Abstract
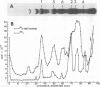
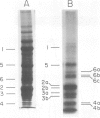
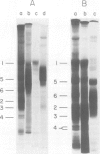
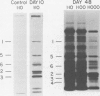
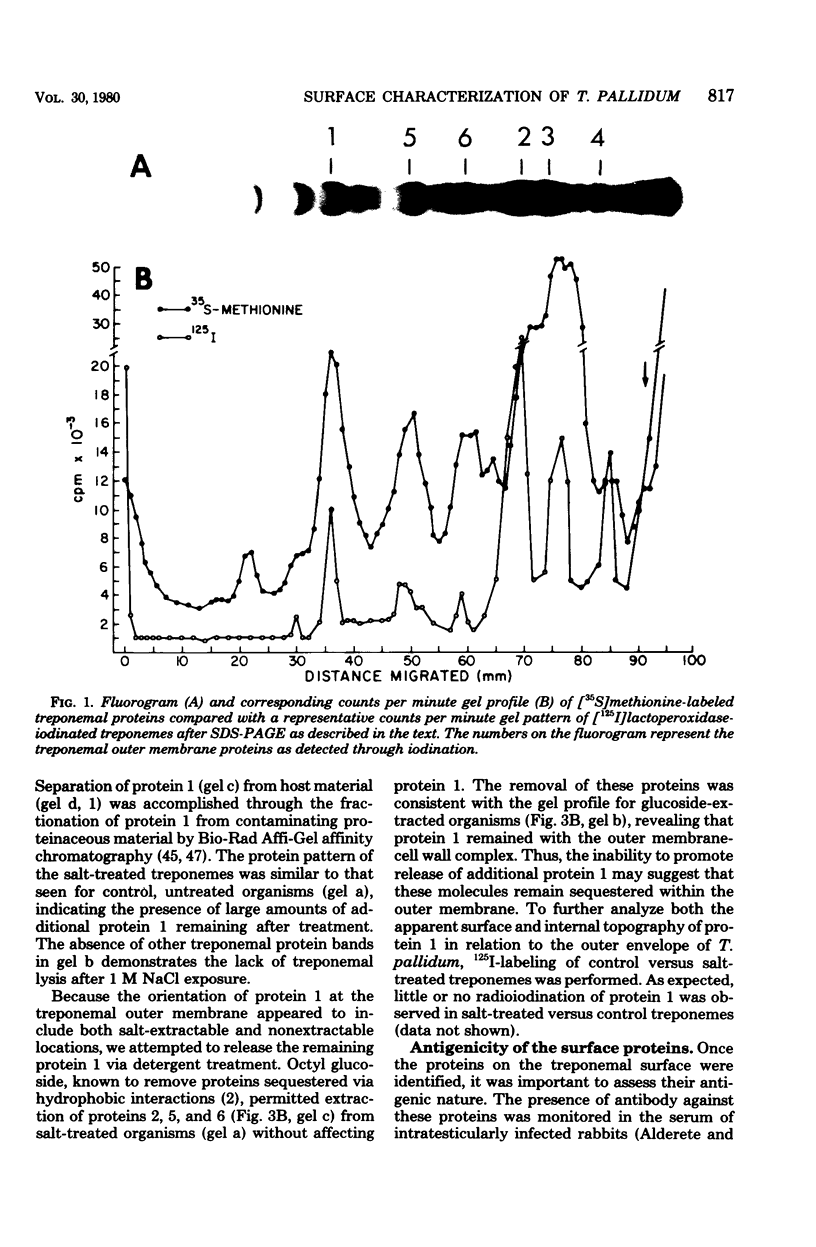
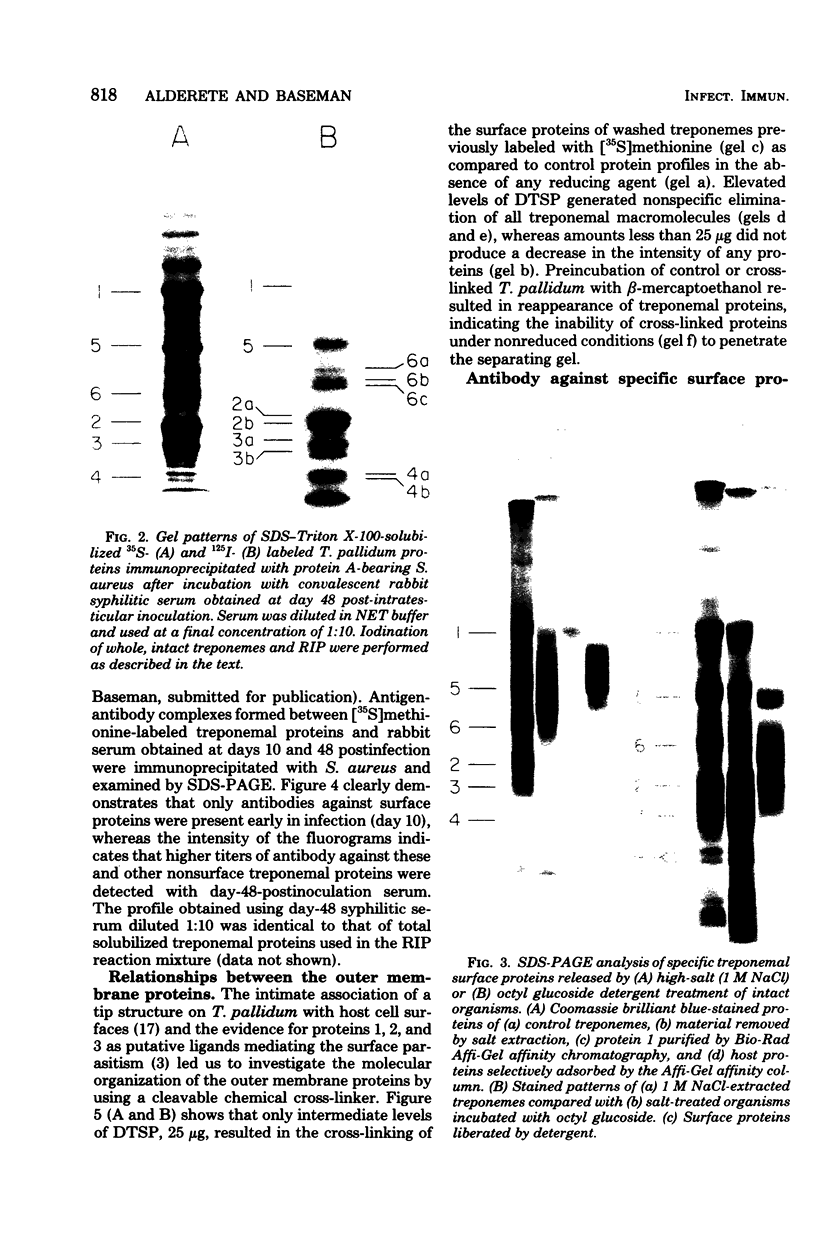
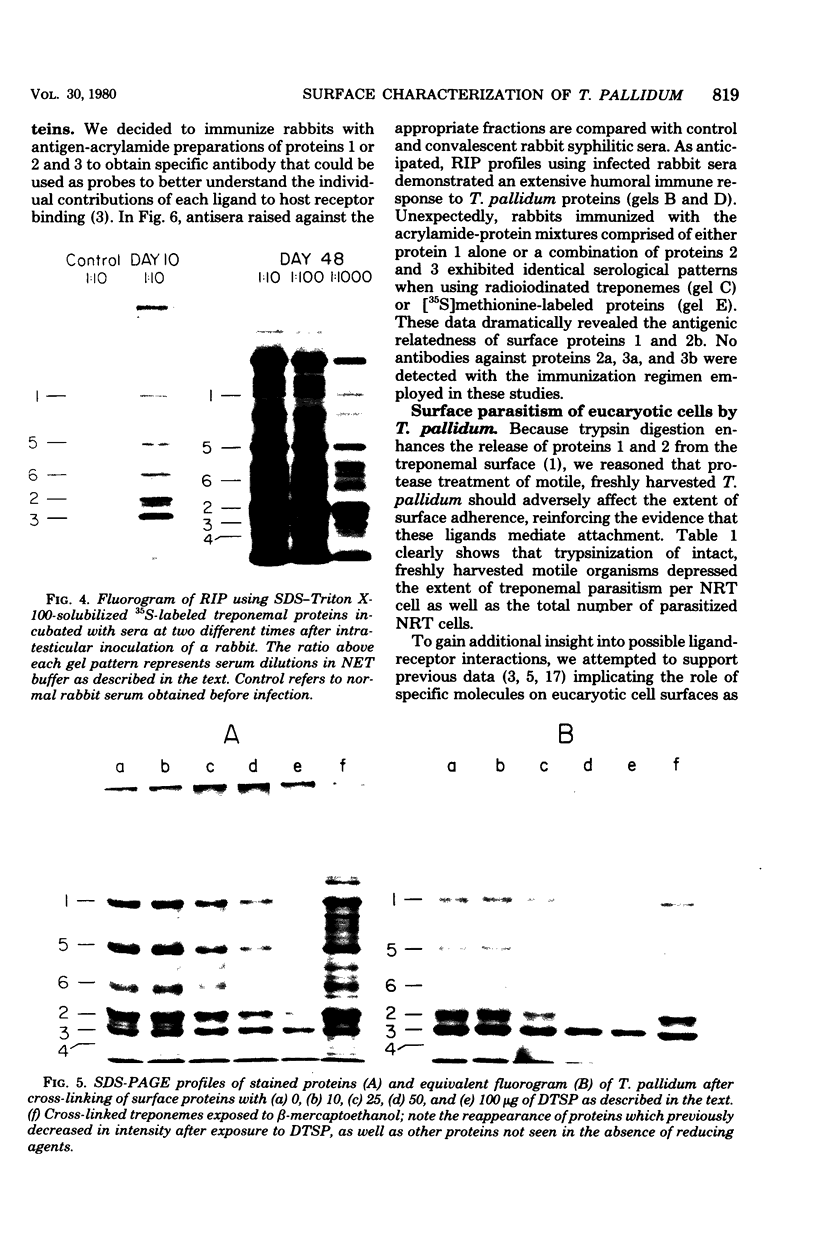
Characterization of the surface of Treponema pallidum was accomplished by [125I]lactoperoxidase-catalyzed iodination of intact organisms and sensitive radioimmunoprecipitation and gel electrophoresis technology. At least 11 outer membrane proteins with molecular weights ranging from 89,000 (89K) to 20K were identified, and all elicited high titers of antibody in experimentally infected rabbits. Proteins of 89.5K, 29.5K, and 25.5K previously implicated as ligands involved in attachment (J. B. Baseman and E. C. Hayes, J. Exp. Med. 151:573-586, 1980) were found to reside on the treponemal surface. Low levels of the 89.5K treponemal protein were released by high salt concentrations, whereas the remaining comigrating material was neither radioiodinated nor released with selective detergents. Other lower-molecular-weight (60K, 45K, and 30K) surface proteins were extracted with octyl glucoside detergent, suggesting their hydrophobic interaction with the external membrane. The molecular organization of surface proteins was studied by employing the cross-linker dithiobis(succinimidyl)-propionate, and data suggested the presence of a highly fluid envelope resulting in random collisions by the surface proteins. The biological function of the treponemal outer envelope proteins was evaluated using, as the indicator system, adherence of T. pallidum to monolayer cultures of eucaryotic cells. Trypsin treatment of motile, freshly harvested organisms decreased the extent of surface parasitism to normal rabbit testicular cells, reinforcing the idea of the proteinaceous nature and role of treponemal ligands for attachment. Other data supported functional and antigenic relatedness among the implicated ligands. Finally, brief periodate treatment of human epithelial (HEp-2) and normal rat testicular cells as well as casein-elicited rabbit peritoneal macrophages significantly reduced the extent of treponemal parasitism, suggesting a role of specific host membrane molecules as mediators of attachment.