Abstract
Background
Lymphocyte to monocyte ratio (LMR) was recently reported as a prognostic factor of pancreatic cancer (PC). However, the prognostic role of LMR in PC remains inconsistent and inconclusive. The aim of this study was to assess the prognostic value of LMR in patients with PC through meta-analysis.
Methods
Eligible studies inquiring into the connection between LMR and survival of patients with PC were collected and extracted by searching PubMed, Embase, Cochrane Library and Web of Science up to May 9, 2017. Pooled hazard ratios (HRs) and the 95% CIs were calculated to assess the prognostic value of LMR on overall survival (OS) and disease-free survival/recurrence-free survival/time to progression (DFS/RFS/TTP).
Results
A total of 1,795 patients with PC from 8 studies were included in the meta-analysis. Pooled analysis indicated that elevated LMR predicted a favorable OS (HR =0.56, 95% CI: 0.38–0.83, P=0.004) and DFS/RFS/TTP in PC patients (HR =0.38, 95% CI: 0.15–0.95, P=0.04). Prognostic values of LMR on OS were observed in subgroups with all ethnicities, treatment with surgery, American Joint Committee on Cancer (AJCC) stage of III–IV, and LMR cut-off value ≥3. In addition, low LMR was significantly connected with gender and AJCC stage.
Conclusion
An elevated LMR is associated with favorable survival in patients with pancreatic cancer.
Keywords: pancreatic cancer, lymphocyte to monocyte ratio (LMR), meta-analysis, prognosis
Introduction
Pancreatic cancer (PC) is one of the most aggressive malignancies, and the fourth leading cause of cancer death worldwide, with an overall 5-year survival rate of <5%.1 Surgical resection is still the primary treatment for patients with operable disease, but most PC patients cannot be diagnosed until advanced stage, and only <20% are operable.2,3 Therefore, it is important to identify a prognostic marker that could contribute to the selection of individual therapeutic strategy and the improvement of prognosis of PC.
Systemic inflammation has been found to be associated with angiogenesis and progression of cancer,4–6 and can be easily measured by peripheral blood-based parameters such as neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR), and lymphocyte to monocyte ratio (LMR), which is associated with prognosis in patients with various types of malignant tumors.7–11 Recently, a number of investigations suggested that LMR predicts clinical outcomes of PC patients.12–14 Nevertheless, the consistency and magnitude of the prognostic impact of LMR for PC patients has not yet been systematically analyzed. Therefore, we conducted a systematic review and meta-analysis to assess the prognostic value of LMR on overall survival (OS) and disease-free survival/recurrence-free survival/time to progression (DFS/RFS/TTP) in PC patients.
Materials and methods
Search strategies and eligible criteria
This analysis was conducted in accordance with the PRISMA statement.41 PubMed, Embase, Cochrane Library, and Web of Science were systematically searched for literature up to May 2017. The following terms were used in the main medical subject heading terms and text words: “PC,” “pancreatic cancer,” “pancreatic carcinoma,” “pancreatic tumor,” “pancreatic neoplasms,” “pancreatic adenocarcinoma,” “PDAC,” “LMR,” “lymphocyte monocyte ratio,” “lymphocyte to monocyte ratio,” “lymphocyte-to-monocyte ratio,” “lymphocyte-monocyte ratio,” “prognosis,” “outcome,” “survival,” “mortality,” “recurrence” “progression,” “metastasis”. References of relevant studies were also scanned for potentially eligible studies.
Inclusion criteria for our analysis were as follows: 1) the diagnosis of PC was confirmed by pathology; 2) described prognostic value of LMR for OS, cancer-specific survival (CSS), RFS, DFS or progression-free survival (PFS); 3) HR with 95% CI was available for data extraction.
The exclusion criteria were as follows: 1) letters, conference abstracts, case reports, editorials, expert opinions and reviews; 2) lacked sufficient data to extract HRs and their 95% CIs; 3) duplicate publications and repeated analyses.
