomy from February 1 to April 30, 2016.
| Characteristics | PVB (n=25) | Control (n=25) | P-value |
|---|---|---|---|
| Age, years | 43.04±11.74 | 45.72±11.69 | 0.423 |
| Range | 27–70 | 22–70 | |
| Gender | |||
| Male | 4 (16) | 5 (20) | 0.713 |
| Female | 21 (84) | 20 (80) | |
| Height, cm | 1.63±0.56 | 1.59±0.66 | 0.094 |
| Weight, kg | 59.68±6.97 | 57.56±7.03 | 0.29 |
| BMI, kg/m2 | 22.53±2.21 | 22.69±1.99 | 0.785 |
| ASA 1 | 13 (52) | 20 (80) | 0.082 |
| ASA 2 | 12 (48) | 5 (20) | |
| Duration of surgery, min | 113.5±16.48 | 107.92±13.24 | 0.192 |
| Duration of anesthesia | 128.32±11.88 | 124.56±8.93 | 0.212 |
Note: Data presented as mean ± SD or n (%).
Abbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index; PVC, pareventricular block.
There were no significant differences concerning postoperative mean arterial blood pressure, heart rate, respiratory rate, and oxygen saturation values between groups (P>0.05).
Postoperative pain scores using NRS
Pain scores within 24 postoperative hours at rest and while coughing or moving are shown in Tables 2 and 3, respectively. The pain intensity at rest and while coughing (movement) reported with the NRS score was significantly lower in the paravertebral group as compared with the control group for each time point (P<0.05) (Tables 2 and 3).
Table 2.
Postoperative NRS at rest over the first 24 postoperative hours in patients who underwent cholecystectomy from February 1 to April 30, 2016, median (25th–75th percentile)
| Characteristics | PVB group | Control | P-value |
|---|---|---|---|
| NRS at 30 min | 0 (0) | 5 (4–6) | <0.001 |
| NRS at 1 h | 1 (1–2) | 4 (3.5–5.5) | <0.001 |
| NRS at 2 h | 2 (1–3) | 5 (4–5) | <0.001 |
| NRS at 6 h | 2 (2–3) | 4 (3–5.5) | <0.001 |
| NRS at 24 h | 4 (3–6) | 5 (5–7) | 0.002 |
Abbreviations: NRS, numerical rating scale; PVB, paraventricular block.
Table 3.
Postoperative NRS at movement or coughing in the postoperative 24 hours in patients who underwent cholecystectomy from February 1 to April 30, 2016, median (25th–75th percentile)
| Characteristics | PVB group | Control | P-value |
|---|---|---|---|
| NRS at 30 min | 1 (0–1.5) | 6 (5–7) | <0.001 |
| NRS at 1 h | 3 (1–4) | 5 (4.5–6) | <0.001 |
| NRS at 2 h | 4 (4–5) | 6 (6–8) | <0.001 |
| NRS at 6 h | 5 (4–5) | 7 (6–8) | <0.001 |
| NRS at 24 h | 4 (4–7) | 6 (5–7.5) | 0.005 |
Abbreviations: NRS, numerical rating scale; PVB, paraventicular block.
Postoperative total analgesic consumption
Postoperative tramadol and morphine consumption and first analgesic request time was checked using the Shapiro–Wilk test and analysis by Mann–Whitney U-test.
The median (25th–75th percentiles) cumulative morphine consumption, including nurse-administered morphine, in the paravertebral group was 0 mg (0–2) and 2.5 mg (2–4) in the control group, with P-value of 0.001. The total tramadol consumption was significantly lower in the PVB group 200 mg (150.00–250) than the control 300 mg (200–350), with P-value of 0.003 (Table 4).
Table 4.
Postoperative total opioid consumption and first analgesic request time over the first 24 postoperative hours in patients who underwent open cholecystectomy from February 1 to April 30, 2016, median (25th–75th percentile)
| Characteristics | PVB group | control | P-value |
|---|---|---|---|
| Total tramadol consumption (mg) | 200 (150–250) | 300 (200–350) | 0.003 |
| Total morphine consumption (mg) | 0 (0–2) | 2.5 (2–4) | <0.001 |
| First analgesic request time (min) | 120 (60–120) | 30 (27.5–30) | <0.001 |
Abbreviation: PVB, paraventricular block.
