Summary
Objectives
Listeria monocytogenes can cause sepsis and meningitis. We report national surveillance data on L. monocytogenes meningitis in the Netherlands, describing incidence changes, genetic epidemiology and fatality rate.
Methods
We analyzed data from the Netherlands Reference Laboratory of Bacterial Meningitis for cases of L. monocytogenes meningitis. Strains were assessed by serotyping and bacterial population structure by multi-locus sequence typing.
Results
A total of 375 cases of Listeria meningitis were identified between 1985 and 2014. Peak incidence rates were observed in neonates (0.61 per 100,000 live births) and older adults (peak at 87 year; 0.53 cases per 100,000 population of the same age). Neonatal listerial meningitis decreased 17-fold from 1.95 per 100,000 live births between 1985 and 1989, to 0.11 per 100,000 live births between 2010 and 2014. Overall case fatality rate was 31%, in a multivariate analysis older age and concomitant bacteremia were associated with mortality (both p < 0.01). Clonal complexes (CC) CC1, CC2 and CC3 decreased over time from respectively 32% to 12%, 33% to 9% and 10% to 2% (all p < 0.001), while CC6 increased from 2% to 26% (p < 0.001).
Conclusions
The incidence of neonatal listerial meningitis has declined over the past 25 years. The genotype CC6 has become the predominant genotype in listerial meningitis in the Netherlands. Mortality of listeria meningitis has remained high.
Keywords: Clonal complex, Epidemiology, Listeria monocytogenes, Meningitis
Highlights
-
•
The incidence of neonatal listerial meningitis declined over the past 25 years.
-
•
CC6 has become the predominant genotype in listerial meningitis in the Netherlands.
-
•
The case fatality rate of listeria meningitis is high.
Background
Listeria monocytogenes is a gram-positive intracellular pathogen causing meningitis and sepsis, which primarily affects elderly and immunocompromised individuals.1, 2, 3 The primary route of infection is through ingestion of infected food. The association between contaminated food and both epidemic and sporadic listeriosis has been well documented.4, 5, 6, 7, 8 Contrary to many other foodborne pathogens, L. monocytogenes can grow in food with low moisture content and high salt concentration, and at refrigeration temperatures.9 L. monocytogenes is the third most common pathogen in bacterial meningitis in adults (5–10% of cases).10, 11, 12, 13, 14 In a recent cohort study, we showed that the rate of unfavorable outcome of L. monocytogenes meningitis increased from 27% in 1998–2002 to 61% in 2006–2012 in the Netherlands, which was associated with infection by Listeria sequence type (ST) 6 isolates.11 Here, we report on 30 years of national surveillance data on invasive L. monocytogenes meningitis in the Netherlands, describing incidence changes, genetic epidemiology and case fatality rate.
Methods
We included episodes of listerial meningitis that were reported to the Netherlands Laboratory for Bacterial Meningitis (NRLBM) from 1 January 1985 to 31 December 2014. Medical microbiology laboratories nationwide submitted bacterial isolates from cerebrospinal fluid (CSF), blood or amniotic fluid to the NRLBM, together with information on patient sex, age and source of the material collected. The NRLBM receives an estimated 90% of all bacterial meningitis isolates.15, 16 Surveillance for cases of culture-positive invasive L. monocytogenes disease by the NRLBM started in 1975. Surveillance was intensified in 2005 and has been obligatory since 2008.17, 18 An episode of listerial meningitis was defined as a patient in whom L. monocytogenes was cultured from CSF. If multiple CSF cultures were positive in one patient, the isolates were manually categorized as a recurrent episode or single episode and in case of a single episode the first CSF isolate was included for further listerial meningitis incidence and genetic analyses. We included a control group of blood isolates from patients in whom a CSF culture was not performed or was negative (non-meningitis patients) to compare the genotype distribution in listerial meningitis to other invasive listerial disease. SPSS was used to randomly select 10% of eligible non-meningitis isolates that were sent to the NRLBM during the observation period.
We obtained demographic data on the age and sex distribution of the Dutch population during the observation period from Statistics Netherlands.19 Patient age was rounded downwards to whole months for patients aged ≤12 months and to whole years over 12 months of age. Incidence was calculated per 100,000 inhabitants of the same age. For grouped comparisons, age was categorized into 0–4 weeks old (neonates), 4 weeks–49 years old (children and young adults), and ≥50 years old (older adults), and incidences were averaged over 5 year time periods. The incidence of neonatal listerial meningitis was calculated using the number of live births in the Netherlands per year.19 For episodes with missing outcome, we collected mortality data through the Municipal Personal Records Database.20 Records from before 1994 were not available in a digital format, and therefore mortality data prior to 1994 was not sought. Death within 2 months after the positive CSF culture was used to calculate the mortality rate due to listerial meningitis. This time frame was chosen based on the clinical data of the MeninGene cohort 2006–2014.14 In this Dutch cohort the longest period between admission and death because of the listeria meningitis was 2 months.