Data extraction and qualitative assessment
The following information of each eligible study was extracted independently by 2 investigators (WL and LT): 1) first author’s name, year of publication, country, ethnicity, survival analysis methods (multivariate, univariate) and survival outcome (OS, CSS, DFS, PFS and TTP); 2) mean age of patients, number of patients (male and female), AJCC stage, treatment, cut-off of LMR, and consideration of ROC for selection of cut-off; 3) HRs as well as their 95% CIs for OS, CSS, DFS, RFS and TTP (Table 1). HRs were extracted from multivariable analyses due to consideration of the confounding factors. If multivariable analyses were not available, HRs from univariable analyses were extracted. Any discrepancies were resolved by discussion to reach a consensus.
Table 1.
Main characteristics of all the studies included in the meta-analysis
| Author | Year Country | Ethnicity | Age, years (mean ± SD) | Sample (male/female) | AJCC stage | Treatment | ROC analysis | Cut-off value | Survival outcome | HR analysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fujiwara et al17 | 2014 Japan | Asian | 66.5±10.9 | 111 (68/43) | I–IV | Surgery | No | 2 | OS/DFS | MV/UV | 7 |
| Li et al14 | 2016 China | Asian | 62.0±2.8 | 144 (77/67) | I–III | Surgery | Yes | 2.86 | OS/RFS | MV/UV | 7 |
| Qi et al18 | 2015 China | Asian | 61.2±10.7 | 211 (134/77) | III–IV | Mixed | No | 3.3 | OS | MV/UV | 6 |
| Qi et al19 | 2016 China | Asian | 58.8±10.7 | 177 (108/69) | III–IV | Chemotherapy | Yes | 3 | OS/TTP | MV/UV | 7 |
| Sierzega et al16 | 2017 Poland | Caucasian | 60 (55–66)* | 442 (260/182) | I–III | Surgery | Yes | 3 | OS | MV/UV | 7 |
| Singh et al13 | 2017 USA | Caucasian | 66.0±8.8 | 97 (97/0) | I–IV | Mixed | No | 2.05 | OS | UV | 6 |
| Yu et al15 | 2017 China | Asian | NA | 139 (83/56) | III–IV | Chemotherapy | No | 3.19 | OS | MV/UV | 7 |
| Stotz et al12 | 2015 Austria | Caucasian | 64.6±10.4 | 474 (256/218) | I–IV | Mixed | Yes | 2.8 | CSS | MV/UV | 8 |
Note:
Median (interquartile range).
Abbreviations: AJCC, American Joint Committee on Cancer; CSS, cancer-specific survival; DFS, disease-free survival; HR, hazard ratio; MV, multivariate; NA, not available; NOS, Newcastle–Ottawa Scale; OS, overall survival; RFS, recurrence-free survival; ROC, receiver operating characteristic; TTP, time to progression; UV, univariate.
The quality assessment of eligible studies was independently performed by 2 reviewers (WL and LT) according to the NOS,20 which included criteria of patient selection (0–4 points), comparability of groups (0–2 points), and outcome assessment (0–3 points). If NOS scores were ≥7, a study was defined as a high-quality study (Table 1).
Statistical analysis
RevMan version 5.3 software (Cochrane Collaboration, Copenhagen, Denmark) was used for statistical analysis. The chi-square-based Q-statistic test and the I2 statistic were performed to assess the inter-study heterogeneity.42 A fixed-effects model was used if there was no significant heterogeneity (P>0.10 for the Q-test and I2<50%). Otherwise, a random-effects model was selected. The aggregated HRs and 95% CIs were applied to access the prognostic value of LMR on OS and DFS/RFS/TTP. The associations between LMR and clinicopathologic features were expressed as odds ratios (ORs) and their 95% CIs. Subgroup analyses were conducted on the following items: ethnicity, treatment, AJCC stage, cut-off for LMR. Sensitivity analyses were conducted by evaluating result stability after sequential omission of each study. All statistical tests were two-tailed, and P<0.05 was defined as statistical significance. Funnel plot asymmetry test was used to estimate the publication bias.
