Abstract
The actin cytoskeleton participates in numerous cellular processes, including less-characterized processes, such as nuclear organization, chromatin remodeling, transcription, and signal transduction. As a key regulator of actin cytoskeletal dynamics, the actin related protein 2/3 complex (Arp2/3 complex) controls multiple developmental processes in a variety of tissues and cell types. To date, the role of the Arp2/3 complex in plant disease resistance signaling is largely unknown. Herein, we identified and characterized wheat ARPC3, TaARPC3, which encodes the C3 subunit of the Arp2/3 complex. Expression of TaARPC3 in the arc18 mutant of Saccharomyces cerevisiae Δarc18 resulted in complementation of stress-induced phenotypes in S. cerevisiae, as well as restore wild-type cell shape malformations. TaARPC3 was found predominantly to be localized in the nucleus and cytoplasm when expressed transiently in wheat protoplast. TaARPC3 was significantly induced in response to avirulent race of Puccinia striiformis f. sp. tritici (Pst). Knock-down of TaARPC3 by virus-induced gene silencing resulted in a reduction of resistance against Pst through a specific reduction in actin cytoskeletal organization. Interestingly, this reduction was found to coincide with a block in reactive oxygen species (ROS) accumulation, the hypersensitive response (HR), an increase in TaCAT1 mRNA accumulation, and the growth of Pst. Taken together, these findings suggest that TaARPC3 is a key subunit of the Arp2/3 complex which is required for wheat resistance against Pst, a process that is associated with the regulation of the actin cytoskeleton.
Keywords: ARPC3, cytoskeleton, wheat, stripe rust, yeast mutant, virus-induced gene silencing (VIGS)
Introduction
The plant actin cytoskeleton forms a contiguous network within all eukaryotic cells and is associated with the function of many cellular processes, such as cell division and development, cell polarity, and organelle movement (Kim et al., 2005; Sparkes et al., 2009; Yokota et al., 2009). During cell growth and development, as well as mechano-stimulation and in response to stress, the actin array undergoes rapid and highly regulated changes (Shimada et al., 2006; Hardham et al., 2007, 2008; Li et al., 2014). Plenty of studies have demonstrated that the actin cytoskeleton acts a pivotal part in plant basic defensive reaction and non-host resistance to various pathogens (Kobayashi et al., 1994; Hardham et al., 2007; Tian et al., 2009). Indeed, some of the earliest work by Kobayashi et al. (1994) indicated that cytoskeleton became radially arranged in mesophyll cells of flax around haustorium or at infection sites before penetration after infected with incompatible isolates of the Melampsora lini. More recently, Henty-Ridilla et al. (2013) mentioned the intrinsic quality and timing of induced changes to the actin microfilaments in Arabidopsis during the infection of pathogens. The early transient overexpress of actin resulting in increasing of actin filaments density and proved to be involved in pathogen-associated molecular pattern (PAMP)-triggered immunity (PTI) by using adapted and non-adapted microbes and treatments with microbial-associated molecular patterns (MAMPs). The microfilament cytoskeleton also participated in race-specific resistance; for example, previous work demonstrated that AtADF4 mediates effector-triggered immunity (ETI) signaling through recognition of signaling activated in response to the recognition of the Pseudomonas effector AvrPphB (Tian et al., 2009).
The organization of the eukaryotic actin cytoskeleton is tightly regulated, and undergoes induced changes in response to a suite of internal and external stimuli. Indeed, a plethora of actin binding proteins (ABPs) have been demonstrated to regulate actin filament remodeling, among which include the actin-related protein 2/3 (Arp2/3) complex, profilin (PRF), actin depolymerizing factor (ADF), adenylate-associated protein (CAP), and Rac (a monomeric Rho-GTPase) (Hussey et al., 2006). A key step in this organization is actin nucleation, the key rate-limiting step necessary to ensure proper filaments formation (Campellone and Welch, 2010). Actin nucleation is regulated in large part by the activity of the Arp2/3 complex, with formins identified as the major actin nucleator (Chesarone and Goode, 2009). In most examples characterized to date, the ARP2/3 complex, consisting of two actin-like proteins (Arp2 and Arp3) and five unrelated subunits (Mullins et al., 1998). The remaining five subunits are commonly named according to their sizes, and in plants are referred to as ARPC1 (actin-related protein complex-1), ARPC2, ARPC3, ARPC4, and ARPC5 (Machesky et al., 1994). Although this complex has been studied more than 20 years (Machesky and Gould, 1999), a full understanding of its regulation in response to the perception of stress and developmental cues has remained elusive, and has been a topic of intense study. Genetic data indicates the Arp2/3 complex is essential in yeast (Winter et al., 1999). However, it remains unclear if each of the functional subunits is essential for the overall function of the Arp2/3 complex. For example, in Saccharomyces cerevisiae, deletion of the ARPC1 or ARPC2 caused lethality and severe reductions in viability. Similarly, the Arp2/3 complex in Arabidopsis has been revealed to control cell morphogenesis, while less is known about its role(s) in plant immunity (Li et al., 2003).
Stripe rust fungi are obligate biotrophic plant pathogens that would not grow without living hosts and cause damage worldwide (Tang and Chen, 2002). Wheat stripe rust, caused by Puccinia striiformis f. sp. tritici (Pst), occurs world-wide, and is one of the most destructive diseases of wheat in many cool and temperate regions (Wellings et al., 2003). Resistance of wheat cultivars against Pst is usually race specific and mediated by gene-for-gene resistance (Flor, 1971). Resistant cultivars are planted in large areas contribute to the disease control. Thus, it is crucial for understanding the mechanism of wheat against Pst and searching for new ways to control the wheat stripe rust disease. Plants defense fungal invasion through innate immune system called zigzag model (Jones and Dangl, 2006). PAMPs recognized as slowly evolving molecules, including non-pathogens, resulting in PTI. These include bursts of calcium and reactive oxygen species (ROS). Pathogens disrupt PTI by delivering effector molecules into their host during infection. In proper order, plants target these pathogen effectors via R proteins that activate ETI. ETI is pathogen strain- or race-specific and is associated with rapid host cell death, termed the hypersensitive response (HR), and leaded to systemic acquired resistance (SAR) in the host.