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Baseman J. B. Surface-associated host proteins on virulent Treponema pallidum. Infect Immun. 1979 Dec;26(3):1048–1056. doi: 10.1128/iai.26.3.1048-1056.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron C., Thompson T. E. Solubilization of bacterial membrane proteins using alkyl glucosides and dioctanoyl phosphatidylcholine. Biochim Biophys Acta. 1975 Mar 25;382(3):276–285. doi: 10.1016/0005-2736(75)90270-9. [DOI] [PubMed] [Google Scholar]
- Baseman J. B., Hayes E. C. Molecular characterization of receptor binding proteins and immunogens of virulent Treponema pallidum. J Exp Med. 1980 Mar 1;151(3):573–586. doi: 10.1084/jem.151.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Hayes N. S. Protein synthesis by Treponema pallidum extracted from infected rabbit tissue. Infect Immun. 1974 Dec;10(6):1350–1355. doi: 10.1128/iai.10.6.1350-1355.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C., Purcell E. M. Physics of chemoreception. Biophys J. 1977 Nov;20(2):193–219. doi: 10.1016/S0006-3495(77)85544-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brause B. D., Roberts R. B. Attachment of virulent Treponema pallidum to human mononuclear phagocytes. Br J Vener Dis. 1978 Aug;54(4):218–224. doi: 10.1136/sti.54.4.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshdat Y., Ofek I., Yashouv-Gan Y., Sharon N., Mirelman D. Isolation of a mannose-specific lectin from Escherichia coli and its role in the adherence of the bacteria to epithelial cells. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1551–1559. doi: 10.1016/0006-291x(78)91179-8. [DOI] [PubMed] [Google Scholar]
- Fieldsteel A. H., Becker F. A., Stout J. G. Prolonged survival of virulent Treponema pallidum (Nichols strain) in cell-free and tissue culture systems. Infect Immun. 1977 Oct;18(1):173–182. doi: 10.1128/iai.18.1.173-182.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieldsteel A. H., Stout J. G., Becker F. A. Comparative behavior of virulent strains of Treponema pallidum and Treponema pertenue in gradient cultures of various mammalian cells. Infect Immun. 1979 May;24(2):337–345. doi: 10.1128/iai.24.2.337-345.