Abstract
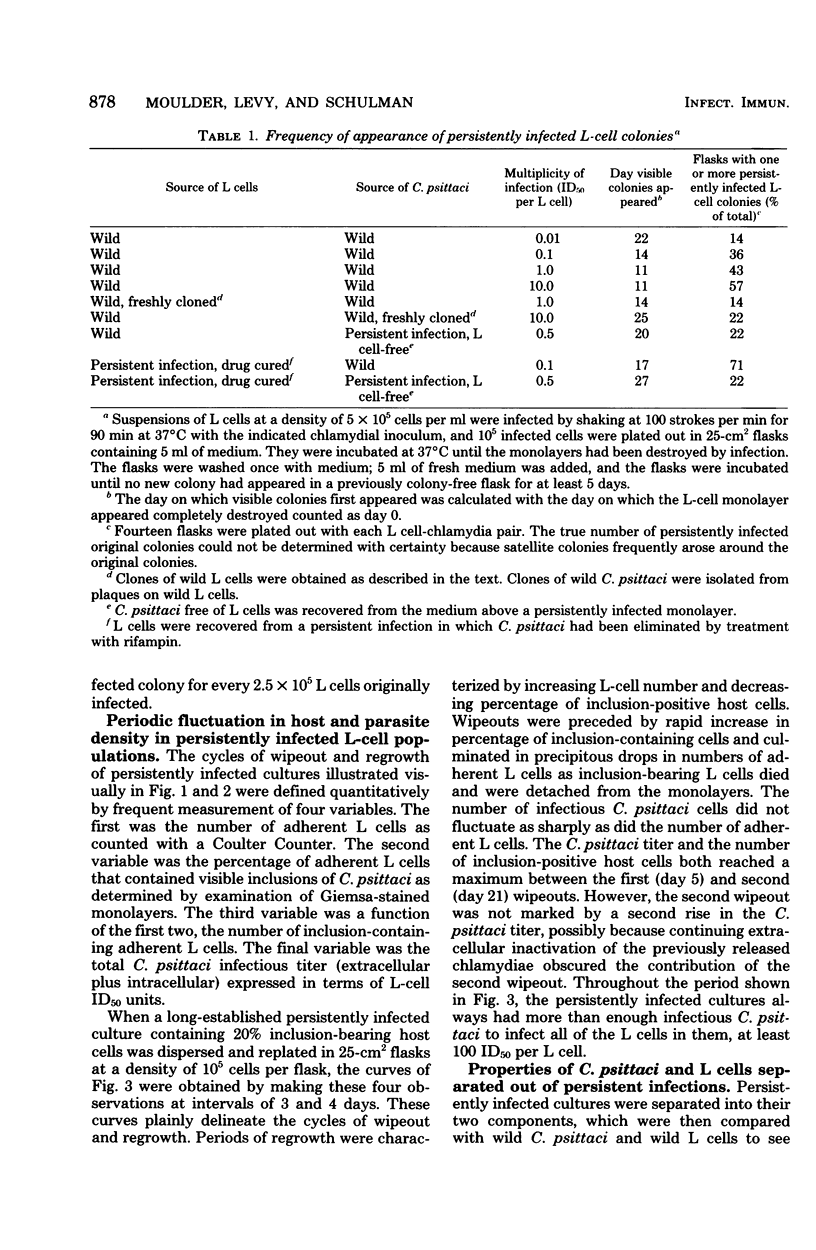
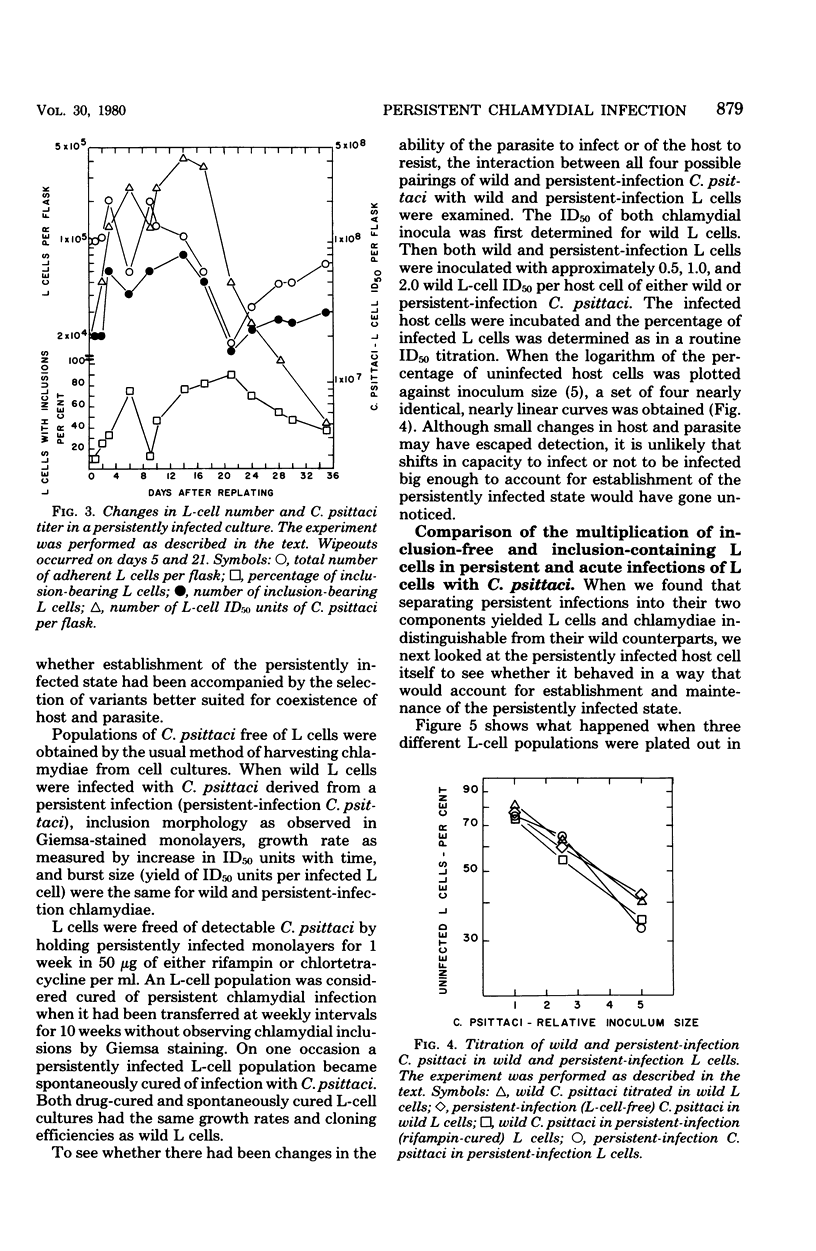
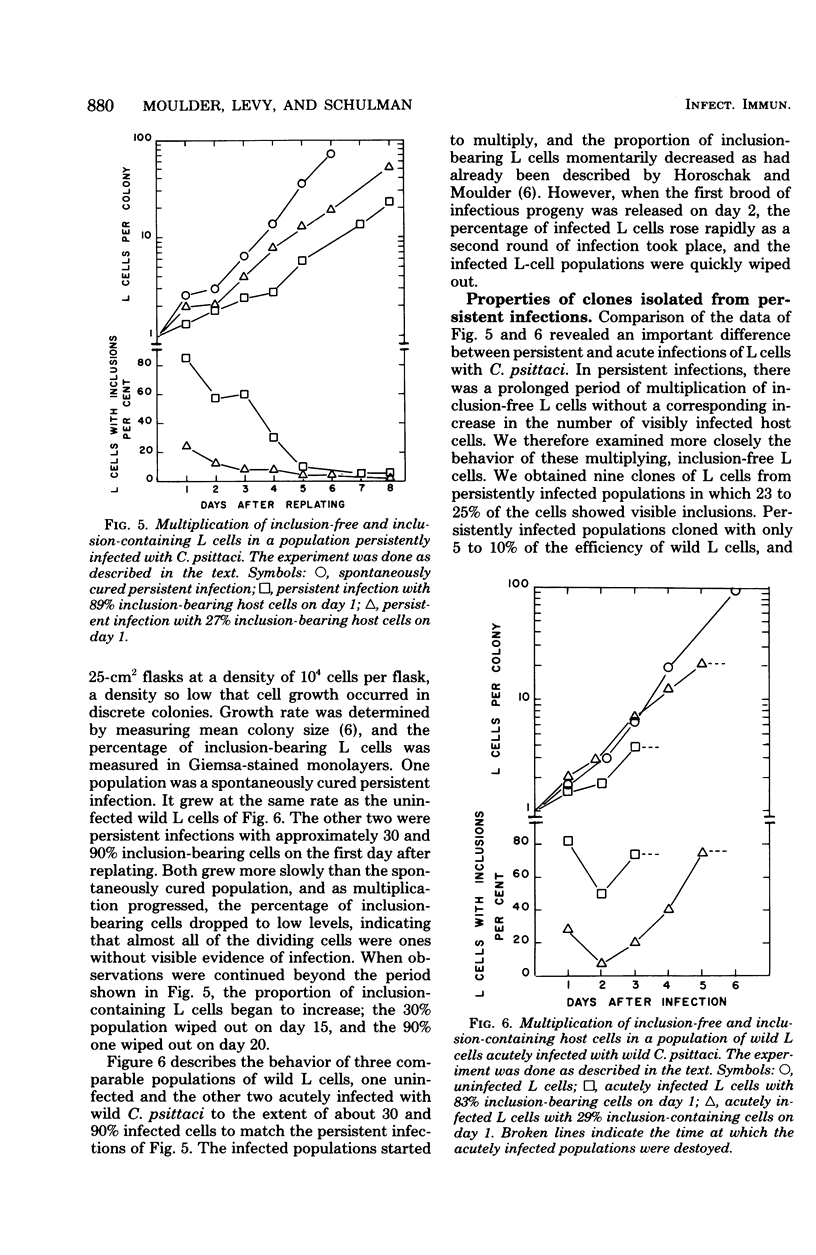
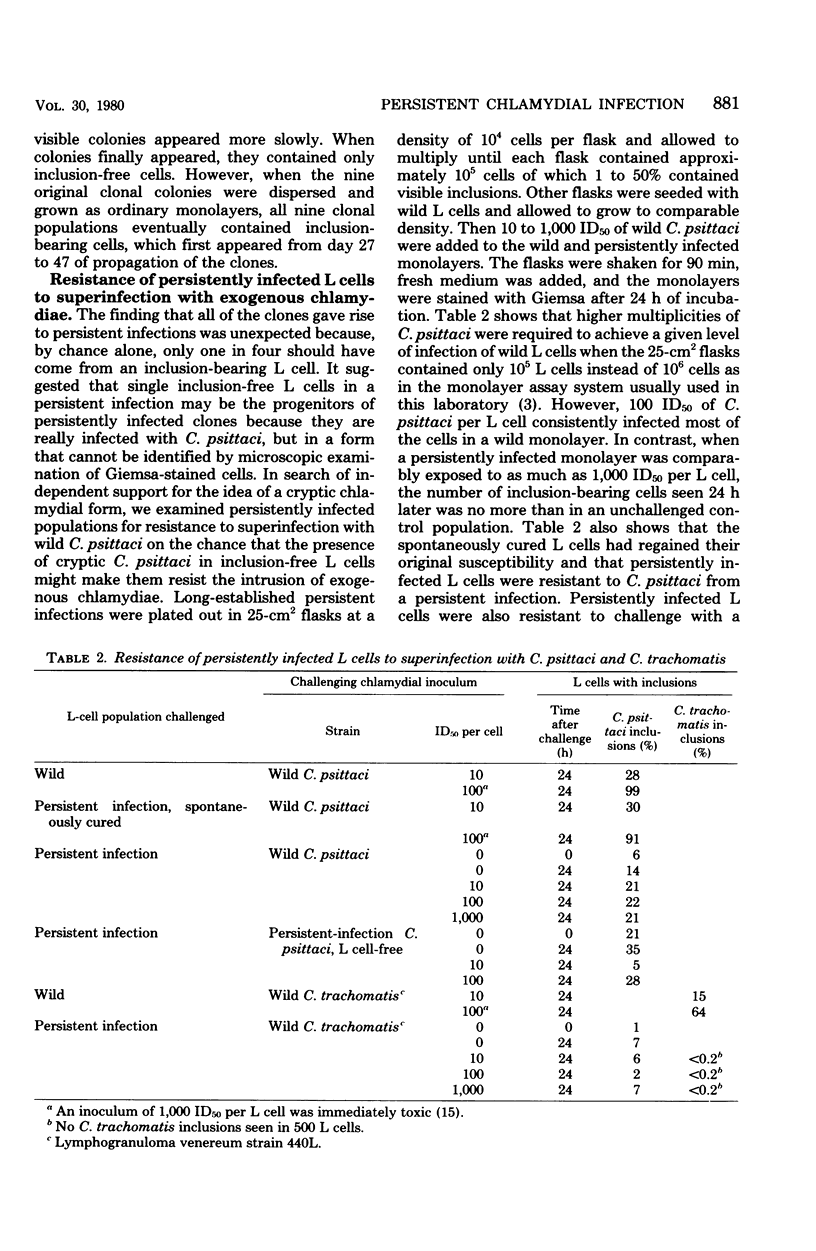
When monolayers of mouse fibroblasts (L cells) were infected with enough Chlamydia psittaci (strain 6BC) to destroy most of the host cells, 1 in every 105 to 106 originally infected cells gave rise to a colony of L cells persistently infected with strain 6BC. In these populations, the density of L cells and 6BC fluctuated periodically and reciprocally as periods of host cell increase were followed by periods of parasite multiplication. Successive cycles of L-cell and 6BC reproduction were sustained indefinitely by periodic transfer to fresh medium. Isolation of L cells and 6BC from persistent infections provided no evidence that there had been any selection of variants better suited for coexistence. Persistently infected populations consisting mainly of inclusion-free L cells yielded only persistently infected clones, grew more slowly, and cloned less efficiently. They were also almost completely resistant to superinfection