Abstract
Background and Purpose
It has recently been suggested that 5‐HT3 receptor blockade enhances the efficacy of selective 5‐HT (serotonin) reuptake inhibitor (SSRI) antidepressants and may reverse stress‐induced deficits in rodents.
Experimental Approach
To further explore this hypothesis, we used mice lacking the 5‐HT3 receptor (Htr3a KO) and their wild‐type (WT) controls to assess their response in behavioural paradigms relevant to anxiety and depression. Mice were studied under basal, antidepressant treatments and chronic social defeat stress (CSDS) conditions.
Key Results
In basal conditions, Htr3a KO mice displayed anxiolytic‐ and antidepressant‐like behaviours in the elevated plus maze, the social interaction and the forced swim tests (FST), but behaved as WT mice in response to acute citalopram in the FST. However, the effects of fluoxetine were blunted in Htr3a KO mice in these same tests. In an in vitro electrophysiological paradigm, a low‐dose citalopram treatment triggered 5‐HT1A receptor desensitization only in the dorsal raphe nucleus of Htr3a KO, although a high dose desensitized 5‐HT1A autoreceptor function equally in Htr3a KO and WT mice, suggesting that citalopram may become effective at lower doses when 5‐HT3 receptors are inactivated. In addition, Htr3a deletion blocked CSDS‐induced modification in the cortical expression of two genes involved in oxidative stress, CaMKIIa and SOD1.
Conclusions and Implications
Taken together, these data show that Htr3a deletion promotes SSRI efficacy and prevents the occurrence of stress‐induced deleterious effects, suggesting that the 5‐HT3 receptor may represent an interesting target for the treatment of stress‐related disorders.
Abbreviations
- 5‐CT
5‐carboxamidotryptamine
- 5‐HIAA
5‐hydroxyindolacetic acid
- SERT (5‐HTT)
5‐HT transporter
- CaMKIIa
calmodulin‐dependent protein kinase IIα
- CSDS
chronic social defeat stress
- DRN
dorsal raphe nucleus
- EPM
elevated plus maze
- FST
forced swim test
- GIRK
G‐protein‐gated inwardly rectifying K+
- KO
knockout
- NTS
nucleus tractus solitarius
- OF
open field test
- SI
social interaction test
- SSRI
selective 5‐HT (serotonin) reuptake inhibitor
- WT
wild‐type
Introduction
Selective 5‐HT (serotonin) reuptake inhibitors (SSRIs) are currently the compounds most prescribed to treat major depression. Although they display a good clinical efficacy, about 50% of patients do not respond correctly to this first‐line therapy (Hamon and Blier, 2013) and they induce dose‐dependent negative side‐effects in several patients that can lead to treatment interruption (Zajecka et al., 2010). Among the new generation of drugs in development, vortioxetine is a recently (2014) released multimodal antidepressant, which, besides being a 5‐HT transporter (SERT; 5‐HTT) inhibitor, a 5‐HT1A receptor agonist and a 5‐HT1B receptor partial agonist is also a 5‐HT7 and 5‐HT3 receptor antagonist (Mork et al., 2012), the latter property being crucial in its mechanism of action (Sanchez et al., 2015). Multimodal actions and in particular those involving 5‐HT3 receptor blockade may facilitate the antidepressant efficacy of 5‐HT reuptake blockers at lower doses.
The 5‐HT3 receptor, the only ligand‐gated cation channel among the 5‐HT receptor family, is located on brain structures relevant to depression and antidepressant responses (Laporte et al., 1992). It has long been argued that 5‐HT3 receptor antagonists possess antidepressant and anxiolytic properties (Martin et al., 1992; Mahesh et al., 2013) and recent data showed that they could potentiate the effects of an SSRI in preclinical behavioural tests (Ramamoorthy et al., 2008; Bétry et al., 2015). Interestingly, chronic vortioxetine has been shown to induce a faster recovery of serotonergic neurons’ firing than fluoxetine treatment (Bétry et al., 2013). These effects of vortioxetine were attributed to its capacity to induce faster 5‐HT1A receptor desensitization, which is usually correlated to an antidepressant's delay of action (Le Poul et al., 1995). However, the fact that in Betry's study, 5‐HT1A receptor desensitization was indirectly measured and the effect of vortioxetine was blocked by 5‐HT3 receptor agonist ligands, which by themselves could induce a rapid desensitization of 5‐HT3 receptors (Thompson and Lummis, 2006) limited the conclusions of these pharmacological studies. It was therefore crucial to determine if the effect of SSRIs is effectively potentiated in the absence of 5‐HT3 receptors. The generation of mutant mice lacking the 5‐HT3 receptor subunit 3A, essential for the receptor channel function, was instrumental in confirming its involvement in anxiety‐related behaviours (Kelley et al., 2003; Bhatnagar et al., 2004). However, although Bhatnagar and Vining (2004) suggested a blunted responsiveness of these mutant mice to acute stress, the effects of chronic stress and antidepressant treatments on 5‐HT3 receptor knockout (Htr3a KO) mice in assays related to depression had not yet been investigated.
Therefore, to thoroughly characterize the role of 5‐HT3 receptors in depression‐related behaviours and antidepressant treatments, Htr3a KO and wild‐type (WT) mice were exposed to acute and chronic SSRI antidepressant treatments and studied in the chronic social defeat stress (CSDS) paradigm, a validated model of depression (see Chaouloff, 2013). We assessed the effects of 5‐HT3 receptor genetic invalidation in behavioural tests related to anxiety and depression and the effects of acute citalopram and fluoxetine in these mutant mice. In vitro electrophysiological studies were used to assess the citalopram‐induced 5‐HT1A autoreceptor desensitization in mice lacking 5‐HT3 receptors. Finally, as oxidative stress is an early event putatively involved in the pathogenesis of depression, in these mutant mice we assessed the effect of the CSDS paradigm on two oxidative stress markers, SOD1 and the calmodulin‐dependent protein kinase IIα (CaMKIIa), recently found to be regulated by 5‐HT3 receptors (Bhatt et al., 2014; Hutchinson et al., 2015).
Methods
Animals
Experiments were performed using male homozygous Htr3a KO and WT littermates born from heterozygous mutants on a C57BL/6 J genetic background (>10 generations) and genotyped as described by Zeitz et al. (2002). After they had been weaned and sexed, males were housed separately in groups of four to six animals per cage and maintained under standard laboratory conditions (22 ± 1°C, 60% relative humidity, 12 h light/dark cycle, food and water available ad libitum). Male mice of each genotype were used at 2 months of age when their body weight equally reached about 25 g. Mice were always used for a single experiment, were all naïve of previous test or treatment and randomly distributed among the different groups (see Experimental protocol in supplementary information). The number of animals (6–15) per experiment was based on a power analysis (Charan and Kantharia, 2013) corrected by the mouse breeding availability. For most of the experiments, no animals were excluded from the results. However, in the splash test, two mice were removed due to the poor quality of the video recording. Some animals were also excluded from CSDS RT‐PCR studies due to sample processing issues. All studies, performed by a blind experimenter, followed the editorial on experimental design and analysis in pharmacology (Curtis et al., 2015). All procedures concerning animal care and treatment were carried out in accordance with the protocols approved by the ethical committee # C2EA ‐05 Charles Darwin for the use of experimental animals and were licensed by the Directorate General for Research and Innovation (French Ministère de l'Enseignement Supérieur et de la Recherche), under protocol authorization # 00966.02. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015).
Measurement of 5‐HT turnover
Tissue levels of 5‐HT and 5‐hydroxyindolacetic acid (5‐HIAA) were determined using HPLC with electrochemical detection (HPLC‐ED) in prefrontal cortex, hippocampus and brainstem levels of both naïve Htr3a KO and WT mice as previously described (Salomon et al., 2006; Mongeau et al., 2010). Those regions have been chosen because of both their strong serotoninergic innervation and of their involvement in the pathogenesis of depression.
Quantitative autoradiography of 5‐HT1A receptor stimulation‐induced [35S]‐GTP‐γ‐S binding
Autoradiographic measurement of 5‐HT1A receptor‐stimulated [35S]‐GTP‐γ‐S binding in the dorsal raphe nucleus (DRN) was performed in naïve Htr3a KO and WT mice as previously described by Froger et al. (2004). [35S]‐GTP‐γ‐S binding is expressed as percentage over the baseline: [(stimulated‐basal)/basal] × 100.