Postoperative first analgesic request time
In the current study, first analgesic request time were significantly higher in the PVB group at 120 minutes (60–120) than in the control group at 30 minutes (27.5–30), P-value <0.05 (Table 4).
Discussion
In this prospective randomized study, unilateral single- injection thoracic PVB decreased postoperative pain, reduced total analgesic consumption, and prolonged the median time for first analgesic request for 24 postoperative hours after open cholecystectomy. The postoperative NRS score was reduced more significantly in the PVB group than in the control group at 30 minutes and 1, 2, 6, and 24 postoperative hours both at rest and on movement. This finding was comparable with previous studies where single-injection TPVB reduced the severity of postoperative pain after open cholecystectomy,9 thoracoscopic surgery,7 breast surgery,19 inguinal hernia repair,20 and rib fractures.21
Single-injection thoracic PVB resulted in pain reduction after cholecystectomy via subcostal incision that could attribute to blockade of somatic and sympathetic pain fibers originating from T5-T1.22 However, unilateral single-shot PVB might provide total visceral anesthesia as vagal nerve and contralateral sympathetic fibers are not targeted by unilateral PVB. Although bilateral PVB using landmark techniques can provide total visceral analgesia, it is highly associated with risk of inadvertent vascular puncture and pleural puncture that could result in development of pneumothorax.23
Low NRS scores seen both at rest and on movement or coughing in the PVB group at 24 hours might not be due to the pharmacological effect of bupivacaine. This, rather, could be related to the preemptive effect of the block, which reduces the nociceptive input to the central nervous system in the first few hours of surgery that could attenuate central sensitization, thereby leading to less postoperative pain.24
Different dematome block levels for cholecystectomy have been reported. In previous studies, after a single injection, a mean sensory level of 2.2 and 1.4 segments above and below the level of injection with the mean value of 4.6 segments25 and sensory analgesia from 1 to 8 dermatomes after a single injection of 0.5% bupivacaine have been reported.26 In our study, patients who had less than 3 dermatomal blocks were excluded. We agreed that this spread is sufficient to block pain sensation after cholecystectomy surgery. Single-injection PVB at T4 is also reported to be as equally effective as multilevel injection for postoperative pain control as shown in a randomized control study conducted on patients undergoing partial mastectomy with lymph node dissection, which possibly minimizes complications associated with multilevel block.27
In this study, the median time for the first analgesic request was significantly prolonged in the single-shot PVB group 120 (60–120) vs controls 30 (27.5–30, P=0.000). Our finding was comparable with previous studies in various procedures such as right lobe donor hepatectomy (P<0.01)28 and breast surgery (P<0.019).19 The similarity might be due to the use of same dose of bupivacaine, 0.3 mL/kg of 0.5%. Although there was prolonged median time of analgesic request in the PVB group in both studies, the median analgesic request time was more prolonged in our study, which could be attributed to the use of fentanyl at the end of surgery. Moreover, this study revealed that the total amount of opioid consumption over the postoperative 24 hours was lower in the PVB group than the control group, with (P<0.05), which was comparable with previous studies conducted on various procedures using the same concentration of bupivacaine and levobupivacaine.28–31
However, there are still controversies regarding the efficacy of single-shot PVB for post cholecystectomy pain relief. A single preincisional thoracic paravertebral injection of bupivacaine 0.5%, 20 mL, before cholecystectomy provides complete pain relief for 1–6 hours.32 On the other hand, another study revealed that TPVB with 0.5% bupivacaine at a 15-mL bolus dose followed by an infusion of 5 mL/h postoperatively was inadequate after cholecystectomy.33 This implies that single-shot PVB alone may not provide adequate postoperative analgesia and that systemic supplementation may be required.
