Abstract
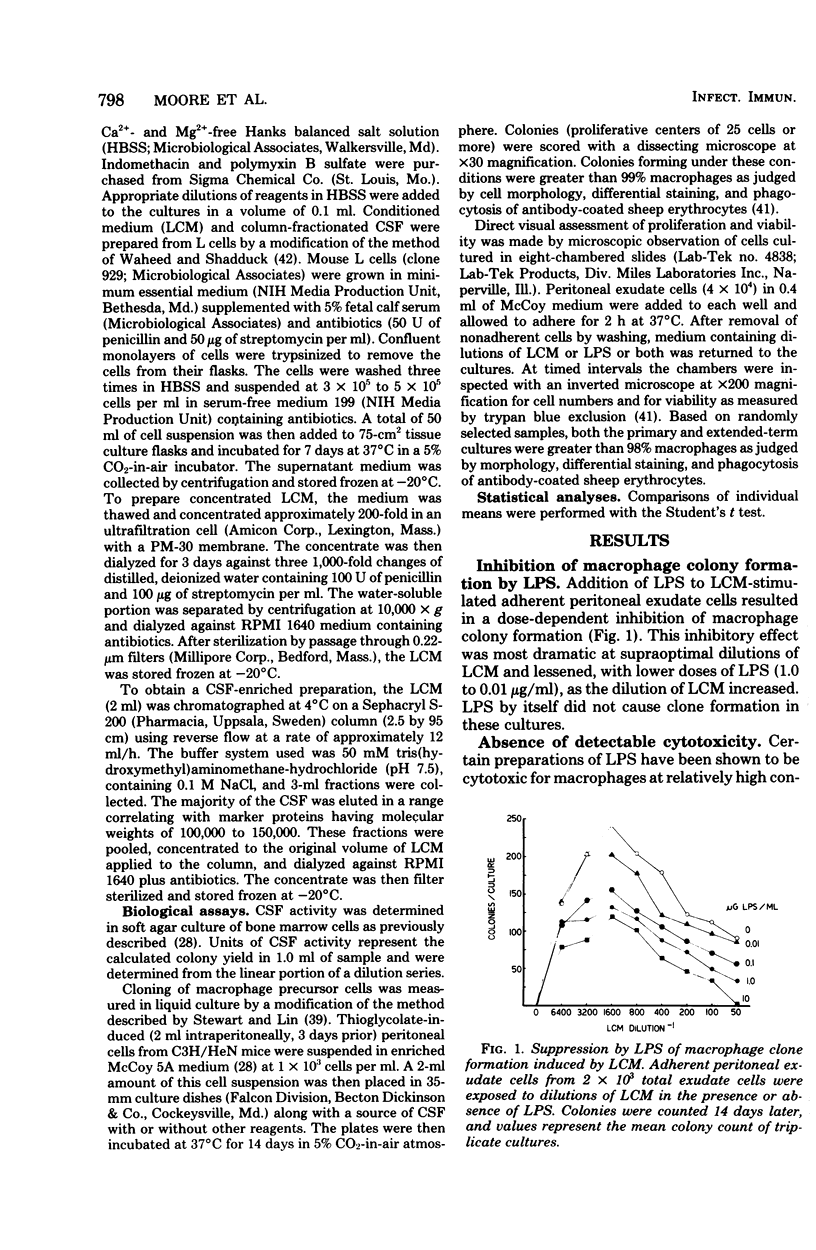
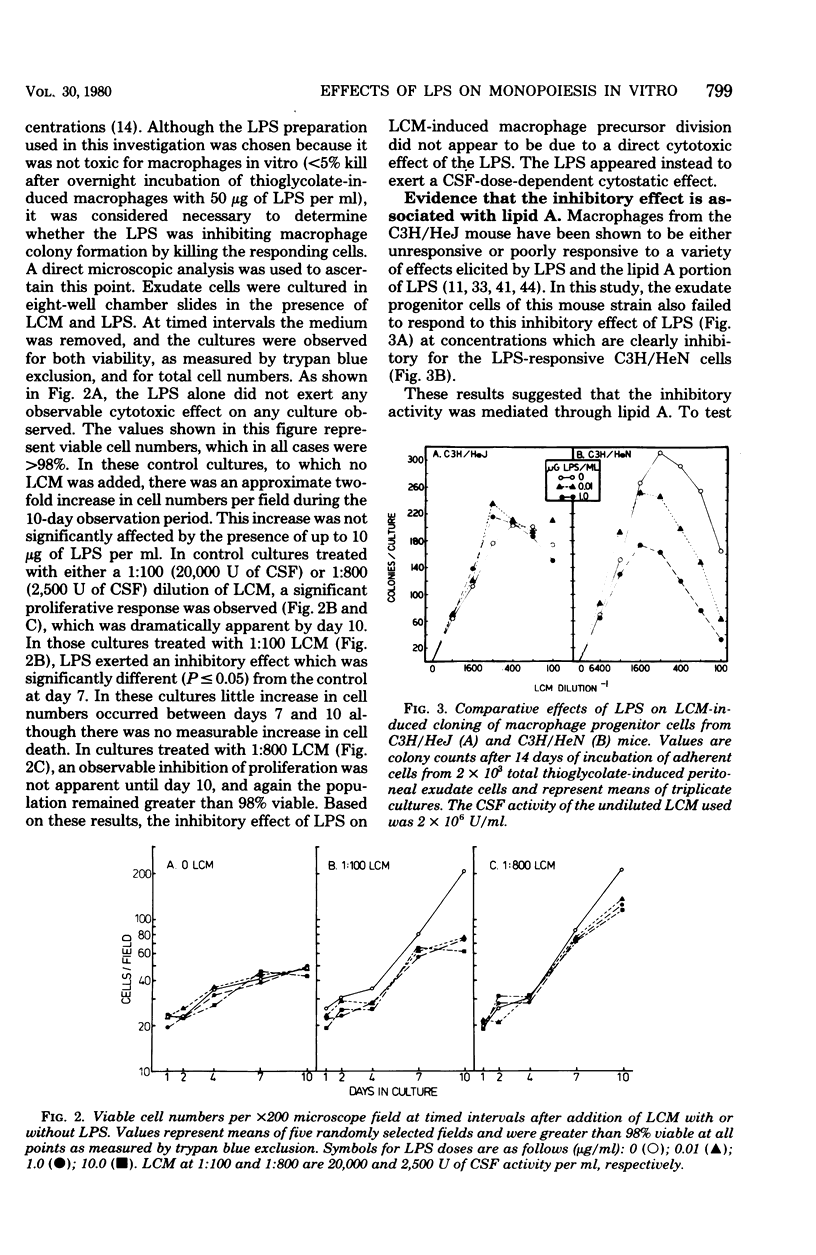
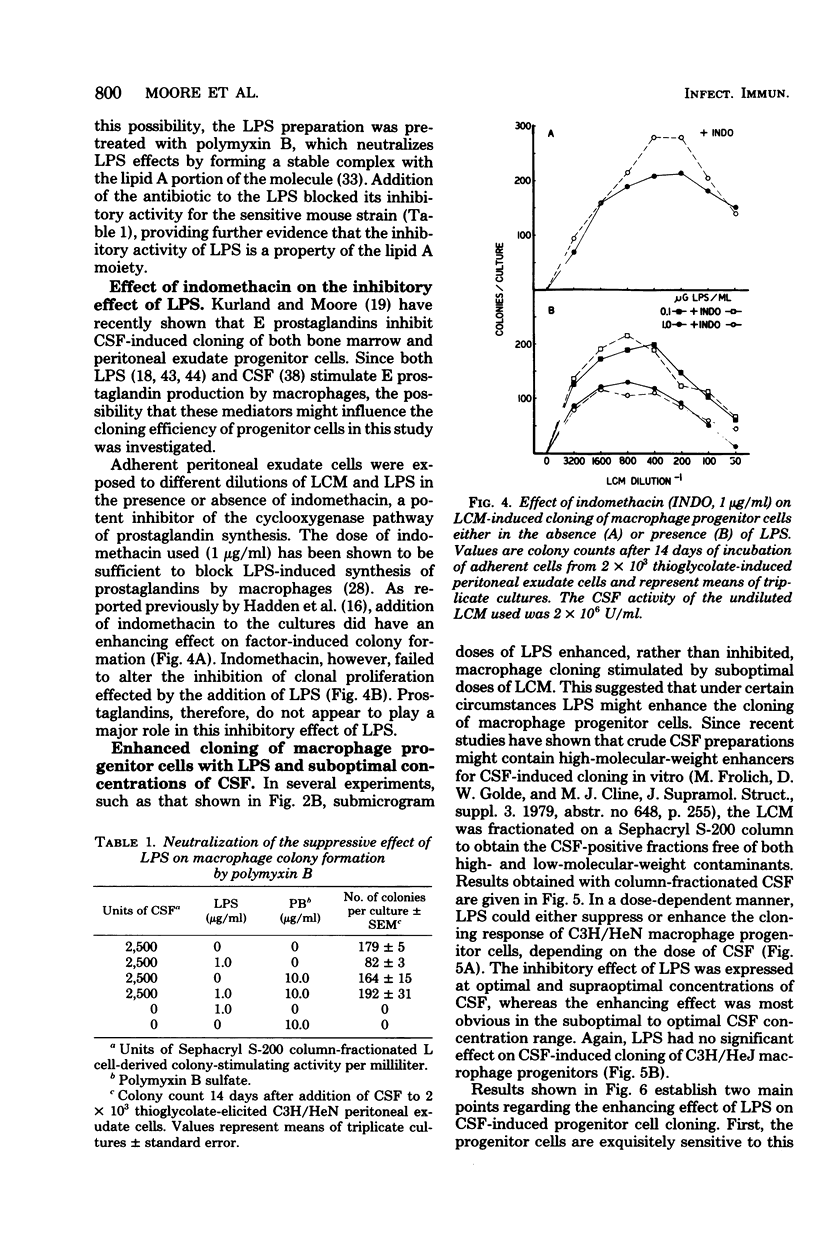
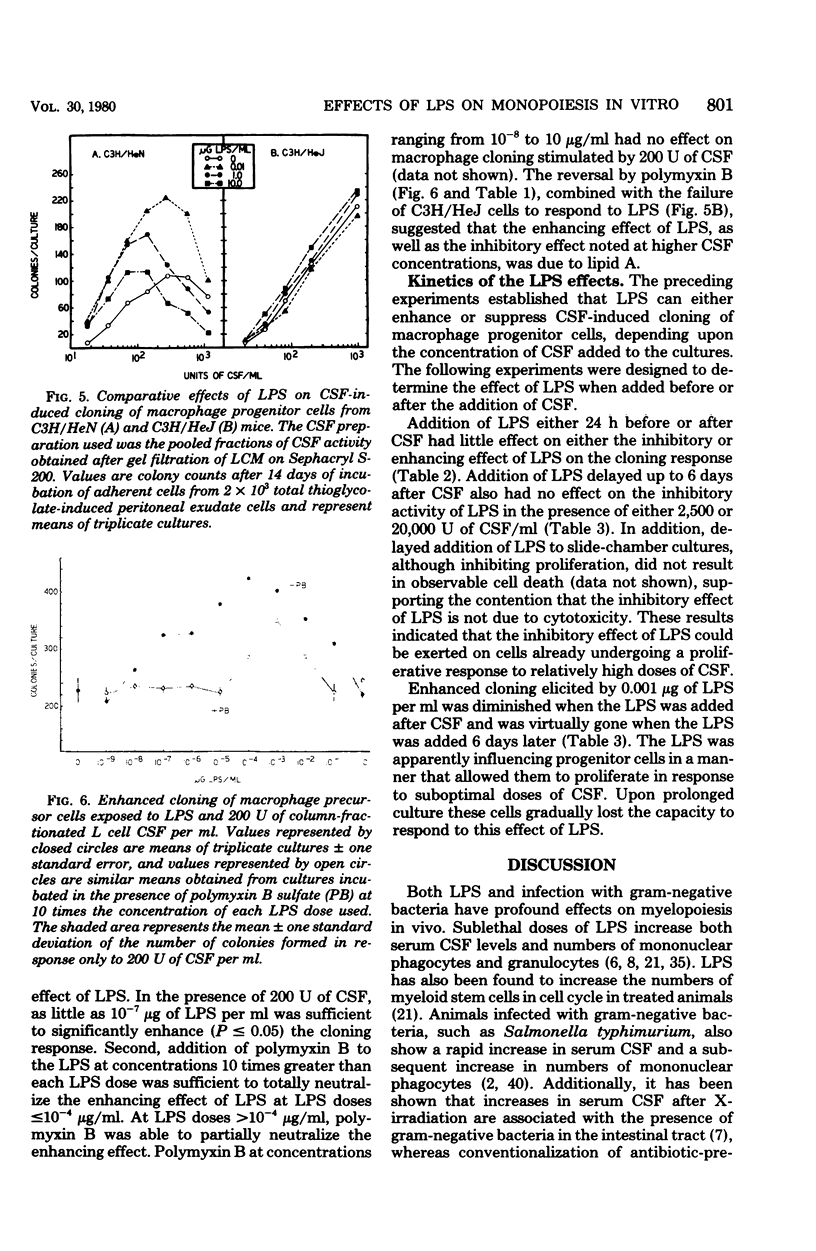
Bacterial lipopolysaccharides (LPS) enhance both production of colony-stimulating factors (CSF) and proliferation of mononuclear phagocytes in vivo. The present study was undertaken to determine whether the effects of LPS on CSF-dependent monopoiesis are due solely to enhanced production of CSF or also to direct effects of LPS on the responding progenitor cell. Addition of LPS to CSF-stimulated macrophage populations had different effects, depending upon the concentration of CSF in the cultures. In the presence of optimal to supraoptimal concentrations of CSF, LPS at doses ≥0.01 μg/ml inhibited macrophage colony formation. This inhibitory activity was not due to cytotoxicity of the LPS and was not mediated through prostaglandin synthesis. In the presence of suboptimal concentrations of CSF, minute concentrations of LPS (10−7 μg/ml) significantly enhanced macrophage colony formation. Both effects of LPS (inhibition and enhancement) appeared to be properties of lipid A since neither effect was noted with cells from LPS-resistant C3H/HeJ mice, whereas both effects could be neutralized by the addition of the antibiotic polymyxin B, which binds to the lipid A portion of LPS. These results suggest that the effects of LPS on monopoiesis in vivo may not be due solely to its capacity to stimulate production of CSF. Rather, LPS may be involved in stimulating monopoiesis both indirectly through stimulation of CSF production and by its effects on the CSF-responsive progenitor cell.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander P., Evans R. Endotoxin and double stranded RNA render macrophages cytotoxic. Nat New Biol. 1971 Jul 21;232(29):76–78. doi: 10.1038/newbio232076a0. [DOI] [PubMed] [Google Scholar]