Quantification of RNA levels by quantitative RT‐PCR
Animals were killed by cervical dislocation, and the prefrontal cortex, hippocampus and blocks containing the raphe or the nucleus tractus solitarius (NTS) were quickly dissected, frozen in liquid nitrogen and kept at −80°C for molecular analysis. Total mRNA was extracted using TRI Reagent Solution (Life Technologies, Saint Aubin, France) following the manufacturer's instructions. Reverse transcription was performed with a High Capacity cDNA Reverse Transcription kit (Applied Biosystem, Courtaboeuf, France) with the following cycling protocol: 25°C for 10 min, 37°C for 2 h and 85°C for 5 s. cDNA samples were stored at −20°C. Amplification reactions were performed with KAPA SYBR FAST qPCR Master Mix (Clinisciences, Nanterre, France) following manufacturer's instructions using the 7300 Real Time PCR System (Applied Biosystem, Courtaboeuf, France). The following cycling protocol was applied: 95°C for 3 min, followed by 40 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 30 s. The following primers were used: 5‐HT1A receptor, ACCCCAACGAGTGCACCATCAG (sense), GCAGGCGGGGACATAGGAG (antisense); 5HT3A subunit, GTGGACTCCTGAGGACTTCGACAA (sense), AGATGTCAAGGCTACAGGCGGTCA (antisense); CaMKIIa (Ca2+/calmodulin‐dependent protein kinase IIa), GAGACCAAAAGCACGGAGAG (sense), GGGTTTGGCTCTTGTATGGA (antisense); SOD1 CACTTCGAGCAGAAGGCAAG (sense), CCCCATACTGATGGACGTGG (antisense). β‐actin (sense: CCACCATGTACCCAGGCATT, antisense: CGGACTCATCGTACTCCTGC) was used as housekeeping gene for normalizing gene expression results. The 2∆∆CT (Delta Delta Comparative Threshold) method was used to quantify the fold change in mRNA expression of Htr3a KO naïve mice with WT naïve mice and socially defeated WT, Control and defeated KO mice groups with the control WT mice group.
Electrophysiological studies
Extracellular recordings of DRN serotonergic neurons firing were made as previously detailed elsewhere (Lanfumey et al., 1999). Briefly, animals were killed by cervical dislocation, and brains from either naïve or SSRI‐treated mice were rapidly removed, and a block of brainstem containing the DRN was cut into 400 μm thick coronal sections using a vibratome and immersed in an ice‐cold artificial CSF (aCSF). Extracellular recordings of characterized 5‐HT1A autoreceptor‐expressing serotonergic neurons in the DRN were obtained using glass microelectrodes filled with 2 M NaCl (10–15 MΩ) (Lanfumey et al., 1999). Baseline activity was recorded for 5–10 min prior to the application of drugs via a three‐way tap system. The duration of each application of the 5‐HT1A receptor agonist ipsapirone (0–0.3 μM) was 3 min. The effect of ipsapirone was evaluated by comparing the mean discharge frequency for 2 min prior to its addition to the superfusing aCSF with that recorded at the peak of drug action. One to three cells were recorded per animal.
Behavioural studies
The behavioural tests were adapted from existing protocols (see Boulle et al., 2014 and Mongeau et al., 2010). Classical tests for measuring depression‐relevant behaviours (forced swim test – FST), locomotor activity (open field – OF) and anxiety‐like behaviours (elevated plus maze – EPM; social interaction test – SI) were performed in different groups of mice.
Elevated plus maze
The apparatus consisted of an elevated plus maze (EPM) composed by two open arms and two closed arms (40 × 5 cm), elevated 50 cm from an infrared floor. Animals were placed at the centre of the plus maze facing a closed arm and left free to explore for 10 min. The number of entries, the time spent in the open arms and the total distance covered in the entire maze were measured using a video tracking system (Viewpoint, Lyon, France).
Social interaction test (SIT)
Mice were habituated for 20 min to the open field (50 × 50 cm) for two consecutive days. The third day, two mice of the same genotype, age and weight but unfamiliar to each other were paired and placed in the open field for 10 min. Total time spent in active social interactions (defined as sniffing, fighting, chasing, grooming or crawling under and over each other) was scored from a recorded video using XNote StopWatch software.
Forced swim test (FST)
Mice were placed for 6 min into Plexiglas cylinders (25 × 15 cm) filled with water (25 ± 0.5°C) to a depth of 15 cm and placed in front of an infrared plate. The immobility (defined as the absence of movement and/or small movements necessary to keep the head above the water) was scored using a video tracking system (Viewpoint, Lyon, France) during the last 4 min of the test. For antidepressant response testing, citalopram (2.5, 5.0 mg·kg−1), fluoxetine (20.0 mg·kg−1) or saline were injected i.p. 30 min before the test.
Chronic social defeat stress (CSDS)
Chronic social defeat stress protocol was adapted from Krishnan et al. (2007), with slight modifications.
The stress procedure was conducted on naïve Htr3a KO and WT mice from day 1 to day 10. Mice were weighted before the beginning of the first defeat session (day 1) and on day 11. Open field and social avoidance tests were performed at day 11 on socially defeated and control animals to measure their anxio‐depressive phenotype. The next day (day 12), the state of their fur and grooming behaviour were evaluated as an index of a depression‐like state (Smolinsky et al., 2009). Finally, mice were killed by cervical dislocation and their brain dissected on day 13 between 09:00 and 12:00 h. Adrenal glands were dissected out and weighed to estimate the effect of stress.
Male CD‐1 retired breeder mice were screened for aggressive behavioural response based on the following criterion: latency to initial aggression less than 60 s in at least two consecutive 180 s screening sessions (Golden et al., 2011). At least 24 h before the first defeat, CD‐1 mice were singly housed in a standard cage on one side of a transparent perforated Plexiglas divider, which allowed sensory but not physical contact. One experimental mouse was exposed to a CD‐1 aggressor mouse for 10 min, during which the intruder WT or Htr3a KO mouse was attacked and displayed subordinate posturing. To avoid physical injury, mice were briefly separated in cases of over‐aggressive behaviour from the CD‐1 mouse. In cases of mild injury, which occurred in less than 25% of the mice (wounds or bites not exceeding 0.2 cm of length), the mice were kept in the study and treated with topical antiseptic (chlorhexidine 0.25%). After the 10 min defeat session, the experimental mouse was placed for the rest of the day on the opposite side of the divider. This procedure was repeated for 10 consecutive days (days 1–10), using a different aggressor CD‐1 mouse each day, between 10:00 and 11:00 h. Control mice were housed in pairs in a similar cage separated by a Plexiglas divider and never exposed to CD‐1 mice. Immediately after the last defeat session, stressed and control mice were singly housed in a new cage.
Open field test (OF)
Twenty‐four hours after the last defeat session, mouse anxiety levels and locomotor activity were tested in the open field. The apparatus consisted of four open arenas (50 × 50 cm) separated by Plexiglas walls and equipped with an infrared floor for automatic animals exploratory behaviour detection. A virtual zone (20 × 20 cm) was delimited in the centre of the open field. Mice were placed in the open field boxes, and left free to explore for 45 min under low light conditions (5 lux). The number of entries, the time spent at the centre and the total distance covered in the entire open field were measured using a video tracking system (Viewpoint, Lyon, France).
Social avoidance testing
Social avoidance was assessed 3 h after the open field test, as previously described (Berton et al., 2006; Krishnan et al., 2007). The test consisted of two consecutive 150 s sessions, under low light conditions (5 lux). In the first session, experimental mice were allowed to explore the open field containing an empty circular wire cage (18 × 9 cm) located at one end of the field. In the second session, conditions were identical except that the circular cage contained a CD‐1 aggressive mouse (defined as ‘target’). A virtual interaction zone (area projecting 8 cm around wire cage) was delimited, and the time spent in this zone was scored during both sessions using a video tracking system (Viewpoint, Lyon, France). Social interaction behaviour was estimated with the time spent in the interaction zone with the target present and with the following interaction ratio: (time spent in the interaction zone in the presence of target / time spent in the interaction zone in the absence of target) x100.
Coat state evaluation
The total score of the coat state resulted from the sum of scores recorded at four different parts of the mouse body: head, neck, dorsal and ventral coat. For each area, a score of 0 was given for a well‐groomed coat and 1 for an unkempt coat. Potential wounds induced by social defeat and treated as described above were not considered for coat state evaluation.
Splash test
The splash test consists in spraying a 10% sucrose solution on the back of mice in their home cage. The sucrose solution induces grooming behaviour. The total time spent in grooming behaviour and the latency for the first grooming event to occur were scored from a recorded video using XNote StopWatch software over a 5 min period.
Drugs
For electrophysiological experiments, Htr3a KO and WT mice were treated either with saline (10 mL·kg−1) or citalopram 5 or 20 mg·kg−1·day−1 i.p. for 14 days.
For antidepressant response testing, citalopram (2.5, 5.0 mg·kg−1), fluoxetine (20.0 mg·kg−1) or saline (10 mL·kg−1) were injected i.p. 30 min before the test.
Citalopram was purchased from Biotrend (Cologne, Germany) and fluoxetine from Santa Cruz (Dallas, USA).
Statistical analysis
All data (displayed as mean ± SEM) were analysed using Prism 5 Software (GraphPad, San Diego, USA). For the comparison of two groups, Student's unpaired t‐test was used, with Welch's correction if needed. All remaining data were compared using a two‐way ANOVA, followed by Bonferroni post hoc test in case of significant factor interaction. The level of significance was set at P < 0.05. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015a,b,c).