Results
Search results and study characteristics
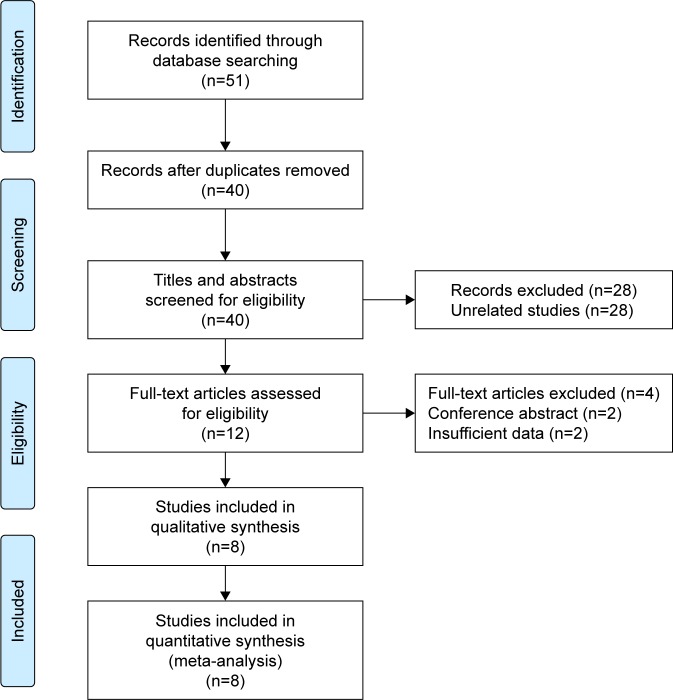
Fifty-one primary records were identified for initial review using searching strategies, of which 11 were duplicates. Forty titles and abstracts were screened for eligibility, and 12 articles were enrolled for further evaluation. After full-text article assessment, 4 articles were excluded due to conference abstract and insufficient data. Finally, 8 studies were selected for the present meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Figure 1).12–19
Figure 1.
Flow diagram of the study selection in the analysis.
A total of 1,795 patients were included for the meta-analysis and characteristics of 8 enrolled studies are shown in Table 1. Two studies were reported by Qi et al and samples came from the same medical center in China, but the enrolled groups were non-repetitive.18,19 All the studies were published between 2014 and 2017 and retrospective sample sizes ranged from 97 to 474. Among the studies, 5 evaluated Asian patients, and 3 Caucasian patients. The treatments were surgery, chemotherapy and mixed methods. Seven studies reported the prognostic value of LMR in OS, and 3 studies in DFS/RFS/TTP. Hazard ratios (HRs) and their 95% CIs were reported in the included studies with multivariable and/or univariable analyses. The LMR cut-off values ranged from 2 to 3.3, and 4 of them were identified with receiver-operating characteristic (ROC) curves. The Newcastle–Ottawa scale (NOS) scores of the included studies ranged from 6 to 8.20
Meta-analysis
The prognostic value of LMR in OS
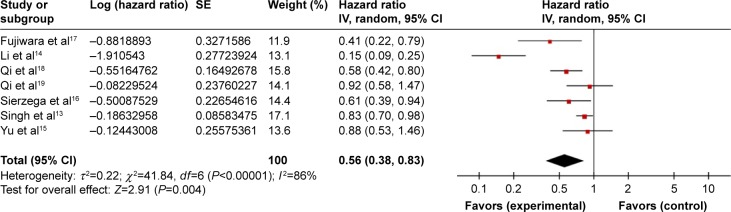
Seven studies evaluated the association between LMR and OS, which included 1,321 patients with PC. The pooled analysis indicated that an elevated LMR predicted a favorable OS (HR =0.56, 95% CI: 0.38–0.83, P=0.004). The heterogeneity of included studies was significant and a random-effects model was used (Ph <0.001; I2=86%) (Figure 2).