Upon arrival in the NRLBM isolates were stored at −80 °C. Cultures were performed by plating the bacteria onto Columbia Agar with 5% sheep blood. L. monocytogenes strains were serotyped by agglutination as described by Seeliger and Höhne1 with the use of O- and H-sera of an Listeria Antisera Set (Denka Seiken Co Ltd, cat.no. 294616). Multi locus sequence typing (MLST) was used to assess the L. monocytogenes population structure. MLST groups bacterial isolates into sequence types based on sequence variation in seven housekeeping genes.21 These seven different loci of the bacterial genome (abcZ, bglA, cat, dapE, dat, ldh, and lhkA) were analyzed using polymerase chain reaction followed by sequencing.22 Allocation occurred according to the Listeria MLST database of the Pasteur Institute [http://bigsdb.pasteur.fr/listeria/].23 Cluster analyses were performed using PHYLOViZ 1.0 based on the goeBURST algorithm.24 Samples from 1998 to 2002 and 2006 to 2012 were previously serotyped and sequence typed in a previous study.11
Fisher's exact test was used to compare categorical outcomes and clonal complex trends per 5-year periods and logistic regression was used to analyze the association between potential predictors and mortality. Odds ratios (ORs) and 95% confidence intervals (CIs) were used to quantify the strength of these associations. Statistical tests were two-sided and a p-value of 0.05 or less was considered significant. Dutch law waivers informed consent for obtaining and analyzing surveillance data.
Results
A total of 1109 L. monocytogenes isolates from CSF or blood were submitted to the NRLBM during the observation period. Twenty-five entries were identified as duplicates and excluded. In 208 (19%) of the remaining 1084 episodes a listerial isolate was cultured from CSF only, and in 167 (15%) from CSF and blood (Fig. 1). These 375 episodes were included as listerial meningitis episodes. No recurrent cases were identified. A control group of 74 (10%) of the 709 non-meningitis isolates were randomly selected to compare genetic epidemiology between meningitis and non-meningitis invasive listerial disease strains. Listerial meningitis occurred in 228 (61%) men, with a median age of 64 (IQR 49–71) in men and a median age of 62 (IQR 40–74) in women (p = 0.46).
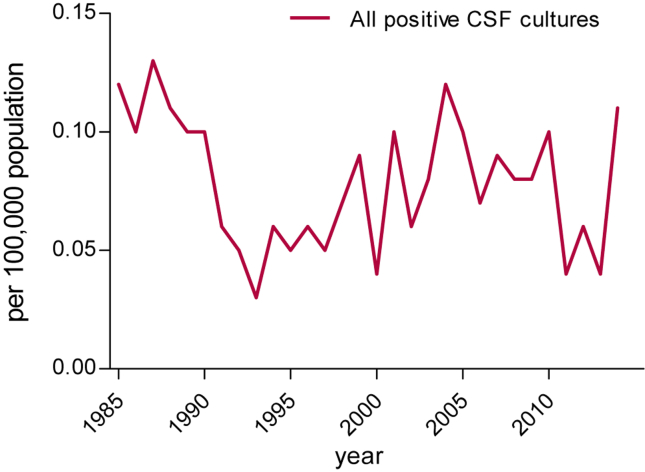
Figure 1.
Annual incidence of listerial meningitis between 1985 and 2014.
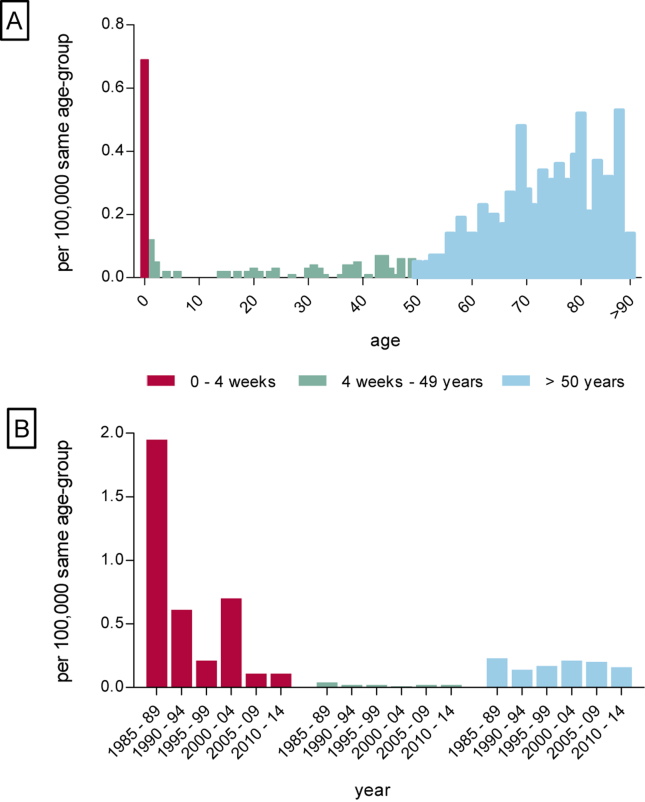
The annual incidence of listerial meningitis varied between 0.03 and 0.13 cases per 100,000 inhabitants during 1985–2014 (Fig. 1). We did not observe epidemic outbreaks or a seasonal pattern in the prevalence of listerial meningitis. The highest incidence was observed in neonates at; 0.61 per 100,000 live births (Fig. 2A). The incidence rates in children and young adults, varied between 0.01 and 0.04 cases per 100,000 same age population. The incidence in the older adults varied between 0.16 and 0.53 cases per 100,000 same age population with a peak incidence of 0.53 per 100,000 in 87-years old. A total of 40 episodes (11%) occurred in children ≤12 months old, 77 (21%) in 12 months–49 years old patients and 258 episodes (69%) in the older adults. Thirty-five (88%) of the 40 cases in children ≤12 months old were neonatal cases. Over the years, the incidence of neonatal listerial meningitis decreased from 1.95 per 100,000 live births in 1985–1989 to 0.11 per 100,000 live births in 2010–2014 (Fig. 2B), constituting a 17-fold decrease. The main decrease occurred between 1985–89 and 1990–94, with a decline from 1.95 to 0.61 per 100,000 live births. Incidence in other age groups remained stable during the studied period (Fig. 2B).