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Mucopolysaccharidase of Treponema pallidum. Infect Immun. 1979 Apr;24(1):261–268. doi: 10.1128/iai.24.1.261-268.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten W., Hindennach I., Henning U. The major proteins of the Escherichia coli outer cell envelope membrane. Characterization of proteins II* and III, comparison of all proteins. Eur J Biochem. 1975 Nov 1;59(1):215–221. doi: 10.1111/j.1432-1033.1975.tb02444.x. [DOI] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm H., Hjelm K., Sjöquist J. Protein A from Staphylococcus aureus. Its isolation by affinity chromatography and its use as an immunosorbent for isolation of immunoglobulins. FEBS Lett. 1972 Nov 15;28(1):73–76. doi: 10.1016/0014-5793(72)80680-x. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium. J Exp Med. 1977 May 1;145(5):1328–1343. doi: 10.1084/jem.145.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Ritzi D. M., Livermore B. P. Outer envelope of virulent Treponema pallidum. Infect Immun. 1973 Aug;8(2):291–295. doi: 10.1128/iai.8.2.291-295.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. C., Wachter M. S., Ritzi D. M. Treponeme outer cell envelope: solubilization and reaggregation. Infect Immun. 1973 Feb;7(2):249–258. doi: 10.1128/iai.7.2.249-258.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston K. H., Gotschlich E. C. Isolation and characterization of the outer membrane of Neisseria gonorrhoeae. J Bacteriol. 1974 Jul;119(1):250–257. doi: 10.1128/jb.119.1.250-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. H., Finn M. A., Thomas J. J., Folger C. Growth and subculture of pathogenic T. pallidum (Nichols strain) in BHK-21 cultured tissue cells. Br J Vener Dis. 1976 Feb;52(1):18–23. doi: 10.1136/sti.52.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehm D. J., Ji T. H. Photochemical cross-linking of cell membranes. A test for natural and random collisional cross-links by millisecond cross-linking. J Biol Chem. 1977 Dec 10;252(23):8524–8531. [PubMed] [Google Scholar]