Figure 2.
Forest plots for the association between LMR and OS.
Abbreviations: LMR, lymphocyte to monocyte ratio; OS, overall survival; SE, standard error.
To minimize the influence of heterogeneity, we conducted 4 subgroup analyses based on ethnicity, treatment, American Joint Committee on Cancer (AJCC) stage, and cut-off value of LMR (Table 2). Subgroup analysis according to ethnicity showed that LMR had a prognostic role of OS in Asians (HR =0.50, 95% CI: 0.27–0.91, P=0.02, random-effects model, I2=87%), and in Caucasians (HR =0.80, 95% CI: 0.68–0.93, P=0.005, fixed-effects model, I2=41%). In the subgroup of the treatment, increased LMR predicted an enhanced OS in patients treated with surgery (HR =0.34, 95% CI: 0.14–0.80, P=0.01, random-effects model, I2=87%), but not chemotherapy (HR =0.90, 95% CI: 0.64–1.27, P=0.56, fixed-effects model, I2=0%) and mixed methods (HR =0.71, 95% CI: 0.50–1.01, P=0.06, random-effects model, I2=74%). When stratified by AJCC stage, the analyses indicated that LMR was a prognostic marker in patients with AJCC stage of III and IV (HR =0.71, 95% CI: 0.56–0.90, P=0.005, fixed-effects model, I2=43%), but not in patients with AJCC stage of I–IV (HR =0.63, 95% CI: 0.32–1.23, P=0.17, random-effects model, I2=76%) and I–III (HR =0.30, 95% CI: 0.08–1.20, P=0.09, random-effects model, I2=94%). In the subgroup of the cut-off value for LMR, the results indicated that increased LMR was significantly associated with favorable OS in studies of cut-off value ≥3 (HR =0.69, 95% CI: 0.56–0.85, P<0.001, fixed-effects model, I2=24%), but not in studies of cut-off value <3 (HR =0.38, 95% CI: 0.12–1.15, P=0.09, random-effects model, I2=95%).
Table 2.
Subgroup analyses for the association between LMR and OS in PC
| Subgroup | No of studies | No of patients | Effects model | HR (95% CI) | P-value | Heterogeneity
|
|
|---|---|---|---|---|---|---|---|
| I2 (%) | Ph | ||||||
| Overall | 7 | 1,321 | Random | 0.56 (0.38–0.83) | 0.004 | 86 | <0.001 |
| Ethnicity | |||||||
| Asian | 5 | 782 | Random | 0.50 (0.27–0.91) | 0.02 | 87 | <0.001 |
| Caucasian | 2 | 539 | Fixed | 0.80 (0.68–0.93) | 0.005 | 41 | 0.19 |
| Treatment | |||||||
| Surgery | 3 | 697 | Random | 0.34 (0.14–0.80) | 0.01 | 87 | <0.001 |
| Chemotherapy | 2 | 316 | Fixed | 0.90 (0.64–1.27) | 0.56 | 0 | 0.90 |
| Mixed | 2 | 308 | Random | 0.71 (0.50–1.01) | 0.06 | 74 | 0.05 |
| AJCC stage | |||||||
| I–IV | 2 | 208 | Random | 0.63 (0.32–1.23) | 0.17 | 76 | 0.04 |
| I–III | 2 | 586 | Random | 0.30 (0.08–1.20) | 0.09 | 94 | <0.001 |
| III–IV | 3 | 527 | Fixed | 0.71 (0.56–0.90) | 0.005 | 43 | 0.17 |
| Cut-off for LMR | |||||||
| ≥3 | 4 | 969 | Fixed | 0.69 (0.56–0.85) | <0.001 | 24 | 0.27 |
| <3 | 3 | 352 | Random | 0.38 (0.12–1.15) | 0.09 | 95 | <0.001 |
Abbreviations: AJCC, American Joint Committee on Cancer; HR, hazard ratio; LMR, lymphocyte to monocyte ratio; OS, overall survival; PC, pancreatic cancer.