with high multiplicities of either 6BC or the lymphogranuloma venereum strain 440L of Chlamydia trachomatis. These properties of persistently infected L cells may be accounted for by assuming that all of the individuals in these populations are cryptically infected with 6BC and that cryptic infection slows the growth of the host cell and makes it immune to infection with exogenous chlamydiae. According to this hypothesis, the fluctuations in host and parasite density occur because some factor periodically sets off the conversion of cryptic chlamydial forms into reticulate bodies that multiply and differentiate into infectious elementary bodies in a conventional chlamydial developmental cycle.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADER J. P., MORGAN H. R. Latent viral infection of cells in tissue culture. VII. Role of water-soluble vitamins in psittacosis virus propagation in L cells. J Exp Med. 1961 Feb 1;113:271–281. doi: 10.1084/jem.113.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne G. I. Kinetics of phagocytosis of Chlamydia psittaci by mouse fibroblasts (L cells): separation of the attachment and ingestion stages. Infect Immun. 1978 Feb;19(2):607–612. doi: 10.1128/iai.19.2.607-612.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
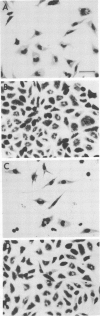
- Horoschak K. D., Moulder J. W. Division of single host cells after infection with chlamydiae. Infect Immun. 1978 Jan;19(1):281–286. doi: 10.1128/iai.19.1.281-286.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg K. R., Horoschak K. D., Moulder J. W. Toxicity of low and moderate multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1977 Nov;18(2):531–541. doi: 10.1128/iai.18.2.531-541.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury D. T. Estimate of the genome size of various microorganisms. J Bacteriol. 1969 Jun;98(3):1400–1401. doi: 10.1128/jb.98.3.1400-1401.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANIRE G. P., GALASSO G. J. Persistent infection of HeLa cells with meningopneumonitis virus. J Immunol. 1959 Nov;83:529–533. [PubMed] [Google Scholar]