In the current study, we describe the characterization of the ARPC3 subunit of the wheat Arp2/3 complex. TaARPC3 was identified as an evolutionarily conserved subunit, and data presented herein demonstrate a role for ARPC3 in regulation of actin cytoskeletal function during plant-pathogen interactions. Complementation of the S. cerevisiae mutant, arc18, with TaARPC3 recovered developmental and actin-associated phenotypes, and in wheat, knock-down of TaARPC3 reduced the resistance to Pst in an actin-dependent manner. Taken together, these results suggest that ARPs also play an important role in host response against fungal pathogens.
Materials and Methods
Plant Materials and Fungal Isolates
Wheat cultivar Suwon11 and Pst races CYR23 and CYR31 were used in this study. The method used for growing and inoculating of wheat seedlings were described by Kang and Li (1984). Suwon11 and CYR23 constituted an incompatible interaction that exhibited a typical HR, while Suwon11 was highly susceptible to CYR31 (Cao et al., 2003). CYR23 was maintained on wheat cv. Mingxian 169, whereas CYR31 maintained on wheat cv. Suwon 11.
To evaluate the expression levels of TaARPC3 in response to Pst infection, wheat leaves were harvested at 0, 6, 12, 18, 24, 36, 48, 72, and 120 hour post-inoculation (hpi) based on previous microscopic observations of the interactions between wheat and Pst (Wang et al., 2007). To analyze the silencing efficiency of virus-induced gene silencing (VIGS) and the relative expression of TaCAT1 and TaSOD, the fourth leaves of TaARPC3-knockdown wheat plants were sampled at 0, 24, 48, and 120 hpi following infection with Pst.
For hormone treatment, the 10-day-old wheat seedlings were sprayed with 500 μM salicylic acid (SA), 100 μM methyl jasmonate, 100 μM ethephon, 100 μM abscisic acid (ABA) and sampled at 0, 2, 6, 12, and 24 hpi. Wheat leaves inoculated with sterile distilled water were used as MOCK-inoculation controls.
Quantitative RT-PCR Analysis (qRT-PCR)
Total RNA was isolated using the Biozol reagent (Invitrogen, Carlsbad, CA, United States). 3 μg of RNA aliquot of each sample was subjected to first strand cDNA synthesis using the oligo (dT)18 primer and Promega Reverse Transcription System (Promega, Madison, WI, United States). The primers (Supplementary Table S3) were designed according to the procedures as described by Livak and Schmittgen (2001) and quantization Real-Time PCR (qRT-PCR) assays were performed on an ABI-7500 system (Applied Biosystems, Foster City, CA, United States). The transcript level of TaARPC3 was quantified using the comparative 2-ΔΔCT method (Livak and Schmittgen, 2001) with the wheat Elongation factor 1 (TaEF-1α) used as control (GenBank accession number Q03033) (Supplementary Table S3). Transcript abundance of each reaction was assessed in triplicate.
Identification and Sequence Analysis of TaARPC3
Arabidopsis thaliana actin related protein complex subunit 3 ARPC3 (Li et al., 2003; Cvrčková et al., 2004) was used to screen (in silico) the EST databases of wheat constructed in our lab (Ma et al., 2009; Wang et al., 2009, 2010). A 1091-bp unisequence (Genbank accession number AK336075.1) exhibiting high homology to the A. thaliana homolog, AtARPC3, was obtained from the cDNA library (Yu et al., 2010). Based on this sequence, we used specific primers TaARPC3-F and TaARPC3-R (Supplementary Table S3) to amplify the sequence of TaARPC3. The cloned open reading frame (ORF) was constructed into pGEM-T Easy (Promega, United States) for sequencing.
The cDNA sequence of TaARPC3 was analyzed in silico using the online BLAST1 and the ORF Finder software2 in NCBI. The amino acid sequence of TaARPC3 was analyzed with Pfam3 and ScanProsite4 for conserved domain identification. Multiple sequence alignments were performed using CLUSTALX2.0 (Chenna et al., 2003) and DNAMAN6.0 (Lynnon BioSoft, United States). A phylogenetic tree based on the neighbor-joining method was performed with the Mega 6 software (Tamura et al., 2007).
Yeast Mutant Complementation Assay
The S. cerevisiae diploid mutant strain Y06714 (MATa; ura3Δ0; leu2Δ0; his3Δ1; met15Δ0; YLR370c::kanMX4) and the wild type strain (WT) BY4741 (MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0) were obtained from EUROSCARF collection. The complete ORF of TaARPC3 was cloned into pDR195. The transformed cell (Δarc18+TaARPC3) was obtained by transforming the reconstructed vector (pDR195-TaARPC3) into the mutant strain Y06714. The transformed cell with empty vector pDR195 (Δarc18+empty) was used as control. Transformants were selected on SC-U medium at 30°C and validated using PCR.
To investigate the yeast cell survival under stress, the transformed cells with pDR195 (Δarc18+empty) and pDR195-TaARPC3 (Δarc18+ TaARPC3) and wild yeast cells (WT BY4741) were cultured on SC medium with 0.1mM H2O2, 2 M Sorbitol, 0.3 M NaCl and 0.1 M CaCl2, respectively. Transformed cells with a starting optical density at 600 nm (OD600) of 0.2 were cultured in yeast medium. The fluorescent dye 4′, 6-diamino-2-phenylindole (DAPI) was added to the cell suspensions at a concentration of 5 μg ml-1 to counter-stain nuclei. The cells were stained with 0.5 μM TRITC-phalloidin to observe F-actin structures (Bereiterhahn and Kajstura, 1988). Cells were incubated at 30°C in phosphate buffer saline (PBS) for 30 min without shaking, followed by washing with sterile distilled water, then resuspended in distilled water. Cells were examined by fluorescence microscopy using an Olympus BX-53 microscope (Olympus, Corp., Tokyo) under blue light excitation by epifluorescence microscopy (excitation filter, 485 nm; dichroic mirror, 510 nm; and barrier filter, 520 nm).