The prognostic value of LMR in DFS/RFS/TTP
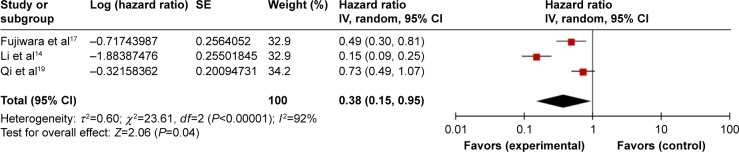
Three studies comprising 432 patients reported HRs for DFS/RFS/TTP. The random-effects model was used for analyses because there was significant heterogeneity (Ph <0.001; I2=92%). Pooled data indicated that elevated LMR was connected with favorable DFS/RFS/TTP in PC patients (HR=0.38, 95% CI: 0.15–0.95, P=0.04) (Figure 3). Subgroup analyses were not carried out because of a deficiency in studies.
Figure 3.
Forest plots for the association between LMR and DFS/PFS/TTP.
Abbreviations: DFS, disease-free survival; LMR, lymphocyte to monocyte ratio; PFS, progression-free survival; TTP, time to progression; SE, standard error.
Clinicopathological parameters and LMR
To investigate the association between LMR and characteristics of PC patients, we identified 5 clinicopathological parameters that included gender, age, location, AJCC stage, and tumor differentiation (Table 3). The pooled analysis showed that low LMR was significantly connected with gender (male vs female; HR =1.46, 95% CI: 1.09–1.97, P=0.01) and AJCC stage (I and II vs III and IV; HR =0.50, 95% CI: 0.37–0.69, P<0.001). No significant difference was found between the level of LMR and age (<65 vs ≥65; HR =0.86, 95% CI: 0.63–1.18, P=0.35), location (head vs body/tail; HR =1.03, 95% CI: 0.64–1.65, P=0.91), and tumor differentiation (poor vs well/moderate; HR =1.25, 95% CI: 0.90–1.73, P=0.18).
Table 3.
Meta-analysis of the association between LMR and clinicopathological parameters of PC
| Characteristics | No of studies | No of patients | OR (95% CI) | P-value | Heterogeneity
|
|
|---|---|---|---|---|---|---|
| I2 (%) | Ph | |||||
| Gender (male vs female) | 3 | 729 | 1.46 (1.09–1.97) | 0.01 | 0 | 0.90 |
| Age (<65 vs ≥65) | 2 | 618 | 0.86 (0.63–1.18) | 0.35 | 39 | 0.20 |
| Location (head vs body/tail) | 3 | 341 | 1.03 (0.64–1.65) | 0.91 | 0 | 0.83 |
| AJCC stage (I–II vs III–IV) | 4 | 822 | 0.50 (0.37–0.69) | <0.001 | 0 | 0.90 |
| Differentiation (poor vs well/moderate) | 2 | 618 | 1.25 (0.90–1.73) | 0.18 | 0 | 0.33 |
Abbreviations: AJCC, American Joint Committee on Cancer; LMR, lymphocyte to monocyte ratio; OR, odds ratio; PC, pancreatic cancer.
Sensitivity analysis and publication bias
We performed sensitivity analyses to assess the stability of the results of analyses considering all the included studies. Every single study was removed to estimate the influence of individual data sets on the combined HR for OS and DFS/RFS/TTP. The result showed that the pooled HR was not obviously influenced by any single study, which indicated that our pooled results were stable.
Funnel plot asymmetry test was used to estimate the publication bias. Visual inspection of funnel plot of OS indicated asymmetry, which may be due to an insufficient number of studies and significant statistical heterogeneity (Figure 4).
Figure 4.