Results
Effect of 5‐HT3 receptor deletion on the serotonergic activity
5‐HT turnover was evaluated in the prefrontal cortex, the hippocampus and the brainstem to investigate the serotonergic activity in naïve Htr3a KO and WT mice. A two‐way ANOVA revealed no brain area × genotype interaction [F(2,32) = 0.79, P = 0.46], but there was a significant overall effect of genotype [F(1,32) = 4.95, P = 0.03], which was associated with an overall diminution of the 5‐HIAA/5‐HT ratio (−9%, prefrontal cortex; −16%, hippocampus; −20%, brainstem; Figure 1A).
Figure 1.
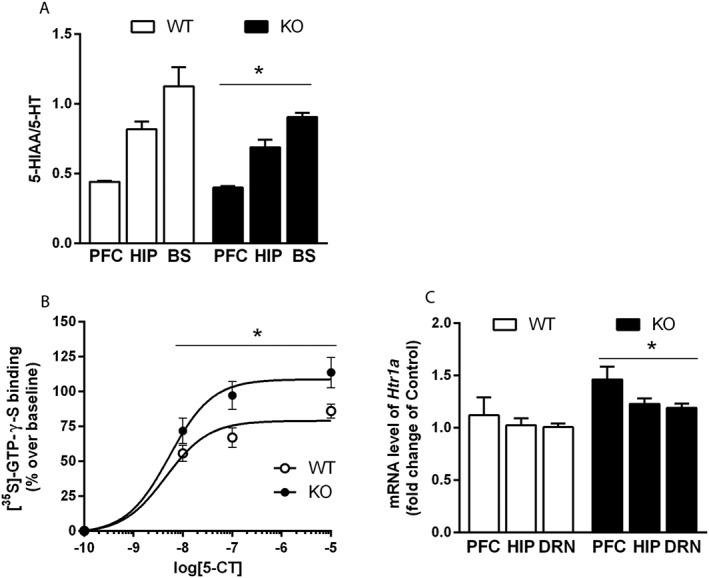
Effect of 5‐HT3 receptor deletion on 5‐HT1A receptor function and 5‐HT turnover (A) 5‐HT turnover, determined by the 5‐HIAA/5‐HT ratio, was evaluated in the prefrontal cortex, the hippocampus and the brainstem of naïve Htr3a KO and WT mice. 5‐HT turnover was significantly decreased in Htr3a KO compared with WT mice. Each bar was the mean ± SEM of n = 7 WT and n = 6 KO mice. *P < 0.05, effect of genotype; two‐way ANOVA. BS, brain stem. (B) 5‐HT1A receptor coupling to G protein was assessed in the DRN of naïve Htr3a KO and WT mice using quantitative autoradiography for measuring 5‐HT1A receptor‐stimulated [35S]‐GTP‐γ‐S binding. Binding of [35S]‐GTP‐γ‐S was increased in the DRN of Htr3a mutants compared with WT mice. Each bar was the mean ± SEM of n = 6 mice per group. *P < 0.05, effect of genotype, two‐way ANOVA. (C) 5‐HT1A receptor gene (Htr1A) mRNA expression was determined by quantitative qRT‐PCR in the raphe, the prefrontal cortex and the hippocampus of naïve Htr3a KO and WT mice. Htr1A expression was higher in Htr3a KO compared with WT mice. Each bar was the mean ± SEM of n = 10 WT and n = 9 KO mice. *P < 0.05, effect of genotype; two‐way ANOVA. β‐actin was used as housekeeping gene. PFC, prefrontal cortex; HIP, hippocampus.
Because 5‐HT1A receptors are known to regulate 5‐HT turnover (Hamon et al., 1991), their coupling capacity to G‐protein was assessed in the DRN of naïve Htr3a KO and WT mice by measuring [35S]‐GTP‐γ‐S binding after stimulation with the 5‐HT1A receptor agonist 5‐carboxamidotryptamine (5‐CT). A two‐way ANOVA revealed a significant effect of genotype [F(1,40) = 13.37, P = 0.0007], linked to an increased [35S]‐GTP‐γ‐S binding of about 30% for each 5‐CT concentration (Figure 1B). No 5‐CT concentration × genotype interaction was found [F(3,40) = 1.85, P = 0.15].
The expression of 5‐HT1A receptor mRNA (Htr1A) was also quantified by qRT‐PCR in several brain regions of naïve Htr3a KO and WT mice. Although there was no brain structure × genotype interaction [F(2,53) = 0.39, P = 0.68], a two‐way ANOVA revealed a significant effect of genotype [F(2,53) = 13.88, P = 0.003], associated with an increased Htr1A expression in each brain structure (+23.4%, prefrontal cortex; +18.5%, hippocampus; +16.7%, raphe; Figure 1C).
Effect of 5‐HT3 receptor deletion on anxiety‐related behaviours
The phenotype of naïve Htr3a KO versus WT mice was assessed in tests relevant to anxiety‐related behaviours. In the EPM (Figure 2A), Htr3a KO mice displayed less anxiety‐like behaviours, defined as a two times higher exploration and time spent in the open arms. Locomotor activity in the entire maze was similar in the two groups (Figure S1A).
Figure 2.
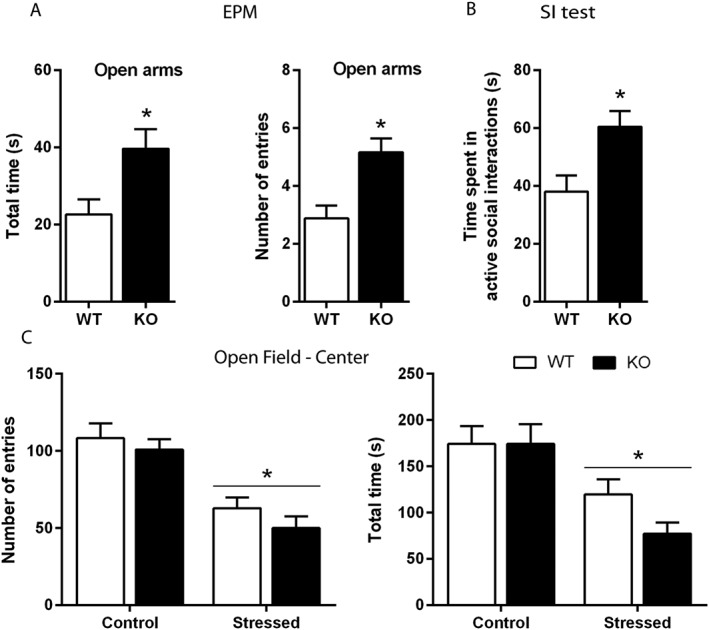
Effect of 5‐HT3 receptor genetic invalidation on anxiety‐related behaviours in naïve and socially defeated mice. (A) Naïve Htr3a KO and WT mice were placed for 10 min in an EPM. The exploration of the open arms (measured as the time spent and the number of entries) was higher in Htr3a KO compared with WT animals. Each bar was the mean ± SEM of n = 8 WT and n = 6 KO mice. *P < 0.05, ‘WT’ versus ‘KO’, Student's unpaired t‐test. (B) The total time spent in active social interactions during a 10 min period was significantly increased in Htr3a KO compared with paired WT mice. Each bar was the mean ± SEM of n = 7 WT and n = 9 KO mice.*P < 0.05 ‘WT’ versus ‘KO’, Student's unpaired t‐test. (C) After 10 days of CSDS, there was a similar decrease in the exploration of the centre of an open field in both Htr3a KO and WT mice, as shown by the diminution in the number of entries and time spent in this zone. Each bar was the mean ± SEM. of n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. *P < 0.05, effect of stress, two‐way ANOVA.
In the SI test, Htr3a KO mice showed an increased time spent in active social interactions compared with control mice (Figure 2B), suggesting again an anxiolytic‐like phenotype. This effect was not related to an increase in locomotion because activity of the two groups did not differ in the open field test (Figure S1B). In addition, in various group of mice subjected to the CSDS procedure for 10 days and submitted to the latter test, a two‐way ANOVA revealed a significant effect of stress (number of entries in the centre, F(1,47) = 37.87, P < 0.0001; time spent in the centre, F(1,47) = 19.80, P < 0.0001 and locomotor activity, F(1,47) = 80.00, P < 0.0001), but no effect of genotype and no significant interaction (Figures 2C and S1B).
Effect of 5‐HT3 receptor deletion on acute SSRI‐induced antidepressant‐like response and in depression‐related behavioural tests
Behaviours of naïve Htr3a KO and WT mice were evaluated in the FST: Htr3a KO mice spent less time immobile than WT mice (Figure 3A, left).
Figure 3.