- MORGAN H. R. Latent viral infection of cells in tissue culture. I. Studies on latent infection of chick embryo tissues with psittacosis virus. J Exp Med. 1956 Jan 1;103(1):37–47. doi: 10.1084/jem.103.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OFFICER J. E., BROWN A. Serial changes in virus and cells in cultures chronically infected with psittacosis virus. Virology. 1961 May;14:88–99. doi: 10.1016/0042-6822(61)90136-2. [DOI] [PubMed] [Google Scholar]
- POLLARD M., SHARON N. Induction of prolonged latency in psittacosis-infected cells by aminopterin. Proc Soc Exp Biol Med. 1963 Jan;112:51–54. doi: 10.3181/00379727-112-27947. [DOI] [PubMed] [Google Scholar]
- Schoenholz W. K. Studies on Bedsonia latency. II. Effect of immune lymphocytes and of rabbit-anti-lymphocyte globulin (RAMLG) on infected macrophages exposed to increased incubation temperature in vitro. Z Immunitatsforsch Allerg Klin Immunol. 1970 May;139(4):359–371. [PubMed] [Google Scholar]
- Schoenholz W. K. Studies on bedsonia latency. I. Induction of latency in rabbit cornea (Sirc) cell culture and its reversal by changes in culture conditions. Z Immunitatsforsch Allerg Klin Immunol. 1968 Jun;135(4):283–293. [PubMed] [Google Scholar]