- BOHME D. H., SCHNEIDER H. A., LEE J. M. Some physiopathological parameters of natural resistance to infection in murine salmonellosis. J Exp Med. 1959 Jul 1;110(1):9–26. doi: 10.1084/jem.110.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S. G. Cellular and molecular mechanisms of action of bacterial endotoxins. Annu Rev Microbiol. 1979;33:67–94. doi: 10.1146/annurev.mi.33.100179.000435. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Metcalf D., Watt S. M. Regulation of hemopoietic cell differentiation and proliferation. J Supramol Struct. 1978;8(4):489–500. doi: 10.1002/jss.400080411. [DOI] [PubMed] [Google Scholar]
- Butler R. C., Abdelnoor A. M., Nowotny A. Bone marrow colony-stimulating factor and tumor resistance-enhancing activity of postendotoxin mouse sera. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2893–2896. doi: 10.1073/pnas.75.6.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. F., Pollard M. Effects of microbial flora on levels of colonay stimulating factor in serums of irradiated CFW mice. Proc Soc Exp Biol Med. 1973 Oct 1;144(1):177–180. doi: 10.3181/00379727-144-37551. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A. Effect of endotoxin and postendotoxin plasma on in vitro granulopoiesis. J Lab Clin Med. 1972 Jun;79(6):1014–1020. [PubMed] [Google Scholar]
- Dienstman S. R., Defendi V. Necessary and sufficient conditions for recruitment of macrophages into the cell cycle. Exp Cell Res. 1978 Aug;115(1):191–199. doi: 10.1016/0014-4827(78)90416-0. [DOI] [PubMed] [Google Scholar]