- King G. J., Swanson J. Studies on gonococcus infection. XV. Identification of surface proteins of Neisseria gonorrhoeae correlated with leukocyte association. Infect Immun. 1978 Aug;21(2):575–584. doi: 10.1128/iai.21.2.575-584.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. R., Monjan A. A., Hardy P. H., Jr, Cole G. A. Abrogation of genetically controlled resistance of mice to Treponema pallidum by irradiation. Nature. 1980 Feb 7;283(5747):572–574. doi: 10.1038/283572a0. [DOI] [PubMed] [Google Scholar]
- Lomant A. J., Fairbanks G. Chemical probes of extended biological structures: synthesis and properties of the cleavable protein cross-linking reagent [35S]dithiobis(succinimidyl propionate). J Mol Biol. 1976 Jun 14;104(1):243–261. doi: 10.1016/0022-2836(76)90011-5. [DOI] [PubMed] [Google Scholar]
- Maret S. M., Baseman J. B., Folds J. D. Cell-mediated immunity in Treponema pallidum infected rabbits: in vitro response of splenic and lymph node lymphocytes to mitogens and specific antigens. Clin Exp Immunol. 1980 Jan;39(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R. M., Fieldsteel A. H. Genetic relationship between Treponema pallidum and Treponema pertenue, two noncultivable human pathogens. J Bacteriol. 1980 Jan;141(1):427–429. doi: 10.1128/jb.141.1.427-429.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen R. B., Wallach D. F. Photoactivated cross-linking of proteins within the erythrocyte membrane core. J Biol Chem. 1976 Dec 10;251(23):7413–7416. [PubMed] [Google Scholar]
- Mitchell G. F. Responses to infection with metazoan and protozoan parasites in mice. Adv Immunol. 1979;28:451–511. doi: 10.1016/s0065-2776(08)60803-2. [DOI] [PubMed] [Google Scholar]
- Moll A., Manning P. A., Timmis K. N. Plasmid-determined resistance to serum bactericidal activity: a major outer membrane protein, the traT gene product, is responsible for plasmid-specified serum resistance in Escherichia coli. Infect Immun. 1980 May;28(2):359–367. doi: 10.1128/iai.28.2.359-367.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nell E. E., Hardy P. H., Jr The use of freeze-preserved treponemes in the Treponema pallidum immobilization test. Cryobiology. 1972 Oct;9(5):404–410. doi: 10.1016/0011-2240(72)90157-5. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- PELTIER A., CHRISTIAN C. L. The presence of the rheumatoid factor in sera from patients with syphilis. Arthritis Rheum. 