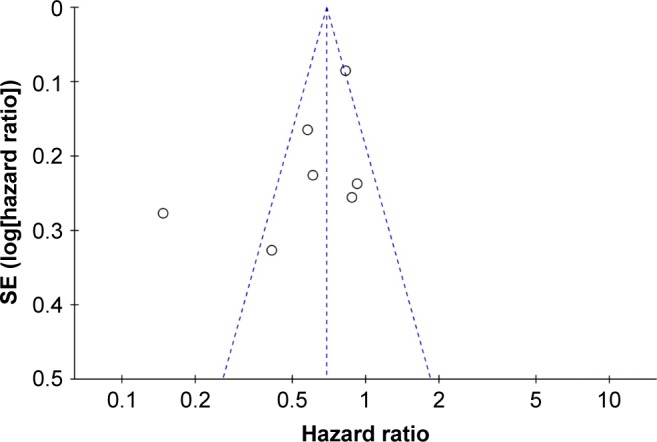
Funnel plot for studies investigated OS in PC.
Abbreviations: OS, overall survival; PC, pancreatic cancer.
Discussion
Inflammation is the seventh hallmark of cancer,21,22 and the bilateral influence of systemic inflammatory status and cancer is involved in the prognosis of PC patients. Inflammation-related prognostic scores utilizing blood count parameters, such as NLR, LMR, and PLR, have gained increasing attention in various malignancies, including PC.10,11,23–25 To the best of our knowledge, this is the first meta-analysis to investigate the prognostic value of LMR in PC.
Our study pooled the currently available evidence and suggested that the elevated LMR significantly increases OS and DFS/RFS/TTP in patients with PC. Because there was significant heterogeneity among studies, subgroup analyses were performed. The subgroup analyses showed that LMR had prognostic value in OS regardless of ethnicity. As for the treatment of PC, our study implied that higher prognostic value of LMR in OS was observed in patients who received surgery. We suggested that the OS of patients receiving chemoradiotherapy can be influenced by unknown factors, such as myelosuppression. Furthermore, the stratified analyses showed that LMR may have more prognostic value for OS in advanced PC patients. The cut-off value of LMR for OS in PC patients was different for previous retrospective studies, and our stratified analyses showed that LMR cut-off value ≥3 may have more discriminative prognostic value for OS. In addition, the association between LMR and clinicopathological parameters of PC patients was analyzed. The pooled data showed that male patients and advanced PC patients (AJCC stage III and IV) were more likely to have low LMR, which indicated poor OS.
The real mechanisms by which LMR predicts the survival outcome of PC patients are still unclear. The systematic inflammation state, which is affected by host immunity, is associated with progression and metastasis of PC.26–28 Tumor infiltrating immune cells play a critical role in cancer progression and metastasis, and have prognostic value in various malignancies.29–31 Tumor-infiltrating lymphocytes are important components of the anti-tumor immune microenvironment, and are the cellular basis of immunosurveillance against tumor cells.32 CD4+ and CD8+ T lymphocytes play an important role in anti-tumor immunity reaction through induction of cytotoxic cell death and inhibition of tumor cell proliferation and migration.33–35 In addition, previous studies have found that lymphocytopenia was a prognostic factor of OS in PC.27,28
Monocytes play an important role in tumor progression. Tumor-associated macrophages (TAMs), which are associated with peripheral blood monocytes, have been found to be correlated with poor survival in several malignancies.36,37 TAMs have also been implicated in promoting tumor invasion and angiogenesis, as well as had immunosuppressive effects on anti-tumor response of lymphocytes by production of growth factors and cytokines.5,39–41 Therefore, a low LMR represented inadequate anti-tumor immunity and a tumor promoted inflammatory microenvironment.