Subcellular Localization of TaARPC3:GFP Fusion Protein in Wheat Protoplasts
The full-length cDNA of TaARPC3 was cloned into the pCaMV35S::GFP vector via XbaI/BamHI digestion and cloning. Protoplast isolation in wheat leaves was performed as previously described (Graham, 2002). The recombinant plasmid pCaMV35S:TaARPC3:GFP, empty vector pCaMV35S:GFP were transformed into wheat protoplasts by PEG4000. The transformed wheat mesophyll protoplasts were incubated in a dark chamber plates at 24°C for 12–36 h. GFP fluorescence was detected with an Olympus FV1000 confocal laser microscope equipped with a 488 nm filter (Olympus, Tokyo, Japan).
BSMV-Mediate Silencing of TaARPC3 in Wheat cv. Suwon 11
For VIGS, a 214-bp fragment of TaARPC3 with NotI and PacI (Supplementary Table S3) was derived from its coding sequence and amplified by RT-PCR to construct the BSMV:γ plasmid for gene silencing (Holzberg et al., 2002). To ensure the specificity for target gene silencing, multi-alignment and BLAST results indicated that the insert sequence of TaARPC3 was located at the non-conservative region and without off target possibility (Supplementary Figure S1 and Table S2). The BSMV RNAs were obtained in vitro from linearized plasmids γ-TaPDS, γ-TaARPC3, γ, α, β (Petty and Jackson, 1990) using the Message T7 transcription kit (Ambion, Austin, TX, United States) according to the manufacturer’s instruction. A 7.5 μl portion of each three transcripts of BSMV viruses (wild type or target gene carrying types) were mixed with 45 μl FES and directly applied by gently sliding the pinched fingers from the base to the tip five times with a gloved finger (Scofield et al., 2005; Bruun-Rasmussen et al., 2007). After inoculation with BSMV at the second leaf stage, plants were placed in the dark chamber for 24 h, and subsequently transferred to a growth chamber at 25 ± 2°C with a 16 h photoperiod and examined for symptoms. In all experiments, the recombinant virus BSMV:TaPDS was used as a positive control, negative control were inoculated with BSMV: γ. When the photo-bleaching phenotype (indicating the PDS gene silence) was observed, the fourth leaves of TaARPC3 silencing group were inoculated with urediospores of Pst race CYR23 and CYR31, respectively. The fourth leaves were collected at 24, 48, and 120 hpi for histological observation and qRT-PCR. The resistance or susceptible phenotypes were visible at 14 dpi.
Fungal Biomass Analyses and Quantification of Disease Severity
Disease severity was assessed by counting the number of uredinia within standardized leaf area sections following infection with Pst. Leaves was collected randomly to avoid bias. The results were exhibited in the mean values of the number of uredinia on knock-down plants compare to the controls. Fungal biomass was analyzed by qRT-PCR as described previously (Livak and Schmittgen, 2001; Cheng et al., 2016). Genomic DNA were performed using the CTAB method (Del et al., 1989) from samples collected at 120 hpi after infected with Pst. Standard curve was generated by the plasmid carried the fragment of PsEF1 and TaEF1 as described (Liu J. et al., 2016; Liu P. et al., 2016) (Supplementary Table S3).
Histological Observation of Host Response and Pathogen Growth
The host response and Pst growth in TaARPC3 know-down plants were observed by light microscopy. Stained leaf segments were fixed and cleared in ethanol/acetic acid (1:1 v/v). Auto-fluorescence of infected mesophyll cells was observed as a necrotic death area with an Olympus BX-51 microscope (Olympus Corp., Tokyo) (excitation filter, 485 nm; dichroic mirror, 510 nm; and barrier filter, 520 nm). Infection sites and lengths of infection hyphae were measured under the blue light excitation. H2O2 accumulation around the infection site was detected by using 3,3-diaminobenzidine (DAB; Amresco, Solon, OH, United States) staining, observed by the differential interference contrast optics. Fifty infection sites were randomly selected for each leaf segment, per treatment. Methods were taken as described previously (Wang et al., 2007), including identification of successful infection sites, hyphae staining and measurement of fungal structures.
Statistical Analyses
Standard errors of deviation were calculated using Microsoft Excel. Statistical significance was assessed by a Student’s t-test (P < 0.05) using SPSS software (SPSS, Inc., Chicago, IL, United States).
Results
TaARPC3 Encodes a Typical Component of Arp2/3 Complex
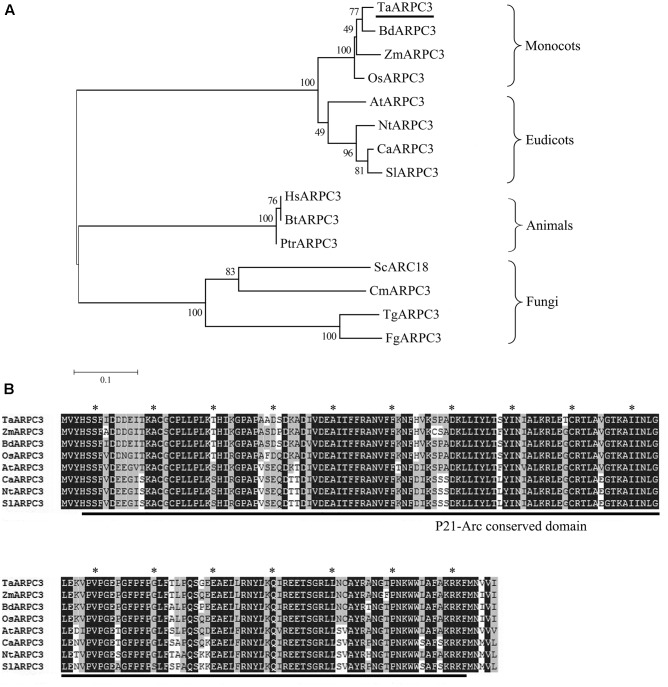
A wheat 526-bp homolog of actin related protein was isolated from wheat cv. Suwon 11 by homology-based cloning and the obtained cDNA was designated as TaARPC3. Blast analysis of TaARPC3 nucleotide sequence in the Triticum aestivum cv. Chinese spring (CS) genome sequence revealed that at least three copies were present, localized on chromosome 7A, 7B, and 7D (Supplementary Figure S1A). The TaARPC3 exhibited 97.84, 92.78, and 97.97% identities with TaARPC3-7A, TaARPC3-7B, TaARPC3-7D, respectively. The predicted ORF of TaARPC3 encodes a protein of 174 amino acid with a hypothetical molecular weight of approximately 21 kDa and an isoelectric point (PI) of 6.71. A phylogenetic analysis of TaARPC3 with BdARPC3, OsARPC3, AtARPC3, ZmARPC3, NtARPC3, CaARPC3, SlARPC3, HsARPC3, BtARPC3, PtrARPC3, MScARPC3, CmARPC3, TgARPC3, and FgARPC3 resulted in that TaARPC3 clusters with ZmARPC3, BdARPC3 and OsARPC3, all of which are members of actin related proteins in monocotyledons (Figure 1A). Nucleic acid sequence analysis revealed that TaARPC3 shared 91% identity with BdARPC3 from Brachypodium distachyon. Multiple amino acid sequences alignment of TaARPC3 with BdARPC3, OsARPC3, AtARPC3, ZmARPC3, NtARPC3, CaARPC3, and SlARPC3 showed that TaARPC3 is predicted to encode proteins with the unique conserved domains of ARP2/3 complex ARPC3 (Figure 1B). These results indicate that TaARPC3 (Genbank accession number KU746328) is a typical member of the Arp2/3 complex in wheat.