- Doe W. F., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. I. Cytolytic effect on tumor target cells. J Exp Med. 1978 Aug 1;148(2):544–556. doi: 10.1084/jem.148.2.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe W. F., Yang S. T., Morrison D. C., Betz S. J., Henson P. M. Macrophage stimulation by bacterial lipopolysaccharides. II. Evidence for differentiation signals delivered by lipid A and by a protein rich fraction of lipopolysaccharides. J Exp Med. 1978 Aug 1;148(2):557–568. doi: 10.1084/jem.148.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves A. C., Bruce W. R. In vitro production of colony-stimulating activity. I. Exposure of mouse peritoneal cells to endotoxin. Cell Tissue Kinet. 1974 Jan;7(1):19–30. doi: 10.1111/j.1365-2184.1974.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Gery I., Gershon R. K., Waksman B. H. Potentiation of the T-lymphocyte response to mitogens. I. The responding cell. J Exp Med. 1972 Jul 1;136(1):128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glode L. M., Jacques A., Mergenhagen S. E., Rosenstreich D. L. Resistance of macrophages from C3H/HeJ mice to the in vitro cytotoxic effects of endotoxin. J Immunol. 1977 Jul;119(1):162–166. [PubMed] [Google Scholar]
- Hadden J. W., Sadlik J. R., Hadden E. M. The induction of macrophage proliferation in vitro by a lymphocyte-produced factor. J Immunol. 1978 Jul;121(1):231–238. [PubMed] [Google Scholar]
- Kurland J. I., Bockman R. Prostaglandin E production by human blood monocytes and mouse peritoneal macrophages. J Exp Med. 1978 Mar 1;147(3):952–957. doi: 10.1084/jem.147.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland J. I., Pelus L. M., Ralph P., Bockman R. S., Moore M. A. Induction of prostaglandin E synthesis in normal and neoplastic macrophages: role for colony-stimulating factor(s) distinct from effects on myeloid progenitor cell proliferation. Proc Natl Acad Sci U S A. 1979 May;76(5):2326–2330. doi: 10.1073/pnas.76.5.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland J., Moore M. A. Modulation of hemopoiesis by prostaglandins. Exp Hematol. 1977 Sep;5(5):357–373. [PubMed] [Google Scholar]