There were several limitations of our study. First, significant heterogeneity was found among studies. Therefore, a random-effects model and subgroup analyses were performed to adjust for heterogeneity. Second, there were not enough eligible studies to focus on the prognostic value of LMR in DFS/RFS/TTP, so the subgroup analyses were not conducted. Third, the cut-off value of LMR was different in each study, based on which stratified analyses on cut-off value for LMR were conducted in our study. Finally, all included studies were retrospective studies.
In conclusion, our meta-analysis showed that an elevated LMR is associated with favorable survival in patients with PC. The LMR could be a generally available and low price prognostic biomarker of PC in clinical practice.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (No 81672862), the Doctoral Fund of the Ministry Education of China (No 20090001110096) and the Capital Characteristic Clinical Application Research and Achievement Promotion Project (No Z171100001017121).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371(22):2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 3.Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adeno-carcinoma. BMJ. 2012;344:e2476. doi: 10.1136/bmj.e2476. [DOI] [PubMed] [Google Scholar]
- 4.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goh BK, Kam JH, Lee SY, et al. Significance of neutrophil-to- lymphocyte ratio, platelet-to-lymphocyte ratio and prognostic nutrition index as preoperative predictors of early mortality after liver resection for huge (& gt;/=10 cm) hepatocellular carcinoma. J Surg Oncol. 2016;113(6):621–627. doi: 10.1002/jso.24197. [DOI] [PubMed] [Google Scholar]
- 8.Glazer ES, Rashid OM, Pimiento JM, Hodul PJ, Malafa MP. Increased neutrophil-to-lymphocyte ratio after neoadjuvant therapy is associated with worse survival after resection of borderline resectable pancreatic ductal adenocarcinoma. Surgery. 2016;160(5):1288–1293. doi: 10.1016/j.surg.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proctor MJ, Morrison DS, Talwar D, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47(17):2633–2641. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 10.Shirai Y, Shiba H, Sakamoto T, et al. Preoperative platelet to lymphocyte ratio predicts outcome of patients with pancreatic ductal adenocarcinoma after pancreatic resection. Surgery. 2015;158(2):360–365. doi: 10.1016/j.surg.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 11.Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte-to-monocyte ratio in patients with solid tumors: a systematic review and meta-analysis. Cancer Treat Rev. 2015;41(10):971–978. doi: 10.1016/j.ctrv.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Stotz M, Szkandera J, Stojakovic T, et al. The lymphocyte to monocyte ratio in peripheral blood represents a novel prognostic marker in patients with pancreatic cancer. Clin Chem Lab Med. 2015;53(3):499–506. doi: 10.1515/cclm-2014-0447. [DOI] [PubMed] [Google Scholar]
- 13.Singh G, Nassri A, Kim D, Zhu H, Ramzan Z. Lymphocyte-to-monocyte ratio can predict mortality in pancreatic adenocarcinoma. World J Gastrointest Pharmacol Ther. 2017;8(1):60–66. doi: 10.4292/wjgpt.v8.i1.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li GJ, Xu HW, Ji JJ, Yang F, Gao BQ. Prognostic value of preoperative lymphocyte-to-monocyte ratio in pancreatic adenocarcinoma. Onco Targets Ther. 2016;9:1085–1092. doi: 10.2147/OTT.S96707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu SL, Xu LT, Qi Q, et al. Serum lactate dehydrogenase predicts prognosis and correlates with systemic inflammatory response in patients with advanced pancreatic cancer after gemcitabine-based chemotherapy. Sci Rep. 2017;7:45194. doi: 10.1038/srep45194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierzega M, Lenart M, Rutkowska M, et al. Preoperative neutrophil-lymphocyte and lymphocyte-monocyte ratios reflect immune cell population rearrangement in resectable pancreatic cancer. Ann Surg Oncol. 2017;24(3):808–815. doi: 10.1245/s10434-016-5634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujiwara Y, Misawa T, Shiba H, et al. Postoperative peripheral absolute blood lymphocyte-to-monocyte ratio predicts therapeutic outcome after pancreatic resection in patients with pancreatic adenocarcinoma. Anticancer Res. 2014;34(9):5163–5168. [PubMed] [Google Scholar]