FIGURE 1.
(A) Phylogenetic analysis of ARPC3 genes. A phylogenetic analysis of ARPC3 in Triticum aestivum (TaARPC3, KU746328), B. distachyon (BdARPC3, XP_003571891), Oryza sativa (OsARPC3, XP_006647107), Arabidopsis thaliana (AtARPC3, NP_564757), Zea mays (ZmARPC3, NP_001136734), Nicotiana tabacum (NtARPC3, XP_016458972.1), Capsicum annuum (CaARPC3, XP_016554076.1), Solanum lycopersicum (SlARPC3, XP_004242738.1), Human species (HsARPC3, NP_001265485), Bos taurus (BtARPC3, NP_001029443.1), Pan troglodytes (PtrARPC3, NP_001238842.1), Saccharomyces cerevisiae (ScARPC3, NP_013474.3), Candida maltose (CmARPC3, EMG45739.1), Trichoderma guizhouense (TgARPC3, OPB39669.1), and Fusarium graminearum (FgARPC3, KIL85740.1). (B) Multiple amino acid sequences alignment of TaARPC3 with AtARPC3, BdARPC3, OsARPC3, and TuARPC3. Amino acid identity (black boxes) and similarity (gray boxes) are shown within the protein kinase domain and the most highly conserved residues are highlighted with asterisk.
TaARPC3 Can Partially Rescue the arc18 Mutant in Saccharomyces cerevisiae
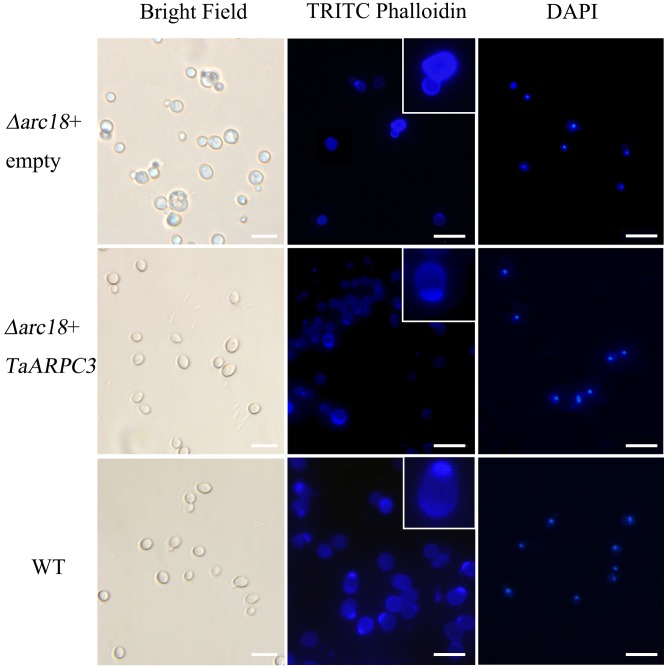
A recent study demonstrated a role for the Arp2/3 complex in the actin-associated control of cell division and polarity in S. cerevisiae (Cabrera et al., 2011). TaARPC3 shared 38% similarity with arc18 (aka ARPC3) from S. cerevisiae (Supplementary Figure S1B). To evaluate the function of TaARPC3, we tested the ability of TaARPC3 to complement the arc18 mutation in yeast. To do this, the TaARPC3 coding sequence into pDR195 vector was transformed into the S. cerevisiae mutant strain Y06714 and heterologously expressed. As shown in Figure 2, S. cerevisiae cells harboring the empty vector pDR195, as well as the wild-type (untransformed) strain, BY4741 (control), showed a similar growth phenotype (i.e., rapid growth on SC medium than the mutant strain). The S. cerevisiae mutant Y06714 (aka, Δarc18) exhibited several morphological defects. As shown, mutant cells vary in size and shape when compared to the wild-type and the complemented strain. Cells were stained with TRITC-phalloidin to assess the subcellular organization of the F-actin, which facilitates observation of F-actin structures. As shown in Figure 2, staining revealed F-actin aggregation in the budding site in the complemented strain, as well as accumulation of actin dots at the septum-forming position. In the mutant strain, actin was observed as concentrated foci at the cell ends, adjacent to the developing septum. No significant difference was observed by fluorescence microscopy of DAPI-stained cells. In total, these data reveal a loss of actin cables and depolarized actin patches, suggesting that TaARPC3 may play a role in maintaining the polarized distribution of actin patches within the cell.
FIGURE 2.