Figure 2.
A. Incidence of listerial meningitis per 100,000 same-age population. B. Incidence over the years in age-groups per 100,000 same-age population.
Serotyping of the 375 CSF isolates showed serotype 4b in 213 episodes (57%), 1/2a in 95 (25%), 1/2b in 44 (12%), 6a in 2 (0.5%), serotype 3c, serotype 4ab, 4c and 4e in 1 episode (0.3%), and 11 serotype 1 isolates (3%) could not further be specified. Serotypes were similar per age group and did not significantly change over time.
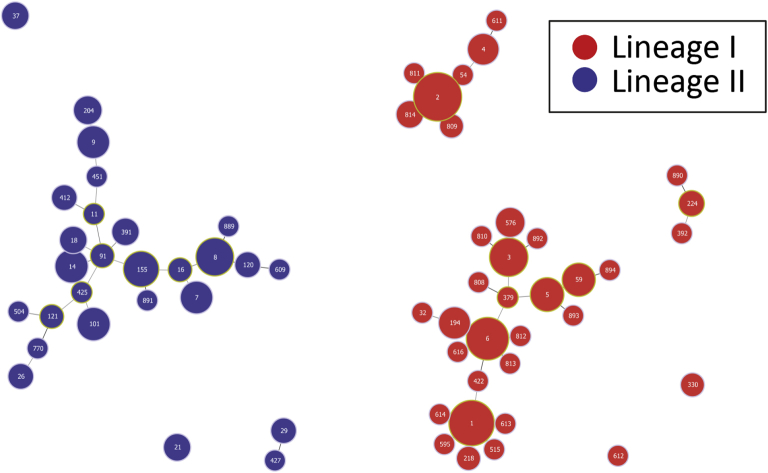
MLST was performed in 371 (99%) of 375 CSF isolates and all 74 non-meningitis isolates from the control group. Overall, 62 unique sequence types were identified (Fig. 3). 268 (72%) of the CSF isolates belonged to lineage I, comprising 32 sequence types and 103 (28%) isolates belonged to lineage II comprising 25 sequence types. The three main sequence types were ST2 in 84 (23%) cases, ST1 in 59 (16%) cases and ST6 in 40 (11%) cases. The CSF isolates grouped into 33 clonal complexes; two isolates ST32 and ST614 were singletons. The main three clonal complexes, all of lineage I were CC2 in 90 (24%) cases, CC1 in 61 (16%) cases and 44 (12%) isolates belonged to CC6 (Table 1). The main serotype in these three clonal complexes was serotype 4b in respectively 97%, 97% and 98% of isolates.
Figure 3.
Population structure analyzed with Phyloviz based on the goeBURST algorithm of L. monocytogenes in 371 CSF isolates and 74 non-meningitis isolates. ST numbers are displayed in the nodes. The node sizes vary linearly with the number of isolates of a given ST. Black and grey links are between single locus variants and double locus variants, respectively.
Table 1.
Distribution of L. monocytogenes lineage, sequence types and serotypes in CSF isolates.
| Lineage N (%) |
CC | N (%) | Serotype 4b N (%) | Serotype 1/2a N (%) | Serotype 1/2b N (%) | Other serotypes N (%) |
|---|---|---|---|---|---|---|
| Lineage I 268 (72%) |
CC1 | 61 (16%) | 59 (97%) | 1 (1.5%) | 1 (1.5%) | |
| CC2 | 90 (24%) | 87 (97%) | 3 (3%) | |||
| CC3 | 23 (6%) | 19 (83%) | 4 (17%) | |||
| CC5 | 10 (3%) | 1 (10%) | 9 (90%) | |||
| CC6 | 44 (12%) | 43 (98%) | 1 (2%) | |||
| CC59 | 10 (3%) | 8 (80%) | 2 (20%) | |||
| Othera | 30 (8%) | 20 (67%) | 2 (6%) | 8 (27%) | ||
| Lineage II 103 (28%) |
CC8 | 25 (7%) | 1 (4%) | 23 (92%) | 1 (4%) | |
| CC14 | 12 (3%) | 12 (100%) | ||||
| CC101 | 10 (3%) | 9 (90%) | 1 (10%) | |||
| CC155 | 12 (3%) | 12 (100%) | ||||
| Othera | 44 (12%) | 36 (82%) | 8 (18%) |
Other isolates < 10 cases.