- MacVittie T. J., Walker R. I. Canine granulopoiesis: alterations induced by suppression of gram-negative flora. Exp Hematol. 1978 Sep;6(8):639–647. [PubMed] [Google Scholar]
- MacVittie T. J., Walker R. I. Endotoxin-induced alterations in canine granulopoiesis: colony-stimulating factor, colony-forming cells in culture, and growth of cells in diffusion chambers. Exp Hematol. 1978 Aug;6(7):613–618. [PubMed] [Google Scholar]
- Maehara N., Ho M. Cellular origin of interferon induced by bacterial lipopolysaccharide. Infect Immun. 1977 Jan;15(1):78–83. doi: 10.1128/iai.15.1.78-83.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Oppenheim J. J., Rosentreich D. L. Characterization of lymphocyte-activating factor (LAF) produced by a macrophage cell line, P388D1. II. Biochemical characterization of LAF induced by activated T cells and LPS. J Immunol. 1978 May;120(5):1504–1508. [PubMed] [Google Scholar]
- Moore R. N., Goodrum K. J., Berry L. J. Mediation of an endotoxic effect by macrophages. J Reticuloendothel Soc. 1976 Mar;19(3):187–197. [PubMed] [Google Scholar]
- Moore R. N., Urbaschek R., Wahl L. M., Mergenhagen S. E. Prostaglandin regulation of colony-stimulating factor production by lipopolysaccharide-stimulated murine leukocytes. Infect Immun. 1979 Nov;26(2):408–414. doi: 10.1128/iai.26.2.408-414.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Jacobs D. M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976 Oct;13(10):813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- Mørland B., Mørland J. Selective induction of lysosomal enzyme activities in mouse peritoneal macrophages. J Reticuloendothel Soc. 1978 Jun;23(6):469–477. [PubMed] [Google Scholar]