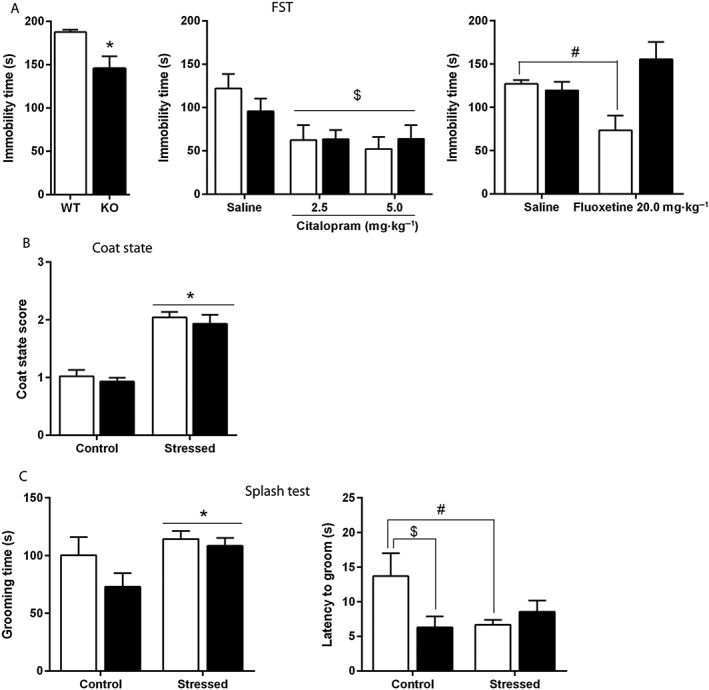
Effect of 5‐HT3 receptor deletion on depression‐related behaviours in naïve, SSRI‐treated and socially defeated mice. (A) At basal level, Htr3a KO mice were less immobile than WT mice in the FST (A, left). Acute administrations of citalopram (2.5, 5.0 mg·kg−1 i.p.) induced a decreased immobility in both genotypes, and no differences were found between mutants and WT mice (A, middle). When injected with fluoxetine (20.0 mg·kg−1 i.p.), WT mice displayed less immobility than saline‐treated paired controls, but this effect was absent in Htr3a KO mice (A, right). Each bar is the mean ± SEM of n = 12 naïve WT, 14 naïve KO (A, left); 7 saline‐treated WT, 9 citalopram 2.5‐treated WT, 9 citalopram 5.0‐treated WT, 13 saline‐treated KO, 8 citalopram 2.5‐treated KO, 9 citalopram 5.0‐treated KO (A, middle); 8 saline‐treated WT, 9 fluoxetine‐treated WT, 8 saline‐treated KO and 7 fluoxetine‐treated KO mice (A, right). *P < 0.05, ‘WT’ versus ‘KO’, Student's unpaired t‐test; $P < 0.05, effect of treatment, two‐way ANOVA; #P < 0.05, ‘WT fluoxetine 20 mg·kg−1’versus ‘WT saline’, two‐way ANOVA followed by Bonferroni post hoc test. (B) After 10 days of CSDS, the coat state score was equally increased in stressed Htr3a KO and WT mice. Each bar was the mean ± SEM of n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. *P < 0.05, effect of stress, two‐way ANOVA. (C) After 10 days of CSDS, the time spent in grooming in a 5 min period following sucrose solution spraying (splash test) was increased in stressed animals (C, left). As for the latency to groom (C, right), a two‐way ANOVA analysis revealed a significant stress × genotype interaction (P < 0.05). Grooming was lower in Htr3a KO compared with WT non‐stressed mice. CSDS induced a significant decrease in the latency to groom in WT mice, but no change in mutant animals. Each bar was the mean ± SEM of n = 11 Control WT, 11 Stressed WT, 11 Control KO and 14 Stressed KO mice. *P < 0.05 effect of stress, two‐way ANOVA; $P < 0.05, ‘Control WT’ versus ‘Control KO’; #P < 0.05, ‘Control WT’ versus ‘Stressed WT’, two‐way ANOVA followed by Bonferroni post hoc test.
Htr3a KO and WT mice responses to acute antidepressant treatments were investigated in this test using two different SSRI: citalopram, a highly selective compound (Hyttel, 1982) in order to block 5‐HTT only, and fluoxetine, which possesses moderate 5‐HT3 receptor antagonist properties (Fan, 1994). Indeed, different classes of antidepressants such as fluoxetine act as functional antagonists at the human 5‐HT3A receptor stably expressed in HEK 293 cells and at endogenous 5‐HT3 receptors of rat hippocampal neurons and of mouse N1E‐115 neuroblastoma cells (Eisensamer et al., 2003). A two‐way ANOVA revealed a significant effect of treatment [FST, F(2,49) = 6.25, P = 0.03] in mice acutely treated with citalopram (2.5 and 5.0 mg·kg−1 i.p). There was no significant effect of genotype or treatment × genotype interaction suggesting a similar response to acute citalopram in Htr3a KO and WT (Figure 3A, middle). In mice acutely treated with fluoxetine (20 mg·kg−1, i.p.), there was a significant treatment × genotype interaction [F(1,28) = 10.04, P < 0.004] in the FST (Figure 3A, right), as fluoxetine induced a decrease in the immobility time in WT mice, but not Htr3a KO mice (P < 0.05 and P > 0.05, respectively, two‐way ANOVA followed by Bonferroni post hoc test).
In groups of mice subjected to the CSDS procedure for 10 days, coat state scoring showed a significant effect of stress in both Htr3a KO and WT mice [F(1,47) = 71.65, P < 0.0001, effect of stress, two‐way ANOVA], but no effect of genotype [F(1,47) = 0.71, P = 0.40] or stress × genotype interaction [F(1,47) = 0.007, P = 0.93] (Figure 3B).
In the splash test (Figure 3C), a two‐way ANOVA indicated a significant overall effect of stress on time spent grooming, which was increased in both stressed Htr3a KO and WT mice [F(1,45) = 5.64, P = 0.02]. There was no effect of genotype [F(1,45) = 2.57, P = 0.12] or stress × genotype interaction [F(1,45) = 1.06, P = 0.31]. However, a significant stress × genotype interaction [F(1,44) = 5.69, P = 0.02] in the latency to groom was found. Bonferroni post hoc test revealed that the latency to groom was lower in control Htr3a KO compared with WT mice. CSDS shortened this latency in stressed WT mice but, interestingly, not in mutant animals.
In addition, in the social avoidance test performed 24 h after the CSDS procedure, a two‐way ANOVA on the time spent in the interaction zone with the target present did not show any stress × genotype interaction nor a significant stress effect [F(1,45) = 0.52, P = 0.47 and F(1,45) = 1.52, P = 0.23, respectively]. Nevertheless, there was a significant effect of genotype [F(1,45) = 4.86, P = 0.03], as both non‐stressed and stressed Htr3a KO mice spent more time interacting with the target than paired controls, suggesting again an anxiolytic‐like behaviour (Table S1).
Effect of 5‐HT3 receptor deletion on 5‐HT neuronal activity and on chronic antidepressant‐induced responses
Basal serotonergic neuronal firing activity was recorded in brainstem slices containing the DRN. This activity did not differ between naïve Htr3a KO and WT mice (Figure 4A). Ipsapirone, a 5‐HT1A receptor agonist, that induce about 50% firing inhibition of DRN 5‐HT neurons at a dose 60 nM (EC50; Lanfumey et al., 1999), produced a similar effect in brainstem slices of Htr3a KO and WT mice (Figure 4B).
Figure 4.
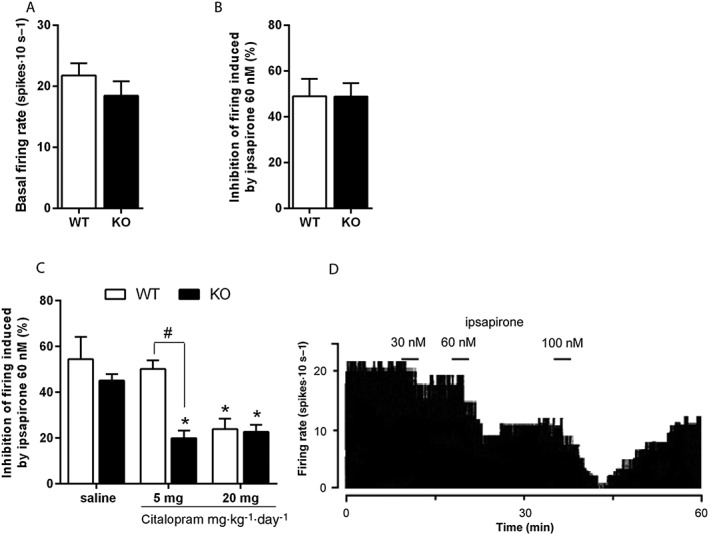
Effect of 5‐HT3 receptor deletion on 5‐HT1A receptor function under chronic citalopram. In basal conditions, no difference in either the basal firing rate of DRN serotonergic neurons (A) or the efficacy of ipsapirone 60 nM (B) to inhibit their activity was observed between naïve Htr3a KO and WT mice. Each bar was the mean ± SEM of n = 13 WT and 10 KO cells (five WT and four KO mice). (C) Effects of chronic citalopram treatment. In Htr3a KO mice, a 14 day treatment with citalopram with either a 5 or 20 mg·kg−1·day−1 regimen decreased 5‐HT firing inhibition induced by ipsapirone 60 nM, while in WT mice, only the 20 mg·kg−1·day−1 regimen decreased ipsapirone‐induced inhibition of firing. Each bar was the mean ± SEM of n = 6 saline‐treated WT, 7 citalopram 5‐treated WT, 5 citalopram 20‐treated WT, 4 saline‐treated KO, 6 citalopram 5‐treated KO and 9 citalopram 20‐treated KO cells (respectively 5, 6, 4, 4, 5 and 6 mice). *P < 0.05, ‘Citalopram’ versus paired ‘Saline’ and #P < 0.05, ‘KO mice treated with citalopram 5 mg·kg−1·day−1’ versus paired ‘WT mice’, two‐way ANOVA followed by Bonferroni post hoc test. (D) Integrated firing rate histogram (in spikes. 10 s−1) showing the effect of increasing concentrations of ipsapirone on the electrical activity of a DRN 5‐HT neurons.