We did not find a significant difference in clonal complex distribution in non-meningitis isolates compared to CSF isolates. Of the 74 non-meningitis isolates, 50 (68%) belonged to lineage I, distributed into 14 sequence types and 24 (32%) isolates among lineage II distributed into 13 sequence types. The main three clonal complexes were CC2 in 18 (24%) cases, CC1 in 9 (12%) cases and CC3 in 5 (7%) cases (Supplementary Table 1).
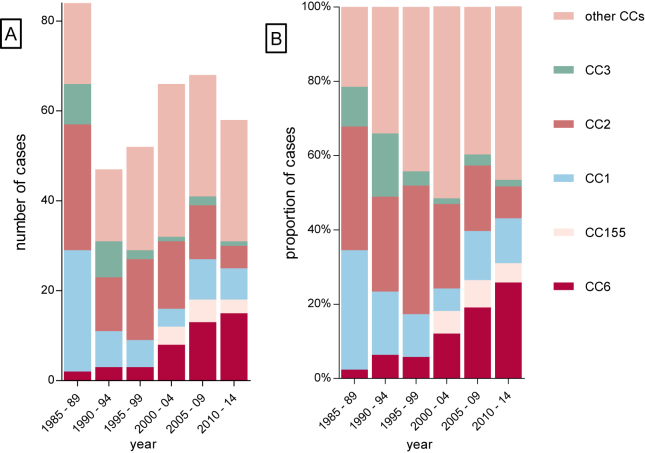
The absolute and relative frequency of CC6 and CC155 among listerial meningitis isolates significantly increased during the observed time period. CC6 was identified in 2% of episodes identified in 1985–1989 compared to 26% of episodes in 2010–2014 (p < 0.001) (Fig. 4). CC155 was not identified before 2000–2005 and found in 5% of episodes between 2010 and 2014 (p = 0.03). CC1, CC2 and CC3 decreased during the observation period from respectively 32% to 12%, 33% to 9% and 10% to 2% of cases (all p < 0.001) (Fig. 4). Clonal complexes were evenly distributed among age groups (Supplementary Fig. 1).
Figure 4.
Distribution of CC in CSF isolates per 5 year, 1985–2014, A number of cases, B percentages.
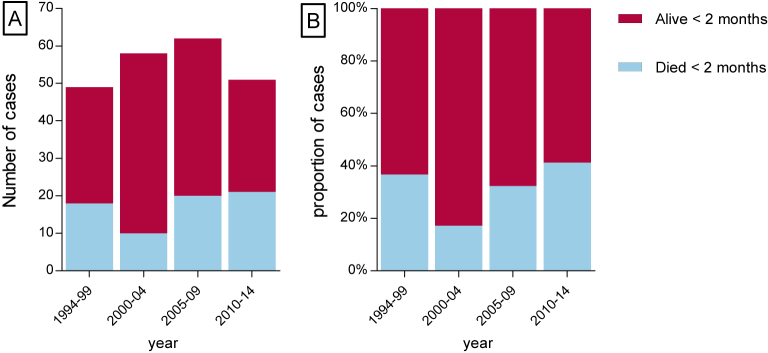
Mortality rate was analyzed in episodes included in the database from 1 January 1994 to 2014. A total of 253 (67%) listerial meningitis episodes were diagnosed in this time period and outcome was retrieved for 220 (87%) patients. A total of 69 patients (31%) died within 2 months after the diagnostic lumbar puncture. Median age in deceased patients was 74 years old (IQR 68–80) and 62 (IQR 45–71) in survivors (p < 0.01) (Table 2), 36 of 64 (56%) deceased patients were male compared to 91 of 143 (64%) in survivors. Concomitant bacteremia was present in 46 (72%) deceased patients compared to 56 (39%) in survivors (p < 0.01). Lineages, serotypes and clonal complexes were uniformly distributed between deceased and surviving patients. The overall fatality rate fluctuated between 17 and 42% per 5-year period (Fig. 5). No deaths occurred in the 11 cases of neonatal meningitis occurring after 1994.
Table 2.
Characteristics of patients and isolate characteristics in listerial meningitis patients who died within 2 months compared to survivors.
| Characteristic | Died <2 months (%) | Alive after 2 months (%) | p-Value |
|---|---|---|---|
| Median age [IQR]a | 74 [65–80] | 62 [45–71] | <0.01 |
| Male | 36/64 (56) | 91/143 (72%) | 0.36 |
| Bacteremia | 46/69 (67) | 56/151 (37) | <0.01 |
| Lineage I | 43/69 (62) | 99/148 (67) | 0.54 |
| Serotype 4b | 36/69 (52) | 77/150 (52) | 0.99 |
| Serotype 1/2a | 26/69 (38) | 47/150 (31) | 0.36 |
| Serotype 1/2b | 5/69 (7) | 21/150 (14) | 0.18 |
| Other serotypes | 2/69 (3) | 5/150 (3) | 0.99 |
| CCb 1 | 6/69 (9) | 17/151 (11) | 0.64 |
| CC 2 | 13/69 (19) | 31/151 (20) | 0.86 |
| CC 3 | 1/69 (1) | 4/151 (3) | 0.99 |
| CC 6 | 14/69 (20) | 22/151 (15) | 0.33 |
| CC 155 | 3/69 (5) | 8/151 (5) | 0.99 |
| Other CC | 32/69 (46) | 69/151 (46) | 0.99 |
IQR = interquartile range.