Furthermore, there are also discrepancies regarding the mean duration between the initial dose and the first top-up of local anesthetic of 7.2±2.7 hours, which shows a need for supplementation after 7 hours.34 In the current study, there were no complications associated with PVB. Complication rates of thoracic PVB are reported to be low,35,36 reported figures (5%),37 and even in another retrospective study that reviewed 156 thoracic PVB cases with multiple-injection technique, complications occurred in only four cases (2.6%).38 In addition, a prospective study evaluating complications after PVBs in 367 patients observed the following complications: vascular puncture (3.8%), hypotension (4.6%), pleural puncture (1.1%), and pneumothorax (0.5%).35 TPVB is technically easy to learn, has a high success rate regardless of the number of blocks performed, and does not appear to be operator dependent.38–40
Limitation of the study
The current study has certain limitations, including shorter duration of postoperative follow-up and inability to accurately identify failed blocks. There was involvement of nurses in postoperative pain management that might have an impact on the magnitude of treatment difference.
Conclusion and recommendation
TPVB is effective in controlling the postoperative pain, prolongs analgesic request time, and has a good opioid-sparing effect in comparison to the traditional general anesthesia alone for patients undergoing open cholecystectomy and deserves more widespread use. We recommend further study, including one with a longer time of follow-up of at least 48 hours postoperatively, to evaluate the effect of PVB after cholecystectomy on clinically important outcomes, such as complication rates and intraoperative hemodynamic changes.
Acknowledgments
We would like to thank the University of Gondar for financial support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Baidya DK, Khanna P, Maitra S. Analgesic efficacy and safety of thoracic paravertebral and epidural analgesia for thoracic surgery: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2014;18(5):626–635. doi: 10.1093/icvts/ivt551. [DOI] [PubMed] [Google Scholar]
- 2.Gan TJ, Robinson SB, Oderda GM, Scranton R, Pepin J, Ramamoorthy S. Impact of postsurgical opioid use and ileus on economic outcomes in gastrointestinal surgeries. Curr Med Res Opin. 2015;31(4):677–686. doi: 10.1185/03007995.2015.1005833. [DOI] [PubMed] [Google Scholar]
- 3.Glissmeyer C, Johnson W, Sherman B, Glissmeyer M, Garreau J, Johnson N. Effect of paravertebral nerve blocks on narcotic use after mastectomy with reconstruction. Am J Surg. 2015;209(5):881–883. doi: 10.1016/j.amjsurg.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Lönnqvist P, Olsson G. Paravertebral vs epidural block in children. Effects on postoperative morphine requirement after renal surgery. Acta Anaesthesiol Scand. 1994;38(4):346–349. doi: 10.1111/j.1399-6576.1994.tb03905.x. [DOI] [PubMed] [Google Scholar]
- 5.Ak K, Gursoy S, Duger C, et al. Thoracic paravertebral block for postoperative pain management in percutaneous nephrolithotomy patients: a randomized controlled clinical trial. Med Princ Pract. 2013;22(3):229–233. doi: 10.1159/000345381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ballantyne JC, Carr DB, Suarez T, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86(3):598–612. doi: 10.1097/00000539-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 7.Vogt A, Stieger DS, Theurillat C, Curatolo M. Single-injection thoracic paravertebral block for postoperative pain treatment after thoracoscopic surgery. Br J Anaesthesia. 2005;95(6):816–821. doi: 10.1093/bja/aei250. [DOI] [PubMed] [Google Scholar]
- 8.Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2010;105(6):842–852. doi: 10.1093/bja/aeq265. [DOI] [PubMed] [Google Scholar]