Htr3a KO and WT animals were treated with citalopram (5 or 20 mg·kg−1·day−1, i.p.) or saline for 14 days, and the activity of 5‐HT neurons in response to ipsapirone 60 nM was assessed. As illustrated in Figure 4C, a two‐way ANOVA showed a significant interaction between genotype and citalopram treatment [F(2,30) = 4.24, P = 0.02]. Bonferroni post hoc tests showed that while a 14 day treatment with 20 mg·kg−1·day−1 citalopram significantly decreased the effect of ipsapirone 60 nM in both Htr3a KO and WT mice, 5 mg·kg−1·day−1 citalopram for 14 days decreased the inhibitory effect of ipsapirone in Htr3a KO mice only, suggesting that at this low dose, citalopram more effectively desensitizes 5‐HT1A receptor in the mutant mice.
Effect of 5‐HT3 receptor deletion on weight alterations and CSDS‐induced cellular stress
In mice subjected to the CSDS procedure, adrenal weight was significantly greater [F(1,37) = 46.27, P < 0.0001, two‐way ANOVA, effect of stress] than in non‐stressed mice for both genotypes (Figure S2A).
Difference in body weight between D1 and D11 (Figure S2B) showed a significant stress × genotype interaction [F(1,44) = 4.69, P = 0.04]. Bonferroni post hoc test showed that stressed WT mice displayed a gain of body weight compared with non‐stressed WT mice, an effect absent in mutant mice.
The effect of CSDS on the 5‐HT3A subunit gene (Htr3a) mRNA expression in WT mice was evaluated using qRT‐PCR in various brain regions including the NTS, a region strongly enriched in 5‐HT3AR (Laporte et al., 1992). A two‐way ANOVA revealed a significant stress × brain area interaction [F(2,48) = 10.25, P = 0.02] in Htr3a gene expression. Post hoc test showed that Htr3a was significantly up‐regulated in the NTS of stressed mice (Table 1).
Table 1.
5‐HT3 receptor subunit 3A gene expression after CSDS in WT mice
| Control WT | Stressed WT | |
|---|---|---|
| Hippocampus | 1.00 ± 0.03 | 1.03 ± 0.03 |
| Prefrontal Cortex | 1.00 ± 0.04 | 1.14 ± 0.04 |
| NTS | 1.01 ± 0.07 | 1.53 ± 0.15a |
A two‐way ANOVA revealed a significant stress × brain area interaction in Htr3a expression, and a post hoc test showed that Htr3a was significantly up‐regulated in the NTS. Each value was the mean ± SEM of n = 7 Control and 10 Stressed WT mice.
P < 0.05 ‘Control WT’ versus ‘Stressed WT’, two‐way ANOVA followed by Bonferroni post hoc test.
The mRNA expression of CaMKIIa and SOD1, genes linked to oxidative stress and regulated by 5‐HT3 receptors (Bhatt et al., 2014; Hutchinson et al., 2015), was also quantified using qRT‐PCR in CSDS‐subjected Htr3a KO and WT mice prefrontal cortex, a region commonly studied in relation with depression‐related behaviours (Ressler and Mayberg, 2007). A two‐way ANOVA showed a significant stress × genotype interaction [F(1,41) = 5.32, P = 0.03] in CaMKIIa gene expression. While the Bonferroni post hoc test revealed that CaMKIIa mRNA level was higher in stressed compared with control WT mice, there was no difference in Htr3a KO mice (Figure 5A). Analysis of the SOD1 gene expression also revealed a significant stress × genotype interaction [F(1,33) = 6.69, P = 0.01]. Post hoc analyses showed that SOD1 mRNA level was lower in control KO than in control WT mice and that stress induced a down‐regulation of this gene only in WT animals (Figure 5B).
Figure 5.
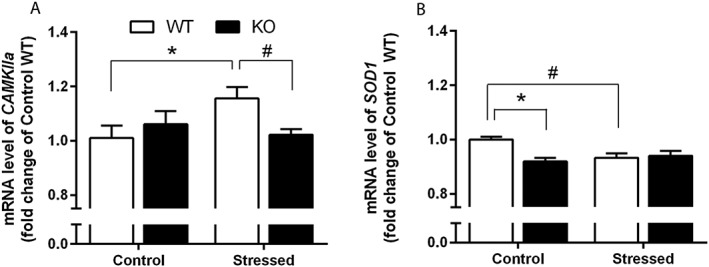
Effect of CSDS on oxidative stress‐related gene expression. (A) CSDS increased CaMKIIa mRNA level in WT mice but not in Htr3a KO mice. Each bar was the mean ± SEM of n = 9 Control WT, 14 Stressed WT, 10 Control KO and 14 Stressed KO mice. *P < 0.05, ‘Control WT’ versus ‘Stressed WT’; #P < 0.05, ‘Stressed WT’ versus ‘Stressed KO’, two‐way ANOVA followed by Bonferroni post hoc test. β‐actin was used as housekeeping gene. (B) SOD1 mRNA level was significantly lower in control KO compared with paired WT mice. CSDS decreased SOD1 gene expression in WT animals but not in mutant mice. Each bar was the mean ± SEM. n = 6 Control WT, 13 Stressed WT, 8 Control KO and 11 Stressed KO mice. *P < 0.05, ‘Control WT’ versus ‘Control KO’; #P < 0.05, ‘Control WT’ versus ‘Stressed WT’, two‐way ANOVA followed by Bonferroni post hoc test. β‐actin was used as housekeeping gene.
Discussion
The pivotal role of 5‐HT1A receptors in both depression‐related behaviours and antidepressant responses have well been established several years ago (see in Celada et al., 2013); however, that of 5‐HT3 receptors remained controversial. The present data clearly show its involvement in anxiety‐ and depression‐related behaviours. Furthermore, they suggest that SSRI treatments may be effective at lower doses when 5‐HT3 receptors are inactivated. 5‐HT3 receptors are thus of considerable interest in the development of new therapeutic strategies with lower levels of 5‐HT reuptake inhibition to increase treatment tolerability while preserving maximal effectiveness (Jakubovski et al., 2016).
Effect of the 5‐HT3A receptor mutation on anxiety‐ and depression‐ related behaviours
The present series of behavioural explorations conducted in Htr3a KO mice generally confirmed that genetic inactivation of 5‐HT3 receptors induces an anxiolytic‐like phenotype (Kelley et al., 2003; Bhatnagar et al., 2004), consistent with the anxiolytic properties of 5‐HT3 receptor antagonists (Mahesh et al., 2013). Nevertheless, the anxiogenic effect generated by social defeat was not altered by the Htr3a mutation. Htr3a KO mice displayed less immobility behaviour than WT mice in the FST, consistently with the effect of 5‐HT3 receptor antagonists in these tests used to screen antidepressant‐like activity (Ramamoorthy et al., 2008; Bétry et al., 2015). Therefore, the bulk of data show that 5‐HT3 receptor inactivation can lead to both anxiolytic‐ and antidepressant‐like behaviours. As expected under acute treatment conditions, the highly selective SSRI citalopram reduced immobility in the FST, and this effect was not altered by the genotype, contrasting with previous studies that had shown that 5‐HT3 receptor antagonists potentiate behavioural effects induced by various SSRIs in the mice FST (Redrobe and Bourin, 1997; Ramamoorthy et al., 2008). Constitutive KO cannot be considered always similar to acute receptor blockade, as is the case with 5‐HT3 receptor antagonists such as ondansetron (Saynor and Dixon, 1989). Several 5‐HT system compensations, like those documented in the present study at the 5‐HT1A receptor level, could intervene during the development of mutant mice (Rudmann and Durham, 1999).
In the FST, fluoxetine induced a significant antidepressant effect only in WT but not in Htr3a KO mice. Interestingly, in addition to its 5‐HTT blocking effect, fluoxetine, in contrast to citalopram, possesses 5‐HT3 receptor antagonist properties within the micromolar affinity range (Fan, 1994). This suggests that in these conditions of treatment, the antidepressant‐like activity of fluoxetine in the FST is at least partly mediated by 5‐HT3 receptors. This result finds an echo in the work of Smit‐Rigter et al. (2012), in which the effect of prenatal exposure to fluoxetine was blunted in Htr3a KO mice in the novelty‐suppressed feeding test, a paradigm frequently used to screen the anxiolytic properties of antidepressant treatments.