CC = clonal complex.
Figure 5.
Deceased cases 1994–2014. A number of cases, B percentages.
In a univariate analysis including all 220 listerial meningitis patients with known outcome, age and bacteremia were associated with death. These variables remained significantly associated with death in a multivariable analysis including age, year of infection, bacteremia, sex and CC6. Overall fatality rate did not increase over time and CC6 was not associated with mortality (see Table 3).
Table 3.
Results of univariate and multivariable analysis in L. monocytogenes meningitis episodes on risk for death within 2 months after diagnosis.
| Variable | Univariate analysis |
Multivariable analysis |
||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Agea | 1.07 (1.04–1.10) | <0.01 | 1.07 (1.04–1.11) | <0.01 |
| Male sex | 0.74 (0.40–1.34) | 0.31 | ||
| Bacteremia | 3.39 (1.86–6.18) | <0.01 | 3.97 (2.00–7.85) | <0.01 |
| Lineage I | 1.22 (0.67–2.22) | 0.51 | ||
| Serotype 4b | 1.03 (0.59–1.83) | 0.91 | ||
| Clonal complex 6b | 1.49 (0.71–3.13) | 0.29 | 1.25 (0.53–2.97) | 0.61 |
| Year of infectiona | 1.03 (0.98–1.09) | 0.23 | 1.04 (0.98–1.11) | 0.18 |
Per one year increase.
Compared to all other clonal complex.
Conclusions
We observed a strong increase of listerial meningitis due to CC6 over the last thirty years in the Netherlands. Previous epidemiological studies showed CC6 is mostly found in Europe and North American countries, and less on other continents.25, 26, 27 In the United States L. monocytogenes of CC6 caused two multistate listeriosis outbreaks in 1998 and 2002 with respectively 50 and 45 listeriosis cases.26 A recent French study found that CC6 was the second most common clonal complex in listerial positive CSF cultures (13% of cases).27 None of these studies however analyzed temporal shifts in listerial genotype. ST6 was previously shown to be associated with unfavorable outcome in listerial meningitis but not with death.11 The results of our study are in line with that finding because we did not find an association between mortality and CC6 or any other clonal complex. Our findings stress the need of continued surveillance studies on this disease, preferably including data on infection source, clinical outcome, and genetic monitoring of the causative pathogen.28
We observed a strong decrease in the incidence of neonatal listerial meningitis. This decline may be explained by improved primary prevention by education of pregnant women to increase their awareness on the dangers of types of food that are frequently contaminated with L. monocytogenes, a decrease in contamination through ready-to-eat food following improvements in the food-processing industry, or both. In the Netherlands the Institute of Public Health and the Environment (RIVM) created more awareness for food restrictions in pregnancy in the ‘90s by educational folders.29 Our results are similar to a study in France showing perinatal invasive listerial infections decreased 12-fold from 1984 to 2011 with the strongest decline between 1986 and 1996.30 In this study a concurrent decrease in pregnancy-related toxoplasmosis was observed, which was suggested to be due to education campaigns causing behavioral changes in pregnant woman.
Several studies have suggested that the decline in listerial infections since the 1990s could also be the result of improvements in the food-processing industry.31 The European Food Safety Authority was established in 2002 to regulate food safety measurements, which should improve the level of protection of the population. Food and safety regulation for the European Union were drafted in 2002 and updated in 2005.32 However, a European surveillance report between 2006 and 2009 showed no overall decrease of listeriosis incidence in 25 European countries after the implementation of these directives.33 With an aging population the susceptibility for listerial meningitis increases,19 and therefore, food and health authorities should remain vigilant for epidemiological changes leading to increased disease severity or incidence.
The emrC efflux transporter has recently been shown to be associated with the emergence of CC 6 in the Netherlands.34 The emrC gene encodes an efflux protein that pumps quaternary ammonium compounds out of the cell and increases the capacity to form a biofilm, resulting in benzalkonium chloride tolerance. Benzalkonium chloride is extensively used in the food-processing industry as a disinfectant and sterilization agent. Reduced susceptibility to benzalkonium chloride may explain the increasing incidence of CC 6 isolates.
The case fatality rate of listerial meningitis is high (30%), and is higher than reported in patients with pneumococcal meningitis.14, 35, 36 The all age mortality fluctuated over time between 17 and 42% per 5-year period and is comparable to other studies showing a 13–36% mortality rate.3, 11, 37, 38, 39, 40 Death in listerial meningitis was associated with older age and concomitant bacteremia. In retrospective studies older age has been described to be associated with death3 and bacteremia has been described to be associated with neurological sequelae.40 A recent prospective study from France found an overall 3-month mortality rate of 30% in neurolisteriosis and 46% in bacteremia.2 This study identified female sex, older age, ongoing neoplasia, more than 5% weight loss, multi-organ failure, decompensated comorbidity, monocytopenia less than 200 cells per μL, and high neutrophil count as independent predictors of death in listerial bacteremia and neurolisteriosis.