- Pabst M. J., Johnston R. B., Jr Increased production of superoxide anion by macrophages exposed in vitro to muramyl dipeptide or lipopolysaccharide. J Exp Med. 1980 Jan 1;151(1):101–114. doi: 10.1084/jem.151.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peavy D. L., Baughn R. E., Musher D. M. Strain-dependent cytotoxic effects of endotoxin for mouse peritoneal macrophages. Infect Immun. 1978 Jul;21(1):310–319. doi: 10.1128/iai.21.1.310-319.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The induction of clones of normal mast cells by a substance from conditioned medium. Exp Cell Res. 1966 Oct;43(3):553–563. doi: 10.1016/0014-4827(66)90026-7. [DOI] [PubMed] [Google Scholar]
- Quesenberry P., Morley A., Stohlman F., Jr, Rickard K., Howard D., Smith M. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972 Feb 3;286(5):227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- Shah R. G., Green S., Moore M. A. Colony stimulating and inhibiting activities in mouse serum after Corynebacterium parvum-endotoxin treatment. J Reticuloendothel Soc. 1978 Jan;23(1):29–41. [PubMed] [Google Scholar]
- Shands J. W., Jr, Peavy D. L., Gormus B. J., McGraw J. In vitro and in vivo effects of endotoxin on mouse peritoneal cells. Infect Immun. 1974 Jan;9(1):106–112. doi: 10.1128/iai.9.1.106-112.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Cifone M., Heard P. M., Defendi V. Factors regulating macrophage production and growth: identity of colony-stimulating factor and macrophage growth factor. J Exp Med. 1976 Mar 1;143(3):631–647. doi: 10.1084/jem.143.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C. C., Lin H. Macrophage growth factor and its relationship to colony stimulating factor. J Reticuloendothel Soc. 1978 Apr;23(4):269–285. [PubMed] [Google Scholar]