We have also assessed a depression‐like behaviour using the stress‐induced fur coat state alteration (Smolinsky et al., 2009). The Htr3a mutation did not affect the stress‐induced behavioural alteration in this paradigm. In the splash test, CSDS induced an overall stimulating effect in grooming in both Htr3a KO and WT animals. These data are consistent with those describing an increase of self‐grooming behaviour in socially defeated rodents (Smolinsky et al., 2009; Denmark et al., 2010), whereas chronic mild stress is usually followed by a decrease in grooming (Boulle et al., 2014) and/or an increased latency for the first grooming event (Siopi et al., 2016). Interestingly, the later parameter was reduced in WT mice, but not in Htr3a KO stressed mice, suggesting that 5‐HT3 receptor genetic inactivation reduced the sensitivity to social defeat stress.
Effect of the 5‐HT3A receptor mutation on chronic antidepressant treatment responses
The delay for SSRI‐induced clinical efficacy is partly linked to the time course of somatodendritic 5‐HT1A receptor desensitization (Le Poul et al., 1995), and 5‐HT3 receptor blockade has recently been proposed to accelerate this phenomenon (Bétry et al., 2013). We thus conducted in vitro single cell recording to directly measure 5‐HT1A receptor responses in Htr3a KO mice.
First, we showed that 5‐HT3 receptor invalidation affected neither basal 5‐HT neuronal firing nor its response to 5‐HT1A receptor stimulation, which is in accord with previous studies where 5‐HT3 receptor antagonists did not modify DRN 5‐HT neuron firing (Adrien et al., 1992). However, this conclusion contrasts with previous results indicating that 5‐HT3 receptor stimulation impaired 5‐HT1A receptor function in mice (Kondaurova et al., 2012) and with the data obtained here at the mRNA expression and G‐protein coupling levels. Indeed, our receptor expression studies demonstrated a 5‐HT1A receptor up‐regulation in Htr3a KO mice, associated with both a higher degree of coupling between 5‐HT1A receptors and G proteins within the DRN and a decrease in 5‐HT turnover. The 5‐HT1A receptor up‐regulation observed in Htr3a KO mice was not translated at the neural activity level under the ipsapirone challenge. The electrophysiological response of raphe 5‐HT cells is not exclusively related to 5‐HT1A autoreceptor activity, as it is also driven by the G‐protein‐gated inwardly rectifying K+ (GIRK) channels coupled to these receptors (Haj‐Dahmane et al., 1991), and thus, a regulation at GIRK itself may have compensated for 5‐HT1A receptor up‐regulation. Further experiments are necessary to address this issue.
Although not modified by the mutation at basal level, 5‐HT1A receptor sensitivity was shown to be differently affected by chronic citalopram treatment. While 5 mg·kg−1 × 14 days citalopram treatment failed to produce any change in 5‐HT1A receptor sensitivity in WT mice, it did produce strong 5‐HT1A receptor desensitization in Htr3a KO mice. These results are in line with those of Bétry et al. (2013) who have hypothesized that the fast action of vortioxetine, an SSRI with strong 5‐HT3 receptor antagonist properties, is mediated by its ability to block 5‐HT3 receptors. Moreover, a recent in vivo electrophysiological study in rats revealed that a 5‐HT3 receptor antagonist (ondansetron) is able to counteract the 5‐HT1A receptor‐mediated inhibitory effect of the SSRI paroxetine on DRN 5‐HT neurons basal firing (Bétry et al., 2015). The latter and present data both argue for an important regulatory action of 5‐HT3 receptors on the 5‐HT1A receptor‐desensitization process induced by SSRI.
Together with Bétry et al. (2013), we therefore suggest a synergistic effect of 5‐HT3 receptor blockade on SSRI‐induced 5‐HT1A receptor desensitization occurring under low 5‐HTT inhibition. Vortioxetine is able – in contrast to classical SSRIs – to increase 5‐HT concentrations at low levels of 5‐HTT occupancy (Pehrson et al., 2013). In our conditions, 5‐HT1A receptor desensitization occurred in Htr3a KO mice, not WT mice, under the low‐dose citalopram regimen (5 mg·kg−1·day−1). The amplitude of this desensitization was similar to that observed in WT mice with the 20 mg·kg−1·day−1 citalopram regimen (Martin et al., 2015), commonly used in preclinical studies (Calcagno and Invernizzi, 2010). Actually, Ferrés‐Coy et al. (2013) demonstrated that short‐term partial reduction of 5‐HTT expression by small interference RNA led to decreased in both 5‐HT1A autoreceptor expression and function. Interestingly, these effects appeared earlier and were of greater amplitude than those induced by fluoxetine at high dose. Therefore, we can hypothesize that 5‐HT3 receptor inactivation may possibly affect 5‐HTT expression or internalization, leading to faster SSRI‐induced effects on 5‐HT neurotransmission when administered at low dose only.
Effect of the 5‐HT3A receptor mutation on CSDS‐induced cellular stress and weight alterations
To further explore the role of 5‐HT receptors in response to stress, we assessed the metabolic and molecular consequences of a CSDS in both Htr3a KO and WT mice.
The increased weight gain observed after CSDS in WT mice is consistent with most of the data reported in the literature (Goto et al., 2014). Interestingly, this weight gain was blunted by the 5‐HT3 receptor KO, similar to what has been observed with 5‐HT3 receptor antagonists that reversed weight gain in response to chronic mild stress (Gupta et al., 2014). Interestingly, the Htr3a KO had reduced weight gain in mice fed with a high‐fat diet, suggesting a higher endogenous metabolism in Htr3a KO mice (Oh et al., 2015).
In WT mice, CSDS up‐regulated Htr3a in the NTS, a structure particularly enriched in 5‐HT3 receptors in the mouse brain (Laporte et al., 1992) and involved in stress‐related changes in feeding (Lam et al., 2009).
In addition, CSDS triggered significant modulations of 5‐HT3 receptor‐related markers involved in the cellular stress response, that is, the antioxidant enzyme SOD1, a marker of oxidative stress shown to be reversed by 5‐HT3 receptor antagonists (Bhatt et al., 2014; Kurhe et al., 2014) and the CaMKIIa, recently found to be involved in 5‐HT3 receptor signalling (Hutchinson et al., 2015). In the prefrontal cortex, a structure known to be involved in mood disorders (Ressler and Mayberg, 2007), CSDS down‐regulated SOD1 in WT mice, as expected from previous stress studies (Bhatt et al., 2014; Kurhe et al., 2014). The decrease in the levels of this antioxidant protective enzyme in stressed mice suggests that stress could induce dysfunction in the antioxidant defence system. Interestingly, these metabolic CSDS effects were not observed in mutant Htr3a KO mice. These data are consistent with those showing that 5‐HT3 receptor antagonists can reverse chronic stress effects on both SOD1 expression and enzymatic activity in rodent brain (Bhatt et al., 2014; Kurhe et al., 2014), supporting the notion of a beneficial effect of the 5‐HT3 receptor inactivation in preventing stress‐induced oxidative stress damages. Moreover, Uchihara et al. (2016) recently demonstrated that in mice, SOD1 overexpression prevented depressive‐like phenotype induced by chronic corticosterone administration. Although, in our study, Htr3a deletion did not prevent all CSDS‐induced anxio‐depressive phenotypes, it can be suggested that its protective effect on SOD1 expression may have contributed to the resistance of Htr3a KO mice to CSDS that we described earlier.
Regarding CaMKIIa, its expression was increased in socially defeated WT but not in Htr3a KO mice. Although the role of CaMKIIa in the responses to stress and to antidepressant drugs remains unclear, it is known to be activated by oxygen radicals, and this hyperactivation is suspected to play an important role in the development of several peripheral pathologies (Robison, 2014). Furthermore, excessive CaMKIIa activity contributes to oxygen radical formation (Anderson, 2015). The absence of stress‐induced CaMKIIa mRNA up‐regulation in Htr3a KO mice could prevent the oxygen radical formation induced by stress in these mice (Hutchinson et al., 2015), corroborating the role of 5‐HT3 receptors in oxidative‐related stress effects.
In summary, our data showed that the genetic inactivation of 5‐HT3 receptors promoted the efficacy of a low dose of SSRI to trigger 5‐HT1A receptor desensitization in the DRN. This is consistent with the anxiolytic‐ and antidepressant‐like effects of deleting 5‐HT3 receptors. In the chronic social stress depressiogenic‐like condition, we observed an up‐regulation of the Htr3a subunit, while deletion of this subunit prevented the modification of oxidative stress markers. Although some caution should be taken when studying unconditional KO, as they might not reflect entirely an acute gene deletion, these results underline the role of 5‐HT3 receptors in stress‐related disorders and the mechanism of action of antidepressant drugs.