Our study has several limitations. First, the reported incidence rates of listerial meningitis will be an underestimation. Only culture confirmed cases were included in this study. In 11%–30% of patients with bacterial meningitis CSF culture is negative.41, 42 In listerial meningitis this percentage may be higher.1, 43 Furthermore, because surveillance for listeria was intensified in 2005, underestimation could have been stronger before than after 2005. If this is the case the decline in neonatal listerial meningitis will have been even larger than identified. Second, the decrease of neonatal listerial meningitis cases could theoretically be caused by changes in clinical practice, for instance due to higher rates of initiation of antibiotic treatment before lumbar puncture, or reduced lumbar puncture rates. However, this seems unlikely since there has been no decline in other neonatal meningitis such as GBS meningitis in the Netherlands over the last decades.44 Third, mortality rates were based on death within 2 months after the diagnostic puncture. This analysis will overestimate the number of deaths due to a listerial meningitis episode as the causal relationship between the listerial meningitis episode and death was not confirmed. Fourth, this study is based on surveillance data available at the NRLBM based on the submitted strains, therefore we do not have detailed clinical data on disease course in these patients. The majority of samples in this surveillance study were not included in prospective meningitis cohort studies that were previously published.11 Fifth, since we only included positive blood cultures in the control group it is unknown which percentage of these patients had a negative or no CSF culture performed. This may underestimate the difference between only blood culture positive and only CSF of CSF and blood positive cultures. Moreover, we have no data on negative blood culture in patients with positive CSF cultures.
In this national surveillance study, we observed a decline in incidence of neonatal listerial meningitis. The genotype CC6 has become the predominant genotype in listerial meningitis in the Netherlands, and the case fatality rate of listerial meningitis remains high.
Funding
This work was supported by the National Institute of Public Health and the Environment to AvdE, the European Union's seventh Framework program (EC-GA no 279185 [EUCLIDS] to DvdB), Netherlands Organization for Health Research and Development (NWO-Vidi grant 2010 to DvdB), Academic Medical Center (AMC Fellowship 2008 to DvdB), and the European Research Council (ERC Starting Grant 2011 to DvdB).
Conflict of interest
All authors have no conflict of interest.
Acknowledgement
Mrs Agaath Arend, Ilse Schuurman, Moniek Feller and Wendy Keijzer are acknowledged for their expert technical assistance in MLST and serotyping. We thank the team of curators of the Institut Pasteur MLST and whole genome MLST databases for curating the data and making them publicly.23
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jinf.2017.04.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ciesielski C.A., Hightower A.W., Parsons S.K., Broome C.V. Listeriosis in the United States: 1980–1982. Arch Intern Med. 1988;148:1416–1419. [PubMed] [Google Scholar]
- 2.Charlier C., Perrodeau E., Leclercq A., Cazenave B., Pilmis B., Henry B. Clinical features and prognostic factors of listeriosis: the MONALISA national prospective cohort study. Lancet Infect Dis. 2017 Jan 28 doi: 10.1016/S1473-3099(16)30521-7. pii: S1473-3099(16)30521-7. [DOI] [PubMed] [Google Scholar]
- 3.Mylonakis E., Hohmann E.L., Calderwood S.B. Central nervous system infection with Listeria monocytogenes. 33 years' experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 1998;77:313–336. doi: 10.1097/00005792-199809000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Fleming D.W., Cochi S.L., MacDonald K.L., Brondum J., Hayes P.S., Plikaytis B.D. Pasteurized milk as a vehicle of infection in an outbreak of listeriosis. N Engl J Med. 1985;312:404–407. doi: 10.1056/NEJM198502143120704. [DOI] [PubMed] [Google Scholar]
- 5.Schlech W.F., III, Lavigne P.M., Bortolussi R.A., Allen A.C., Haldane E.V., Wort A.J. Epidemic listeriosis–evidence for transmission by food. N Engl J Med. 1983;308:203–206. doi: 10.1056/NEJM198301273080407. [DOI] [PubMed] [Google Scholar]