Complementation of TaARPC3 in yeast recovers the loss of actin morphology. Cell morphology observed using brightfield microscopy. Cells were stained with TRITC-phalloidin and DAPI. Enlarged views indicate the accumulation of F-actin. Bar, 10 μm.
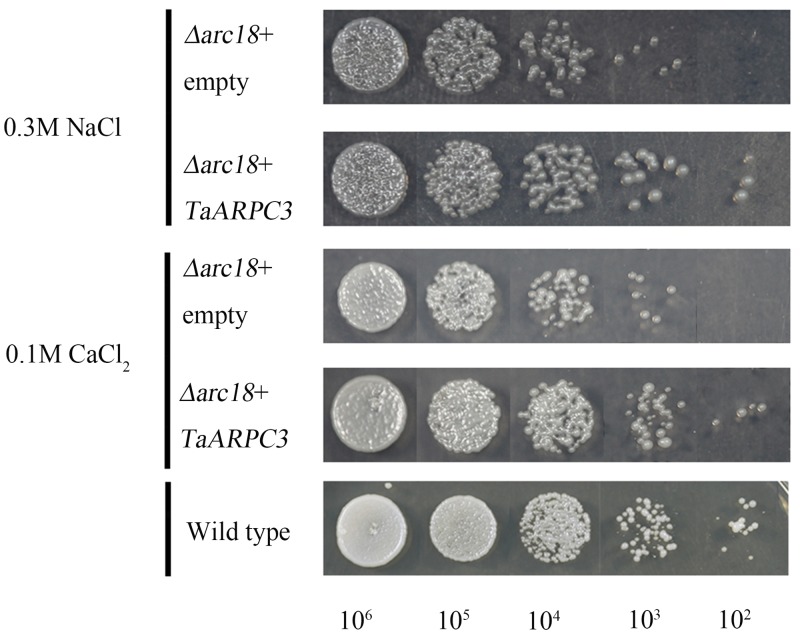
The Arp2/3 complex has been demonstrated to be required for cellular response to various abiotic stresses. To determine if TaARPC3 could complement the S. cerevisiae mutant phenotypes with respect to the perception of cell stress, we tested the function of TaARPC3 in S. cerevisiae under a variety of cellular stresses. As shown in Figure 3, we observed a significant increase in the growth rate of the complemented strain Y06714 compared with the S. cerevisiae arpc3 mutant carrying the empty expression vector pDR195. In SC-U media containing 0.1 mM H2O2 (oxidative stress), 2 M sorbitol (osmotic stress), 0.3 M NaCl (salt stress), and 0.1 M CaCl2, similar results were observed (Figure 3 and Supplementary Figure S2). Yeast cells expressing pDR195 and pDR195-TaARPC3 grew more slowly than WT cells on stress-inducing medium, while the complemented strain containing pDR195-TaARPC3 grew at near wild-type levels (Figure 3 and Supplementary Figure S2), indicating complementation by TaARPC3. Taken together, these results indicate that the TaARPC3 participates in abiotic stress signaling.
FIGURE 3.
Effects of expression of TaARPC3 in yeast cells. Survival of the mutant yeast cells Δarc18 expressing empty pDR195 vector (Δarc18+empty) or pDR195-TaARPC3 (Δarc18+TaARPC3) were spotted on solid medium in combination with different stress treatment. 0.3 M NaCl, solid medium containing 0.3 M NaCl; 0.1 M CaCl2, solid medium containing 0.1 M CaCl2. The final densities were 106, 105, 104, 103, and 102 (cell/ml) following dilution with sterile water.
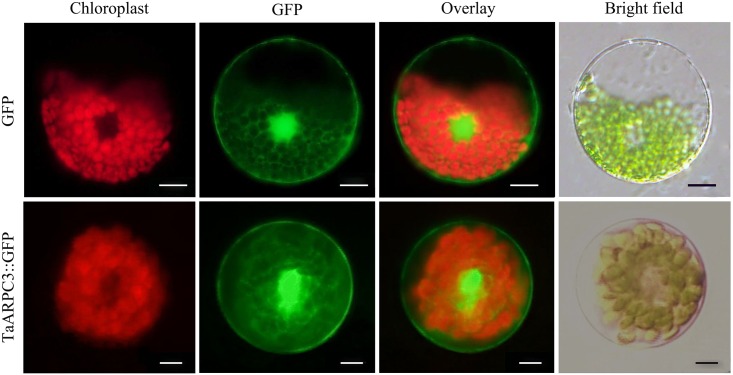
TaARPC3:GFP Fusion Protein Was Found in the Nucleus and Cytoplasm in Wheat Protoplast
To determine the subcellular localization of TaARPC3 in wheat, the green fluorescent protein (GFP) gene was fused with TaARPC3, and then expressed in wheat protoplasts. As showed in Figure 4, empty pCaMV35S:GFP vector, as a control, revealed a diffuse, non-specific cellular address, while cells expressing GFP-tagged TaARPC3 was localized predominantly in the nucleus and cytoplasm.
FIGURE 4.
Subcellular localization of the TaARPC3 protein. Expression of TaARPC3-GFP fusion protein and only GFP (control) in wheat seedlings protoplasts. The green channel shows the localization of GFP and TaARPC3-GFP. Bar, 20 μm.
TaARPC3 Is Differentially Induced by Pst Infection and Hormone Treatment
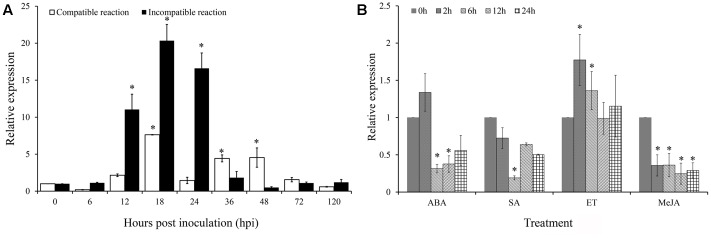
To determine the expression profiles of TaARPC3 mRNA, we next monitored the expression of TaARPC3 during the interaction between wheat and Pst using qRT-PCR. As shown in Figure 5A, during an incompatible interaction, TaARPC3 transcripts were up-regulated as early as 12 hpi, with peak expression at 18 hpi; transcript levels gradually reduced to pre-inoculation levels at 36–120 hpi. During a compatible interaction, TaARPC3 was also induced from the 12 hpi to 48 hpi; however, the transcript levels of TaARPC3 were much higher during an incompatible interaction than that during a compatible interaction at 12–24 hpi. These results support our hypothesis that TaARPC3 is likely to be associated with resistance of wheat against Pst infection.