Author contributions
V.M., R.M. and L.L. conceived and designed the experiments. V.M., A.R., C.B. and J.P.T. performed the experiments. T.M. and B.F. provided technical support. C.S.C. analysed the data. V.M., R.M. and L.L. wrote the manuscript. All authors reviewed the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Social avoidance test. In the social avoidance test, there were no differences in terms of time interacting with the target between stressed and control animals. However, non‐stressed and stressed Htr3a KO mice spent significantly more time than paired WT mice in the interaction zone when the target was present. Each value was the mean ± S.E.M. of n = n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. * P < 0.05, effect of genotype, 2‐way ANOVA.
Figure S1 A: Locomotor activity in the elevated plus maze. Animals from both genotypes displayed a similar locomotion in the EPM. Each bar was the mean ± S.E.M. of n = 8 WT and 6 KO mice. B. Locomotion of Htr3a KO and WT mice submitted to CSDS in the open field. Both stressed mutant and wild‐type animals displayed a similar hypolocomotion in the open field. Each bar was the mean ± S.E.M. of n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. *P < 0.05, effect of stress, 2‐way ANOVA.
Figure S2 Effect of CSDS on body and adrenal weight. (A) Effect of stress on adrenal gland weight. Stress similarly increased adrenal gland weight in both genotypes. There was no effect of genotype nor an interaction between the stress and genotype factors. Each bar was the mean ± S.E.M. of n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. * P < 0.05, effect of stress (two‐way ANOVA). (B) Body weight gain was higher in stressed WT compared to non‐stressed paired mice. Stress had no effect in Htr3a KO mice. Each bar was the mean ± S.E.M. of n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. * P < 0.05, ‘Control WT’ versus ‘Stressed WT’, # P < 0.05, ‘Stressed WT’ versus ‘Stressed KO’, 2‐way ANOVA followed by Bonferroni post hoc test.
Acknowledgements
This work was supported by the Institut National de la Santé et de la Recherche Médicale (Inserm, France) and Agence Nationale de la Recherche (ANR) (2011‐BSV‐017‐01). We thank Mme N. Bazin for genotyping the mice.
Martin, V. , Riffaud, A. , Marday, T. , Brouillard, C. , Franc, B. , Tassin, J.‐P. , Sevoz‐Couche, C. , Mongeau, R. , and Lanfumey, L. (2017) Response of Htr3a knockout mice to antidepressant treatment and chronic stress. British Journal of Pharmacology, 174: 2471–2483. doi: 10.1111/bph.13857.
References
- Adrien J, Tissier MH, Lanfumey L, Haj‐Dahmane S, Jolas T, Franc B et al. (1992). Central action of 5‐HT3 receptor ligands in the regulation of sleep‐wakefulness and raphe neuronal activity in the rat. Neuropharmacology 31: 519–529. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Peters JA, Kelly E, Marrion N, Benson HE, Faccenda E et al. (2015b) The Concise Guide to PHARMACOLOGY 2015/16: Ligand‐gated ion channels. Br J Pharmacol 172: 5870–5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ME (2015). Oxidant stress promotes disease by activating CaMKII. J Mol Cell Cardiol 89: 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ et al. (2006). Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science 311: 864–868. [DOI] [PubMed] [Google Scholar]
- Bétry C, Pehrson AL, Etievant A, Ebert B, Sanchez C, Haddjeri N (2013). The rapid recovery of 5‐HT cell firing induced by the antidepressant vortioxetine involves 5‐HT(3) receptor antagonism. Int J Neuropsychopharmacol 16: 1115–1127. [DOI] [PubMed] [Google Scholar]
- Bétry C, Overstreet D, Haddjeri N, Pehrson AL, Bundgaard C, Sanchez C et al. (2015). A 5‐HT3 receptor antagonist potentiates the behavioral, neurochemical and electrophysiological actions of an SSRI antidepressant. Pharmacol Biochem Behav 131: 136–142. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C (2004). Pituitary‐adrenal activity in acute and chronically stressed male and female mice lacking the 5‐HT‐3A receptor. Stress 7: 251–256. [DOI] [PubMed] [Google Scholar]
- Bhatnagar S, Sun LM, Raber J, Maren S, Julius D, Dallman MF (2004). Changes in anxiety‐related behaviors and hypothalamic‐pituitary‐adrenal activity in mice lacking the 5‐HT‐3A receptor. Physiol Behav 81: 545–555. [DOI] [PubMed] [Google Scholar]
- Bhatt S, Mahesh R, Jindal A, Devadoss T (2014). Protective effects of a novel 5‐HT3 receptor antagonist, N‐n‐butyl‐3‐methoxy quinoxaline‐2‐carboxamide (6o) against chronic unpredictable mild stress‐induced behavioral changes and biochemical alterations. Pharmacol Biochem Behav 122: 234–239. [DOI] [PubMed] [Google Scholar]
- Boulle F, Massart R, Stragier E, Paizanis E, Zaidan L, Marday S et al. (2014). Hippocampal and behavioral dysfunctions in a mouse model of environmental stress: normalization by agomelatine. Transl Psychiatry 4: e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagno E, Invernizzi RW (2010). Strain‐dependent serotonin neuron feedback control: role of serotonin 2C receptors. J Neurochem 114: 1701–1710. [DOI] [PubMed] [Google Scholar]
- Celada P, Bortolozzi A, Artigas F (2013). Serotonin 5‐HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs 27: 703–716. [DOI] [PubMed] [Google Scholar]
- Chaouloff F (2013). Social stress models in depression research: what do they tell us? Cell Tissue Res 354: 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan J, Kantharia ND (2013). How to calculate sample size in animal studies? J Pharm Pharmacol 4: 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denmark A, Tien D, Wong K, Chung A, Cachat J, Goodspeed J et al. (2010). The effects of chronic social defeat stress on mouse self‐grooming behavior and its patterning. Behav Brain Res 208: 553–559. [DOI] [PubMed] [Google Scholar]
- Eisensamer B, Rammes G, Gimpl G, Shapa M, Ferrari U, Hapfelmeier G et al. (2003). Antidepressants are functional antagonists at the serotonin type 3 (5‐HT3) receptor. Mol Psychiatry 8: 994–1007. [DOI] [PubMed] [Google Scholar]
- Fan P (1994). Effects of antidepressants on the inward current mediated by 5‐HT3 receptors in rat nodose ganglion neurones. Br J Pharmacol 112: 741–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrés‐Coy A, Pilar‐Cuellar F, Vidal R, Paz V, Masana M, Cortés R et al. (2013). RNAi‐mediated serotonin transporter suppression rapidly increases serotonergic neurotransmission and hippocampal neurogenesis. Transl Psychiatry 3: e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froger N, Palazzo E, Boni C, Hanoun N, Saurini F, Joubert C et al. (2004). Neurochemical and behavioral alterations in glucocorticoid receptor‐impaired transgenic mice after chronic mild stress. J Neurosci 24: 2787–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE 3rd, Berton O, Russo SJ (2011). A standardized protocol for repeated social defeat stress in mice. Nat Protoc 6: 1183–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto T, Kubota Y, Tanaka Y, Iio W, Moriya N, Toyoda A (2014). Subchronic and mild social defeat stress accelerates food intake and body weight gain with polydipsia‐like features in mice. Behav Brain Res 270: 339–348. [DOI] [PubMed] [Google Scholar]
- Gupta D, Radhakrishnan M, Kurhe Y (2014). 5HT3 receptor antagonist (ondansetron) reverses depressive behavior evoked by chronic unpredictable stress in mice: modulation of hypothalamic‐pituitary‐adrenocortical and brain serotonergic system. Pharmacol Biochem Behav 124: 129–136. [DOI] [PubMed] [Google Scholar]
- Haj‐Dahmane S, Hamon M, Lanfumey L (1991). K+ channel and 5‐hydroxytryptamine1A autoreceptor interactions in the rat dorsal raphe nucleus: an in vitro electrophysiological study. Neuroscience 41: 495–505. [DOI] [PubMed] [Google Scholar]
- Hamon M, Blier P (2013). Monoamine neurocircuitry in depression and strategies for new treatments. Prog Neuropsychopharmacol Biol Psychiatry 45: 54–63. [DOI] [PubMed] [Google Scholar]
- Hamon M, El Mestikawy S, Lanfumey L, Adrien J, Fattaccini CM, Gozlan H (1991). Somatodendritic 5‐HT1A autoreceptors in the dorsal raphe nucleus – pharmacological and functional properties In: Langer SZ, Galzin AM, Costentin J. (eds). Presynaptic Receptors and Neuronal Transporters, Adv Biosciences, 82 Pergamon Press: Oxford, pp. 71–74. [Google Scholar]
- Hutchinson TE, Zhong W, Chebolu S, Wilson SM, Darmani NA (2015). L‐type calcium channels contribute to 5‐HT3‐receptor‐evoked CaMKIIalpha and ERK activation and induction of emesis in the least shrew (Cryptotis parva). Eur J Pharmacol 755: 110–118. [DOI] [PubMed] [Google Scholar]
- Hyttel J (1982). Citalopram – pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry 6: 277–295. [DOI] [PubMed] [Google Scholar]