- 6.Bula C.J., Bille J., Glauser M.P. An epidemic of food-borne listeriosis in western Switzerland: description of 57 cases involving adults. Clin Infect Dis. 1995;20:66–72. doi: 10.1093/clinids/20.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Linnan M.J., Mascola L., Lou X.D., Goulet V., May S., Salminen C. Epidemic listeriosis associated with Mexican-style cheese. N Engl J Med. 1988;319:823–828. doi: 10.1056/NEJM198809293191303. [DOI] [PubMed] [Google Scholar]
- 8.Schuchat A., Deaver K.A., Wenger J.D., Plikaytis B.D., Mascola L., Pinner R.W. Role of foods in sporadic listeriosis. I. Case-control study of dietary risk factors. The Listeria Study Group. JAMA. 1992;267:2041–2045. [PubMed] [Google Scholar]
- 9.Pradhan A.K., Ivanek R., Grohn Y.T., Geornaras I., Sofos J.N., Wiedmann M. Quantitative risk assessment for Listeria monocytogenes in selected categories of deli meats: impact of lactate and diacetate on listeriosis cases and deaths. J Food Prot. 2009;72:978–989. doi: 10.4315/0362-028x-72.5.978. [DOI] [PubMed] [Google Scholar]
- 10.Moon S.Y., Chung D.R., Kim S.W., Chang H.H., Lee H., Jung D.S. Changing etiology of community-acquired bacterial meningitis in adults: a nationwide multicenter study in Korea. Eur J Clin Microbiol Infect Dis. 2010;29:793–800. doi: 10.1007/s10096-010-0929-8. [DOI] [PubMed] [Google Scholar]
- 11.Koopmans M.M., Brouwer M.C., Bijlsma M.W., Bovenkerk S., Keijzers W., van der Ende A. Listeria monocytogenes sequence type 6 and increased rate of unfavorable outcome in meningitis: epidemiologic cohort study. Clin Infect Dis. 2013;57:247–253. doi: 10.1093/cid/cit250. [DOI] [PubMed] [Google Scholar]
- 12.Brouwer M.C., van de Beek D., Heckenberg S.G., Spanjaard L., de Gans J. Community-acquired Listeria monocytogenes meningitis in adults. Clin Infect Dis. 2006;43:1233–1238. doi: 10.1086/508462. [DOI] [PubMed] [Google Scholar]
- 13.Thigpen M.C., Whitney C.G., Messonnier N.E., Zell E.R., Lynfield R., Hadler J.L. Bacterial meningitis in the United States, 1998–2007. N Engl J Med. 2011;364:2016–2025. doi: 10.1056/NEJMoa1005384. [DOI] [PubMed] [Google Scholar]
- 14.Bijlsma M.W., Brouwer M.C., Kasanmoentalib E.S., Kloek A.T., Lucas M.J., Tanck M.W. Community-acquired bacterial meningitis in adults in the Netherlands, 2006–14: a prospective cohort study. Lancet Infect Dis. 2016;16:339–347. doi: 10.1016/S1473-3099(15)00430-2. [DOI] [PubMed] [Google Scholar]
- 15.Netherlands reference laboratory for bacterial meningitis (AMC/RIVM) – bacterial meningitis in the Netherlands; annual report 2008. University of Amsterdam; Amsterdam: 2009. [Google Scholar]
- 16.Bijlsma M.W., Bekker V., Brouwer M.C., Spanjaard L., van de Beek D., van der Ende A. Epidemiology of invasive meningococcal disease in the Netherlands, 1960–2012: an analysis of national surveillance data. Lancet Infect Dis. 2014;14:805–812. doi: 10.1016/S1473-3099(14)70806-0. [DOI] [PubMed] [Google Scholar]
- 17.Doorduyn Y., de Jager C.M., van der Zwaluw W.K., Wannet W.J., van der Ende A., Spanjaard L. First results of the active surveillance of Listeria monocytogenes infections in the Netherlands reveal higher than expected incidence. Euro Surveill. 2006;11:E060420. doi: 10.2807/esw.11.16.02945-en. [DOI] [PubMed] [Google Scholar]
- 18.Friesema I.H.M., de Jager C.M., van der Zwaluw W.K., Notermans D.W., van Heerwaarden C.A.M., Heuvelink A.E. Intensieve surveillance van Listeria monocytogenes in Nederland in 2009. Infectiebulletin. 2012;22:67–71. [Google Scholar]
- 19.Centraal Bureau voor Statistiek – Data available at: http://statline.cbs.nl/Statweb/, [Accessed 22 October 2015].
- 20.Governmental website on the Municipal Personal Records Database https://www.government.nl/topics/identification-documents/contents/the-municipal-personal-records-database, [Accessed 23 October 2015].
- 21.Ragon M., Wirth T., Hollandt F., Lavenir R., Lecuit M., Le M.A. A new perspective on Listeria monocytogenes evolution. PLoS Pathog. 2008;4:E1000146. doi: 10.1371/journal.ppat.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jadhav S., Bhave M., Palombo E.A. Methods used for the detection and subtyping of Listeria monocytogenes. J Microbiol Methods. 2012;88:327–341. doi: 10.1016/j.mimet.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Institut Pasteur – Listeria monocytogenes MLST database. Data available at: http://www.pasteur.fr/recherche/genopole/PF8/mlst/Lmono.html, [Accessed 20 September 2015]. The website has changed to http://bigsdb.pasteur.fr/listeria/.