FIGURE 5.
Quantitative Real-Time PCR expression analyze of TaARPC3 in wheat leaves. (A) The expression level of TaARPC3 in wheat leaves was induced in incompatible reaction between wheat-Pst interaction. (B) The transcription of TaARPC3 in wheat response to ET, ABA, MeJA, and SA treatment. Error bars represent the variations among three independent replicates. The data were normalized to wheat the TaEF-1a gene. Wheat leaves treated with distilled water were included as a control. Asterisks indicate significant difference (P < 0.05) from 0 hour after inoculation using Student’s t-test.
To extend these assays, we next evaluated the expression of TaARPC3 as a function of hormone perception and signaling. To do this, wheat seedlings were treated with ethylene (ET), ABA, SA, and jasmonic acid (JA) to evaluate the possible linkages between immune signaling, susceptibility, and hormone biosynthesis. As shown in Figure 5B, when seedlings were treated with ET, the expression level of TaARPC3 was significantly induced at 12 hpi post-treatment (hpt), with an approximate 2.5-fold change in expression, after which time, transcript levels returned to levels similar to control plants (Figure 5B). After treatment with ABA, TaARPC3 transcript levels were slightly induced at up to 2 hpi, and after 2 hpi, were down-regulated (Figure 5B). Treatment with SA and MeJA was observed to down-regulate TaARPC3 expression.
Silencing of TaARPC3 Results in a Decrease in Resistance against Pst
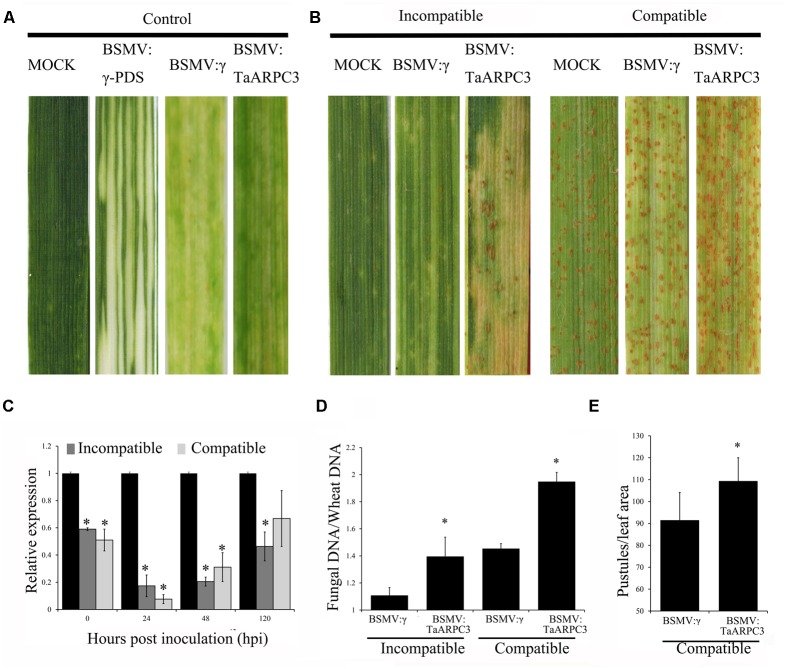
The VIGS system is a rapid and effective reverse-genetic approach to characterize gene function in wheat (Yin et al., 2011). To functional the TaARPC3 in wheat-Pst interaction, we knocked down TaARPC3 using the VIGS and analyzed the impact of this on wheat response to pathogen infection. As exhibited in Figure 6A, 10 days after BSMV inoculation, mild chlorotic mosaic phenotypes were observed on the fourth leaves in BSMV-inoculated plants, with leaves inoculated BSMV:γ-PDS displaying obvious signs of photobleaching (Figure 6A); this indicates effective gene silencing. Next, the fourth leaves of wheat with BSMV-inoculated were inoculated with the avirulent race (CYR23) and virulent race (CYR31) to Suwon 11, respectively (Figure 6B). After 15 days, CYR23 elicited a pronounced HR on leaves inoculated with BSMV:γ, BSMV:TaARPC3-inocuated leaves, with uredia production observed on BSMV:TaARPC3-inocuated leaves (Figure 6B). When leaves were challenged with Pst CYR31, leaves inoculated with BSMV:TaARPC3 show more uredia produced than observed in mock plants and those inoculated with BSMV:γ (Figure 6B). To confirm silencing efficiency, the transcript level of TaARPC3 in TaARPC3 knock-down plants were evaluated by qRT-PCR. We observed that the relative expression at each time point was significantly suppressed as much as 93% (Figure 6C). Next, to quantify the disease severity in the silenced plants, fungal biomass was found to be significantly increased (ca. 1.34-fold increased compared to control infected with CYR23; Figure 6D). The same result was observed after inoculated with CYR31. Quantification of the expansion in uredinia development in leaves was observed to increased 1.19-fold compared with controls treated with BSMV::γ alone (Figure 6E). These results indicate that TaARPC3 is required for defense signaling in wheat during Pst infection.
FIGURE 6.