- Trudgett A., McNeill T. A., Killen M. Granulocyte-macrophage precursor cell and colony-stimulating factor responses of mice infected with Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):450–455. doi: 10.1128/iai.8.3.450-455.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Marshall S. T., Rosenstreich D. L. Analysis of the effects of lipopolysaccharide on macrophages: differential phagocytic responses of C3H/HeN and C3H/HeJ macrophages in vitro. Infect Immun. 1979 Jul;25(1):328–336. doi: 10.1128/iai.25.1.328-336.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A., Shadduck R. K. Purification and properties of L cell-derived colony-stimulating factor. J Lab Clin Med. 1979 Jul;94(1):180–193. [PubMed] [Google Scholar]
- Wahl L. M., Olsen C. E., Sandberg A. L., Mergenhagen S. E. Prostaglandin regulation of macrophage collagenase production. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4955–4958. doi: 10.1073/pnas.74.11.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Rosenstreich D. L., Glode L. M., Sandberg A. L., Mergenhagen S. E. Defective prostaglandin synthesis by C3H/HeJ mouse macrophages stimulated with endotoxin preparations. Infect Immun. 1979 Jan;23(1):8–13. doi: 10.1128/iai.23.1.8-13.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl L. M., Wahl S. M., Mergenhagen S. E., Martin G. R. Collagenase production by endotoxin-activated macrophages. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3598–3601. doi: 10.1073/pnas.71.9.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J. B., Chapman H. A., Jr, Hibbs J. B., Jr Characterization of the effects of endotoxin on macrophage tumor cell killing. J Immunol. 1978 Jul;121(1):72–80. [PubMed] [Google Scholar]
- van 't Hull E., Schellekens H., Löwenberg B., de Vries M. J. Influence of interferon preparations on the proliferative capacity of human and mouse bone marrow cells in vitro. Cancer Res. 1978 Apr;38(4):911–914. [PubMed] [Google Scholar]