- 24.Francisco A.P., Bugalho M., Ramirez M., Carrico J.A. Global optimal eBURST analysis of multilocus typing data using a graphic matroid approach. BMC Bioinforma. 2009;10:152. doi: 10.1186/1471-2105-10-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chenal-Francisque V., Lopez J., Cantinelli T., Caro V., Tran C., Leclercq A. Worldwide distribution of major clones of Listeria monocytogenes. Emerg Infect Dis. 2011;17:1110–1112. doi: 10.3201/eid1706.101778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantinelli T., Chenal-Francisque V., Diancourt L., Frezal L., Leclercq A., Wirth T. “Epidemic clones” of Listeria monocytogenes are widespread and ancient clonal groups. J Clin Microbiol. 2013;51:3770–3779. doi: 10.1128/JCM.01874-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maury M.M., Tsai Y.H., Charlier C., Touchon M., Chenal-Francisque V., Leclercq A. Uncovering Listeria monocytogenes hypervirulence by harnessing its biodiversity. Nat Genet. 2016;48:308–313. doi: 10.1038/ng.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van de Beek D. Progress and challenges in bacterial meningitis. Lancet. 2012;380:1623–1624. doi: 10.1016/S0140-6736(12)61808-X. [DOI] [PubMed] [Google Scholar]
- 29.National Institute for Public Health and the Environment website http://www.rivm.nl/en, [Accessed 14 November 2015].
- 30.Girard D., Leclercq A., Laurent E., Lecuit M., de Valk H., Goulet V. Pregnancy-related listeriosis in France, 1984 to 2011, with a focus on 606 cases from 1999 to 2011. Euro Surveill. 2014;19:E20909. doi: 10.2807/1560-7917.es2014.19.38.20909. [DOI] [PubMed] [Google Scholar]
- 31.Voetsch A.C., Angulo F.J., Jones T.F., Moore M.R., Nadon C., McCarthy P. Reduction in the incidence of invasive listeriosis in foodborne diseases active surveillance network sites, 1996–2003. Clin Infect Dis. 2007;44:513–520. doi: 10.1086/511006. [DOI] [PubMed] [Google Scholar]
- 32.European Food Commission – Commision regulation (EC) No 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Website http://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:02005R2073-20140601&rid=1, [Accessed 14 November 2015].
- 33.Surveillance of six priority food- and waterborne diseases in the EU/EEA 2006–2009. Website http://ecdc.europa.eu/en/publications/Publications/food-and-waterborne-diseases-surveillance-report.pdf, [Accessed 16 November 2015].
- 34.Kremer P.H., Lees J.A., Koopmans M.M., Ferwerda B., Arends A.W., Feller M.M. Benzalkonium tolerance genes and outcome in Listeria monocytogenes meningitis. Clin Microbiol Infect. 2017;23(4):265.e1–265.e7. doi: 10.1016/j.cmi.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van de Beek D., de Gans J., Tunkel A.R., Wijdicks E.F. Community-acquired bacterial meningitis in adults. N Engl J Med. 2006;354:44–53. doi: 10.1056/NEJMra052116. [DOI] [PubMed] [Google Scholar]
- 36.de Gans J., van de Beek D. European dexamethasone in adulthood bacterial meningitis study I. Dexamethasone in adults with bacterial meningitis. N Engl J Med. 2002;347:1549–1556. doi: 10.1056/NEJMoa021334. [DOI] [PubMed] [Google Scholar]
- 37.Pelegrin I., Moragas M., Suarez C., Ribera A., Verdaguer R., Martinez-Yelamos S. Listeria monocytogenes meningoencephalitis in adults: analysis of factors related to unfavourable outcome. Infection. 2014;42:817–827. doi: 10.1007/s15010-014-0636-y. [DOI] [PubMed] [Google Scholar]
- 38.Dzupova O., Rozsypal H., Smiskova D., Benes J. Listeria monocytogenes meningitis in adults: the Czech Republic experience. Biomed Res Int. 2013:1–4. doi: 10.1155/2013/846186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amaya-Villar R., Garcia-Cabrera E., Sulleiro-Igual E., Fernandez-Viladrich P., Fontanals-Aymerich D., Catalan-Alonso P. Three-year multicenter surveillance of community-acquired Listeria monocytogenes meningitis in adults. BMC Infect Dis. 2010;10:324. doi: 10.1186/1471-2334-10-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arslan F., Meynet E., Sunbul M., Sipahi O.R., Kurtaran B., Kaya S. The clinical features, diagnosis, treatment, and prognosis of neuroinvasive listeriosis: a multinational study. Eur J Clin Microbiol Infect Dis. 2015;34:1213–1221. doi: 10.1007/s10096-015-2346-5. [DOI] [PubMed] [Google Scholar]
- 41.van de Beek D., de Gans J., Spanjaard L., Weisfelt M., Reitsma J.B., Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;351:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 42.Durand M.L., Calderwood S.B., Weber D.J., Miller S.I., Southwick F.S., Caviness V.S., Jr. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med. 1993;328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 43.Gellin B.G., Broome C.V. Listeriosis. JAMA. 1989;261:1313–1320. [PubMed] [Google Scholar]
- 44.Bekker V., Bijlsma M.W., van de Beek D., Kuijpers T.W., van der Ende A. Incidence of invasive group B streptococcal disease and pathogen genotype distribution in newborn babies in the Netherlands over 25 years: a nationwide surveillance study. Lancet Infect Dis. 2014;14:1083–1089. doi: 10.1016/S1473-3099(14)70919-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.