- 18.Qi Q, Geng Y, Sun M, Wang P, Chen Z. Clinical implications of systemic inflammatory response markers as independent prognostic factors for advanced pancreatic cancer. Pancreatology. 2015;15(2):145–150. doi: 10.1016/j.pan.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer Am Cancer Soc. 2016;122(14):2158–2167. doi: 10.1002/cncr.30057. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Mantovani A. Cancer: inflaming metastasis. Nature. 2009;457(7225):36–37. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 23.Ben Q, An W, Wang L, Wang W, Yu L, Yuan Y. Validation of the pretreatment neutrophil-lymphocyte ratio as a predictor of overall survival in a cohort of patients with pancreatic ductal adenocarcinoma. Pancreas. 2015;44(3):471–477. doi: 10.1097/MPA.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 24.Zhao QT, Yuan Z, Zhang H, et al. Prognostic role of platelet to lymphocyte ratio in non-small cell lung cancers: a meta-analysis including 3,720 patients. Int J Cancer. 2016;139(1):164–170. doi: 10.1002/ijc.30060. [DOI] [PubMed] [Google Scholar]
- 25.Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204–1212. doi: 10.1158/1055-9965.EPI-14-0146. [DOI] [PubMed] [Google Scholar]
- 26.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 27.von Bernstorff W, Voss M, Freichel S, et al. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7(Suppl 3):925s–932s. [PubMed] [Google Scholar]
- 28.Fogar P, Sperti C, Basso D, et al. Decreased total lymphocyte counts in pancreatic cancer: an index of adverse outcome. Pancreas. 2006;32(1):22–28. doi: 10.1097/01.mpa.0000188305.90290.50. [DOI] [PubMed] [Google Scholar]
- 29.Man YG, Stojadinovic A, Mason J, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013;4(1):84–95. doi: 10.7150/jca.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milne K, Alexander C, Webb JR, et al. Absolute lymphocyte count is associated with survival in ovarian cancer independent of tumor-infiltrating lymphocytes. J Transl Med. 2012;10:33. doi: 10.1186/1479-5876-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haruki K, Shiba H, Fujiwara Y, et al. Perioperative change in peripheral blood monocyte count may predict prognosis in patients with colorectal liver metastasis after hepatic resection. J Surg Oncol. 2012;106(1):31–35. doi: 10.1002/jso.23033. [DOI] [PubMed] [Google Scholar]
- 32.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Zikos TA, Donnenberg AD, Landreneau RJ, Luketich JD, Donnenberg VS. Lung T-cell subset composition at the time of surgical resection is a prognostic indicator in non-small cell lung cancer. Cancer Immunol Immunother. 2011;60(6):819–827. doi: 10.1007/s00262-011-0996-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minami T, Minami T, Shimizu N, et al. Identification of programmed death ligand 1-derived peptides capable of inducing cancer-reactive cytotoxic T lymphocytes from HLA-A24+ patients with renal cell carcinoma. J Immunother. 2015;38(7):285–291. doi: 10.1097/CJI.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411(6835):380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 36.Sasaki A, Kai S, Endo Y, et al. Prognostic value of preoperative peripheral blood monocyte count in patients with colorectal liver metastasis after liver resection. J Gastrointest Surg. 2007;11(5):596–602. doi: 10.1007/s11605-007-0140-0. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki A, Iwashita Y, Shibata K, Matsumoto T, Ohta M, Kitano S. Prognostic value of preoperative peripheral blood monocyte count in patients with hepatocellular carcinoma. Surgery. 2006;139(6):755–764. doi: 10.1016/j.surg.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4(1):71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 40.Lee HW, Choi HJ, Ha SJ, Lee KT, Kwon YG. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim Biophys Acta. 2013;1835(2):170–179. doi: 10.1016/j.bbcan.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]