- 9.Paleczny J, Zipser P, Pysz M. Paravertebral block for open cholecystectomy. Anestezjol Intens Ter. 2009;41(2):89–93. Polish. [PubMed] [Google Scholar]
- 10.Perkins FM, Kahlet H. Chronic pain as an outcome of surgery. A review of predictive factors. Anesthesiology. 2000;93:1123–1133. doi: 10.1097/00000542-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 11.Cheema S, Richardson J, McGurgan P. Factors affecting the spread of bupivacaine in the adult thoracic paravertebral space. Anaesthesia. 2003;58(7):684–687. doi: 10.1046/j.1365-2044.2003.03189_1.x. [DOI] [PubMed] [Google Scholar]
- 12.Naja MZ, Gustafsson AC, Ziade MF, et al. Distance between the skin and the thoracic paravertebral space. Anaesthesia. 2005;60:680–684. doi: 10.1111/j.1365-2044.2005.04232.x. [DOI] [PubMed] [Google Scholar]
- 13.Cutshall C, Hutchins J. Ultrasound-guided continuous thoracic paravertebral catheter management of acute rib pain secondary to cystic fibrosis exacerbation in a pediatric patient. A A Case Rep. 2015;4(3):29–30. doi: 10.1213/XAA.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 14.Matinian VV, Belousova EI, Saltanov AI. Ultrasound guided catheterization of thoracic paravertebral space. Anesteziol Reanimatol. 2014;59(5):57–58. Russian. [PubMed] [Google Scholar]
- 15.Bugelli GJ, Samra WA, Stuart-Smith K. Simple mastectomy and axillary lymph node biopsy performed under paravertebral block and light sedation in a patient with severe cardiorespiratory comorbidities: proposed management of choice in high-risk breast surgery patients. Clin Breast Cancer. 2013;13(2):153–155. doi: 10.1016/j.clbc.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Moawad HE, Mousa SA, El-Hefnawy AS. Single-dose paravertebral blockade versus epidural blockade for pain relief after open renal surgery: a prospective randomized study. Saudi J Anaesth. 2013;7(1):61–67. doi: 10.4103/1658-354X.109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang W, Fang C, Li J, et al. Single-dose, bilateral paravertebral block plus intravenous sufentanil analgesia in patients with esophageal cancer undergoing combined thoracoscopic-laparoscopic esophagectomy: a safe and effective alternative. J Cardiothorac Vasc Anesth. 2014;28(4):978–984. doi: 10.1053/j.jvca.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Lonnqvist PA, MacKenzie J, Soni AK, Conacher ID. Paravertebral blockade. Failure rate and complications. Anaesthesia. 1995;50:813–815. doi: 10.1111/j.1365-2044.1995.tb06148.x. [DOI] [PubMed] [Google Scholar]
- 19.Kairaluoma PM, Bachmann MS, Korpinen AK, Rosenberg PH, Pere PJ. Single-injection paravertebral block before general anesthesia enhances analgesia after breast cancer surgery with and without associated lymph node biopsy. Anesth Analg. 2004;99(6):1837–1843. doi: 10.1213/01.ANE.0000136775.15566.87. [DOI] [PubMed] [Google Scholar]
- 20.Klein SM, Pietrobon R, Nielsen KC, et al. Paravertebral somatic nerve block compared with peripheral nerve blocks for outpatient inguinal herniorrhaphy. Reg Anesth Pain Med. 2002;27(5):476–480. doi: 10.1053/rapm.2002.35147. [DOI] [PubMed] [Google Scholar]
- 21.Karmakar MK, Chui PT, Joynt GM, Ho AM. Thoracic paravertebral block for management of pain associated with multiple fractured ribs in patients with concomitant lumbar spinal trauma. Reg Anesth Pain Med. 2001;26(2):169–173. doi: 10.1053/rapm.2001.21086. [DOI] [PubMed] [Google Scholar]
- 22.Berthoud HR. Anatomy and function of sensory hepatic nerves. Anat Rec A Discov Mol Cell Evol Biol. 2004;280(1):827–835. doi: 10.1002/ar.a.20088. [DOI] [PubMed] [Google Scholar]
- 23.Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade incidence of failed block and complications. Anaesthesia. 2001;56(12):1181–1201. doi: 10.1046/j.1365-2044.2001.02084-2.x. [DOI] [PubMed] [Google Scholar]