The instantaneous silencing of TaARPC3 by BSMV-VIGS method. (A) Wheat leaves treated with 1× Fes buffer (MOCK) shown no phenotypic changes. Mild chlorotic mosaic symptoms were detected on the plants inoculated with BSMV:γ, BSMV:PDS or BSMV:TaARPC3. (B) Phenotypes of the fourth leaves infected with urediospores of Pst CYR23 (avirulent) or CYR31 (virulent). Representative leaves were photographed at 15 dpi. (C) Relative expression levels of TaARPC3 in knockdown plants challenged with CYR23 or CYR31 at 0, 24, 48, and 120 dpi by qRT-PCR assay. The relative transcript level of TaARPC3 was calculated by the comparative threshold method (2-ΔΔCT). Error bars represent the standard deviations among three independent replicates. Asterisks indicate significant difference (P < 0.05) in relative expression as compared to mock inoculated samples. (D) Fungal biomass analysis assay in TaARPC3-knowdown plants after inoculation CYR23. Asterisks indicate significant difference (P < 0.05) in relative expression as compared to BSMV:inoculated. (E) Quantification of the uredinial density in the TaARPC3-knowdown plants after inoculation CYR31. Asterisks indicate significant difference (P < 0.05) in relative expression as compared to BSMV:inoculated.
Host Response and Pst Growth in Knock-Down Plants
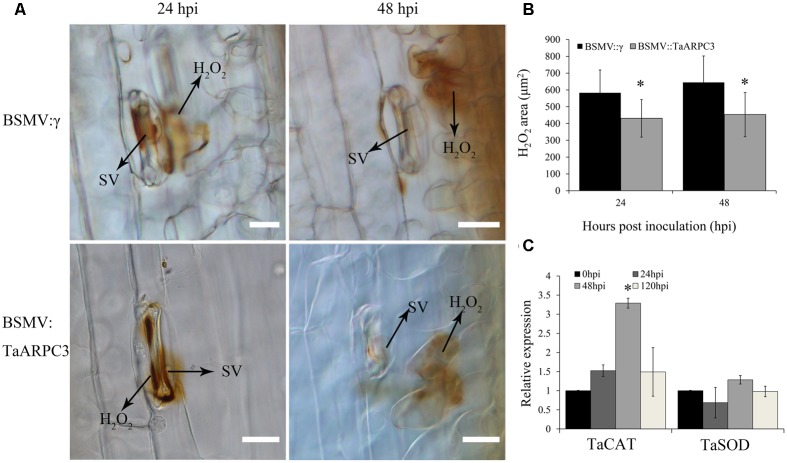
To further understand the how TaARPC3 participates in wheat resistance, H2O2 accumulation was evaluated. As shown in Figure 7, H2O2 production in TaARPC3-silenced wheat showed a significant reduction compared with BSMV:γ-treated plants after CYR23 inoculation (Figures 7A,B). To confirm a decrease in ROS accumulation, we next assayed the expression levels of selected genes involved in the ROS signaling pathway [e.g., catalase (TaCAT) and superoxide dismutase (TaSOD)] in TaARPC3-knockdown plants following infection with Pst. As shown in Figure 7C, we observed an increase in the accumulation of TaCAT in TaARPC3-knockdown plants compared with mock-inoculated or BSMV:γ control plants. Conversely, TaSOD transcripts showed no significant change after TaARPC3 silencing.
FIGURE 7.
H2O2 accumulation observations of know-down plants that inoculated with incompatibility race CYR23. (A) H2O2 accumulation was measured at infection sites by microscopy. HMC, haustorial mother cell; IH, infection hypha; HR, hypersensitive response; SV, sub-stomatal vesicle. Bar, 20 μm. (B) The area of H2O2 staining by 3,3′-diaminobenzidine (DAB) was measured by DP-BSW software in TaARPC3 knockdown plants at 24 and 48 hpi after inoculation. (C) Relative expression of TaCAT and TaSOD in TaARPC3 knockdown plants. Error bars represent the variations among three independent replicates. Asterisks indicate significant difference (P < 0.05) from BSMV:γ using Student’s t-test.
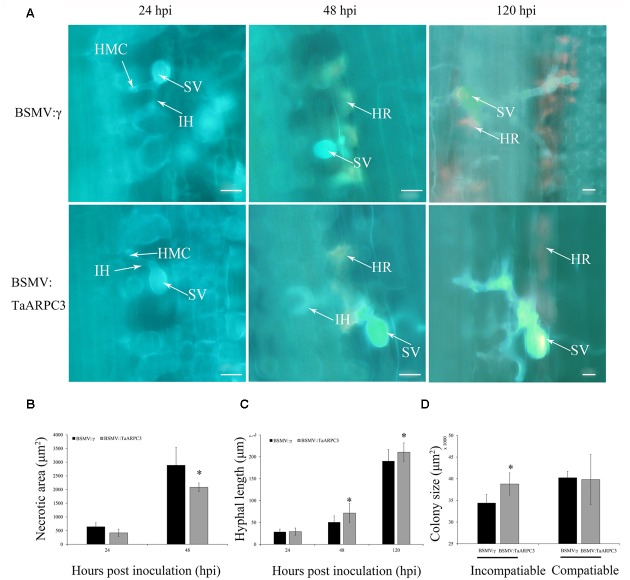
In addition to the induction of H2O2 accumulation, we also observed that pathogen-induced cell death exhibited a similar decrease in the TaARPC3-knowdown plants (Figure 8A), as evaluated by microscopy (Figure 8B). A histological examination of Pst growth was performed in both control and silenced plants by analyzing the length of hyphae and the number of hyphal branches and the formation of haustorial mother cells at infection sites (Supplementary Table S1). In short, we found that the hyphal branch between the BSMV:TaARPC3 and BSMV:γ plants at 24 hpi showed no significant change after inoculation with both CYR23 and CYR 31 (Supplementary Table S1), while the hyphal length was longer in TaARPC3 knock-down plants (Figure 8C and Supplementary Table S1). In addition, the colony area per infection site in the incompatible interaction was obviously larger in TaARPC3 silenced plants compare with the control group at 120 hpi in incompatible interaction (Figure 8D and Supplementary Table S1). In total, these results indicated that a reduction in TaARPC3 expression compromises resistance signaling in response to Pst infection.
FIGURE 8.
Histological observations of necrotic cell death in wheat leaves treated with BSMV and infected with incompatibility race CYR23. (A) Necrotic cell death was observed by epifluorescence at 24, 48, and 120 hpi. HMC, haustorial mother cell; IH, infection hypha; HR, hypersensitive response; SV, sub-stomatal vesicle. Bar, 20 μm. (B) Necrotic cell death was quantified as the area of auto-fluorescence. (C) Hyphal lengths were quantified from the junction of the sub-stomatal vesicle to the hyphal tip. (D) Colony size was measured as the area of auto-fluorescence. Asterisks indicate a significant differences (P < 0.05) from BSMV:γ using the Student’s t-test.