1959 Feb;2(1):1–7. doi: 10.1002/1529-0131(195902)2:1<1::aid-art1780020102>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Pavis C. S., Folds J. D., Baseman J. B. Cell-mediated immunity during syphilis. Br J Vener Dis. 1978 Jun;54(3):144–150. doi: 10.1136/sti.54.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perine P. L., Weiser R. S., Klebanoff S. J. Immunity to syphilis. I. Passive transfer in rabbits with hyperimmune serum. Infect Immun. 1973 Nov;8(5):787–790. doi: 10.1128/iai.8.5.787-790.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reithmeier R. A., Bragg P. D. Cross-linking of the proteins in the outer membrane of Escherichia coli. Biochim Biophys Acta. 1977 Apr 18;466(2):245–256. doi: 10.1016/0005-2736(77)90222-x. [DOI] [PubMed] [Google Scholar]
- Sandok P. L., Jenkin H. M., Matthews H. M., Roberts M. S. Unsustained multiplication of treponema pallidum (nichols virulent strain) in vitro in the presence of oxygen. Infect Immun. 1978 Feb;19(2):421–429. doi: 10.1128/iai.19.2.421-429.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., Chan J. K., Le Frock J. L. Endemic syphilis: passive transfer of resistance with serum and cells in hamsters. J Infect Dis. 1979 Sep;140(3):378–383. doi: 10.1093/infdis/140.3.378. [DOI] [PubMed] [Google Scholar]
- Titus R. G., Weiser R. S. Experimental syphilis in the rabbit: passive transfer of immunity with immunoglobulin G from immune serum. J Infect Dis. 1979 Dec;140(6):904–913. doi: 10.1093/infdis/140.6.904. [DOI] [PubMed] [Google Scholar]
- Travis J., Pannell R. Selective removal of albumin from plasma by affinity chromatography. Clin Chim Acta. 1973 Nov 23;49(1):49–52. doi: 10.1016/0009-8981(73)90341-0. [DOI] [PubMed] [Google Scholar]
- Ware J. L., Folds J. D., Baseman J. B. Serum of rabbits infected with Treponema pallidum (Nichols) inhibits in vitro transformation of normal rabbit lymphocytes. Cell Immunol. 1979 Feb;42(2):363–372. doi: 10.1016/0008-8749(79)90201-6. [DOI] [PubMed] [Google Scholar]
- Wille L. E. A simple and rapid method for isolation of serum lipoproteins from the other serum protein constituents. Clin Chim Acta. 1976 Sep 6;71(2):355–357. doi: 10.1016/0009-8981(76)90554-4. [DOI] [PubMed] [Google Scholar]