- 24.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288(5472):1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 25.Naja M, Ziade M, Rajab ME, Tayara KE, Lönnqvist P. Varying anatomical injection points within the thoracic paravertebral space: effect on spread of solution and nerve blockade. Anaesthesia. 2004;59(5):459–463. doi: 10.1111/j.1365-2044.2004.03705.x. [DOI] [PubMed] [Google Scholar]
- 26.Moller JF, Nikolajsen L, Rodt SA, Ronning H, Carlsson PS. Thoracic paravertebral block for breast cancer surgery: a randomized double-blind study. Anesth Analg. 2007;105(6):1848–1851. doi: 10.1213/01.ane.0000286135.21333.fd. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama MT. A randomized controlled study of the effects of single or multilevel paravertebral block on postoperative analgesia in partial mastectomy with lymph node dissection. Anaesth Pain Intens Care. 2015;19(4):4637. [Google Scholar]
- 28.Moussa A. Opioid saving strategy: bilateral single-site thoracic paravertebral block in right lobe donor hepatectomy. Middle East J Anesthesiol. 2008;19(4):789–801. [PubMed] [Google Scholar]
- 29.Franceschini D, Lipartiti M, Giusti P. Effect of acute and chronic tramadol on [3 H]-norepinephrine-uptake in rat cortical synaptosomes. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(3):485–496. doi: 10.1016/s0278-5846(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 30.Yılmaz Ö, Saraçoğlu A, Bezen O, Şengül T. Effects of a thoracic paravertebral block on postoperative analgesia in patients undergoing modified radical mastectomy. Agri. 2014;26(4):179–183. doi: 10.5505/agri.2014.65982. [DOI] [PubMed] [Google Scholar]
- 31.Breschan C, Jost R, Krumpholz R, et al. A prospective study comparing the analgesic efficacy of levobupivacaine, ropivacaine and bupivacaine in pediatric patients undergoing caudal blockade. Pediatr Anesth. 2005;15(4):301–306. doi: 10.1111/j.1460-9592.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 32.Giesecke K, Hamberger B, Järnberg PO, Klingstedt C. Paravertebral block during cholecystectomy: effects on circulatory and hormonal responses. Br J Anaesth. 1988;61(6):652–656. doi: 10.1093/bja/61.6.652. [DOI] [PubMed] [Google Scholar]
- 33.Bigler D, Dirkes W, Hansen R, Rosenberg J, Kehlet H. Effects of thoracic paravertebral block with bupivacaine versus combined thoracic epidural block with bupivacaine and morphine on pain and pulmonary function after cholecystectomy. Acta Anaesthesiol Scand. 1989;33(7):561–564. doi: 10.1111/j.1399-6576.1989.tb02966.x. [DOI] [PubMed] [Google Scholar]
- 34.Murphy D. Continuous intercostal nerve blockade for pain relief following cholecystectomy. Br J Anaesthesia. 1983;55(6):521–524. doi: 10.1093/bja/55.6.521. [DOI] [PubMed] [Google Scholar]
- 35.Lönnqvist P, MacKenzie J, Soni A, Conacher I. Paravertebral blockade. Anaesthesia. 1995;50(9):813–815. doi: 10.1111/j.1365-2044.1995.tb06148.x. [DOI] [PubMed] [Google Scholar]
- 36.Richardson J, Sabanathan S, Mearns A, Sides C, Goulden C. Post-thoracotomy neuralgia. Pain Clinic. 1994;7(2):87–97. [Google Scholar]
- 37.Richardson J, Sabanathan S. Thoracic paravertebral analgesia. Acta Anaesthesiol Scand. 1995;39(8):1005–1015. doi: 10.1111/j.1399-6576.1995.tb04219.x. [DOI] [PubMed] [Google Scholar]
- 38.Coveney E, Weltz CR, Greengrass R, et al. Use of paravertebral block anesthesia in the surgical management of breast cancer: experience in 156 cases. Ann Surgery. 1998;227(4):496. doi: 10.1097/00000658-199804000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pusch F, Freitag H, Weinstabl C, Obwegeser R, Huber E, Wildling E. Single injection paravertebral block compared to general anaesthesia in breast surgery. Acta Anaesthesiol Scand. 1999;43(7):770–774. doi: 10.1034/j.1399-6576.1999.430714.x. [DOI] [PubMed] [Google Scholar]
- 40.Kirvelä O, Antila H. Thoracic paravertebral block in chronic postoperative pain. Reg Anesth Pain Med. 1992;17(6):348–350. [PubMed] [Google Scholar]