- Jakubovski E, Varigonda AL, Freemantle N, Taylor MJ, Bloch MH (2016). Systematic review and meta‐analysis: dose‐response relationship of selective serotonin reuptake inhibitors in major depressive disorder. Am J Psychiatry 173: 174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley SP, Bratt AM, Hodge CW (2003). Targeted gene deletion of the 5‐HT3A receptor subunit produces an anxiolytic phenotype in mice. Eur J Pharmacol 461: 19–25. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondaurova EM, Naumenko VS, Popova NK (2012). Effect of chronic activation of 5‐HT3 receptors on 5‐HT3, 5‐HT(1A) and 5‐HT(2A) receptors functional activity and expression of key genes of the brain serotonin system. Neurosci Lett 522: 52–56. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ et al. (2007). Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131: 391–404. [DOI] [PubMed] [Google Scholar]
- Kurhe Y, Radhakrishnan M, Gupta D, Devadoss T (2014). QCM‐4 a novel 5‐HT3 antagonist attenuates the behavioral and biochemical alterations on chronic unpredictable mild stress model of depression in Swiss albino mice. J Pharm Pharmacol 66: 122–132. [DOI] [PubMed] [Google Scholar]
- Lam DD, Zhou L, Vegge A, Xiu PY, Christensen BT, Osundiji MA et al. (2009). Distribution and neurochemical characterization of neurons within the nucleus of the solitary tract responsive to serotonin agonist‐induced hypophagia. Behav Brain Res 196: 139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfumey L, Pardon MC, Laaris N, Joubert C, Hanoun N, Hamon M et al. (1999). 5‐HT1A autoreceptor desensitization by chronic ultramild stress in mice. Neuroreport 10: 3369–3374. [DOI] [PubMed] [Google Scholar]
- Laporte AM, Koscielniak T, Ponchant M, Verge D, Hamon M, Gozlan H (1992). Quantitative autoradiographic mapping of 5‐HT3 receptors in the rat CNS using [125I]iodo‐zacopride and [3H]zacopride as radioligands. Synapse 10: 271–281. [DOI] [PubMed] [Google Scholar]
- Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L (1995). Early desensitization of somato‐dendritic 5‐HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol 352: 141–148. [DOI] [PubMed] [Google Scholar]
- Mahesh R, Dhar AK, Jindal A, Bhatt S (2013). 2‐(4‐substituted piperazin‐1‐yl)‐1,8‐naphthyridine‐3‐carboxylic acids: novel 5‐HT3 receptor antagonists with anxiolytic‐like activity in rodent behavioral models. Can J Physiol Pharmacol 91: 848–854. [DOI] [PubMed] [Google Scholar]
- Martin P, Gozlan H, Puech AJ (1992). 5‐HT3 receptor antagonists reverse helpless behaviour in rats. Eur J Pharmacol 212: 73–78. [DOI] [PubMed] [Google Scholar]
- Martin V, Riffaud A, Brouillard C, Lanfumey L (2015). Role of 5‐HT3 receptors in depression‐related behaviors and antidepressant treatments. Eur Neuropsychopharmacol 25 (suppl. II): S555. [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongeau R, Martin CB, Chevarin C, Maldonado R, Hamon M, Robledo P et al. (2010). 5‐HT2C receptor activation prevents stress‐induced enhancement of brain 5‐HT turnover and extracellular levels in the mouse brain: modulation by chronic paroxetine treatment. J Neurochem 115: 438–449. [DOI] [PubMed] [Google Scholar]
- Mork A, Pehrson A, Brennum LT, Nielsen SM, Zhong H, Lassen AB et al. (2012). Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther 340: 666–675. [DOI] [PubMed] [Google Scholar]
- Oh CM, Namkung J, Go Y, Shong KE, Kim K, Kim H et al. (2015). Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nat Commun 6: 6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson AL, Cremers T, Bétry C, van der Hart MG, Jørgensen L, Madsen M et al. (2013). Lu AA21004, a novel multimodal antidepressant, produces regionally selective increases of multiple neurotransmitters‐a rat microdialysis and electrophysiology study. Eur Neuropsychopharmacol 23: 133–145. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy R, Radhakrishnan M, Borah M (2008). Antidepressant‐like effects of serotonin type‐3 antagonist, ondansetron: an investigation in behaviour‐based rodent models. Behav Pharmacol 19: 29–40. [DOI] [PubMed] [Google Scholar]
- Redrobe JP, Bourin M (1997). Partial role of 5‐HT2 and 5‐HT3 receptors in the activity of antidepressants in the mouse forced swimming test. Eur J Pharmacol 325: 129–135. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS (2007). Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 10: 1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison AJ (2014). Emerging role of CaMKII in neuropsychiatric disease. Trends Neurosci 37: 653–662. [DOI] [PubMed] [Google Scholar]
- Rudmann DG, Durham SK (1999). Utilization of genetically altered animals in the pharmaceutical industry. Toxicol Pathol 27: 111–114. [DOI] [PubMed] [Google Scholar]
- Salomon L, Lanteri C, Glowinski J, Tassin JP (2006). Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci U S A 103: 7476–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Asin KE, Artigas F (2015). Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther 145: 43–57. [DOI] [PubMed] [Google Scholar]
- Saynor DA, Dixon CM (1989). The metabolism of ondansetron. Eur J Cancer Clin Oncol 25: S75–S77. [PubMed] [Google Scholar]
- Siopi E, Denizet M, Gabellec MM, de Chaumont F, Olivo‐Marin JC, Guilloux JP (2016). Anxiety‐ and depression‐like states lead to pronounced olfactory deficits and impaired adult neurogenesis in mice. J Neurosci 36: 518–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit‐Rigter LA, Noorlander CW, von Oerthel L, Chameau P, Smidt MP, van Hooft JA (2012). Prenatal fluoxetine exposure induces life‐long serotonin 5‐HT(3) receptor‐dependent cortical abnormalities and anxiety‐like behaviour. Neuropharmacology 62: 865–870. [DOI] [PubMed] [Google Scholar]
- Smolinsky AN, Bergner CL, LaPorte JL, Kalueff AV (2009). Analysis of grooming behavior and its utility in studying animal stress, anxiety, and depression In: Gould TD. (ed). Mood and Anxiety Related Phenotypes in Mice, Neuromethods 42 Humana Press: New York, pp. 29–36. [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Lummis SC (2006). 5‐HT3 receptors. Curr Pharm Des 12: 3615–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchihara Y, Tanaka K, Asano T, Tamura F, Mizushima T (2016). Superoxide dismutase overexpression protects against glucocorticoid‐induced depressive‐like behavioral phenotypes in mice. Biochem Biophys Res Commun 22: 873–877. [DOI] [PubMed] [Google Scholar]
- Zajecka J, Schatzberg A, Stahl S, Shah A, Caputo A, Post A (2010). Efficacy and safety of agomelatine in the treatment of major depressive disorder: a multicenter, randomized, double‐blind, placebo‐controlled trial. J Clin Psychopharmacol 30: 135–144. [DOI] [PubMed] [Google Scholar]
- Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L et al. (2002). The 5‐HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci 22: 1010–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Social avoidance test. In the social avoidance test, there were no differences in terms of time interacting with the target between stressed and control animals. However, non‐stressed and stressed Htr3a KO mice spent significantly more time than paired WT mice in the interaction zone when the target was present. Each value was the mean ± S.E.M. of n = n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. * P < 0.05, effect of genotype, 2‐way ANOVA.
Figure S1 A: Locomotor activity in the elevated plus maze. Animals from both genotypes displayed a similar locomotion in the EPM. Each bar was the mean ± S.E.M. of n = 8 WT and 6 KO mice. B. Locomotion of Htr3a KO and WT mice submitted to CSDS in the open field. Both stressed mutant and wild‐type animals displayed a similar hypolocomotion in the open field. Each bar was the mean ± S.E.M. of n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. *P < 0.05, effect of stress, 2‐way ANOVA.
Figure S2 Effect of CSDS on body and adrenal weight. (A) Effect of stress on adrenal gland weight. Stress similarly increased adrenal gland weight in both genotypes. There was no effect of genotype nor an interaction between the stress and genotype factors. Each bar was the mean ± S.E.M. of n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. * P < 0.05, effect of stress (two‐way ANOVA). (B) Body weight gain was higher in stressed WT compared to non‐stressed paired mice. Stress had no effect in Htr3a KO mice. Each bar was the mean ± S.E.M. of n = 11 Control WT, 12 Stressed WT, 11 Control KO and 15 Stressed KO mice. * P < 0.05, ‘Control WT’ versus ‘Stressed WT’, # P < 0.05, ‘Stressed WT’ versus ‘Stressed KO’, 2‐way ANOVA followed by Bonferroni post hoc test.