Discussion
In the current study, we isolated the wheat TaARPC3 gene, with duplication across the chromosomes 7A, 7B, and 7D, which has a similar conserved P21-Arc domain to that found in numerous other ARPC3 proteins from a wide variety of monocotyledonous plants. As a function of plant defense signaling, our data extend our current understanding in this area and provide a foundation from which to extend studies both in wheat, as well as in numerous other plants, to define the role of this complex during immune signaling and the control of actin cytoskeletal organization during host infection by pathogens.
Plant cells responding to fungal attack undergo numerous changes within a multitude of cellular processes. Among these, rearrangements of the cytoskeleton have been demonstrated to play a major role in the progress of fungal penetration and host resistance. For example, the actin cytoskeleton has been demonstrated to act as a key downstream effector of external and internal stimuli during a wide range of biological phenomena in plants (Vantard and Blanchoin, 2002). Indeed, it is widely accepted that Arp2/3 functions as a regulator of actin filament dynamics by polymerizing the branched F-actin and promoting actin nucleation of actin filament (Higgs and Pollard, 2001; May, 2001; Welch and Mullins, 2002). However, its role in fungal defense is less defined. In this study, we analyzed the function of TaARPC3 in wheat against Pst. Numerous studies have shown that the actin cytoskeleton is associated with plant disease resistance (Tian et al., 2009; Porter et al., 2012; Fu et al., 2014; Tang et al., 2014; Zhang et al., 2016). As highlighted herein, our current work demonstrates that TaARPC3 is required for resistance signaling in wheat following Pst infection. As a biotrophic parasite, Pst is depending on haustoria to absorb nutrition from the host wheat (Kang et al., 2002). The substomatal vesicle within the stomatal cavity as a symbol of an infection site was formed at 6–8 hpi, the primary infection produce hypha, haustorial mother cell, and haustorium initial at 12–18 hpi. From 24 to 144 hpi, hyphae branch and haustoria formed greatly and developed into mycelium. Indeed, and as a function of the specific regulation of resistance at distinct stages of infection, we observed that the expression of TaARPC3 in an incompatible interaction was induced during the initial stages of infection; while in the compatible interaction, we observed only a slight delay in this response. At present, we do not know whether TaARPC3 patriciates in either ETI, PTI, or both layers of immunity. Moreover, the function of this complex, both as a regulator of cortical actin function and as a downstream regulator (e.g., nuclear) of cytoskeletal function remains to be fully defined.
TaARPC3 was found predominantly localized within the nucleus and cytoplasm in wheat protoplasts. In the budding yeast, arc18 is recruited to the mitochondria and the arc18 deletion mutant shows impaired mitochondrial transport (Fehrenbacher et al., 2005). While in most of higher plants, ARP2/3 coats the surface of biochemically and morphologically distinct organelles, in Arabidopsis, AtARP3 subunit is present in nuclei, the mitochondria, and chloroplasts (Zhang et al., 2013). Within these subcellular sites, AtARPC4 was found to be associated with microsomes and the nucleus (Kotchoni et al., 2009; Zhang et al., 2013), while AtARPC2 was found bound directly to micro-tubulins, and interacted with both actin filaments and microtubules (Havelková et al., 2015). To explain these differences, we hypothesize that one function of the Arp2/3 complex may be to regulate organelle positioning and the patterns of organelle transport as a distinct function of each of the different subunits. As a function of the work presented herein, we posit that TaARPC3 may participate in resistance signaling in wheat through regulated changes in nuclear actin and/or organelle positioning.
In addition to nuclear- and organizational-specific changes in the cell, numerous studies have demonstrated a link between Arp2/3, actin, and second messenger perception pathways, including those triggered by hormones, Ca2+, and cAMP (Eun et al., 2001; Hepler et al., 2001; Moutinho et al., 2001; Friml et al., 2002). Herein, we observed an induction of TaARPC3 upon exogenous ET application, and conversely, a reduction in TaARPC3 expression following MeJA treatment, suggesting that TaARPC3 may be an effector associated with ET and JA signaling pathways. Taken together with our observation of an enhancement in yeast cell response to environmental stress stimuli and the induced expression pattern of TaARPC3 under avirulent Pst, it is reasonable to hypothesize that the Arp2/3 complex functions at the nexus of biotic and abiotic stress pathways. Coupled with our demonstration of its role in actin cytoskeletal organization, our data support this proposed role for Arp2/3, linking cell stress, signaling, and the organization of the actin cytoskeleton.
The abiotic and biotic stresses that induce an oxidative burst response has been shown to likely function as a protective mechanism during pathogen infection and environmental stress (Apel and Hirt, 2004; Bhattacharjee, 2005). In plants, the ROS burst, one of the earliest signal events, happens during the early stages of plant–pathogen interactions (Garcia-Brugger et al., 2006). As reported by Wang et al. (2007), the ROS bursts were detected at 12–24 hpi, in the wheat-Pst incompatible interaction. As demonstrated herein, the time point that TaARPC3 was highly induced correlated with the induction of the ROS burst, a processes that is associated with both abiotic and biotic stress signaling. Further, in TaARPC3-silenced plants, the expression levels of TaCAT were also induced. Numerous reports have revealed that changes in cytoskeletal organization can lead to reactive oxygen bursts and subsequent cell death (Gourlay and Ayscough, 2005). For example, in yeast, a reduction in actin dynamics was shown to lead to a loss of mitochondrial membrane potential and elevated ROS levels (Gourlay et al., 2004). Similarly, overexpression of the phosphodiesterase PDE2 rescues actin dynamics was shown to reduce oxidative stress sensitivity (Gourlay and Ayscough, 2006). As a possible mechanism for this response, cells bearing mutations in ARP2 or arc15 genes show decreased velocities of mitochondrial movement and a concomitant loss of all directed movement in mitochondrial morphology (Boldogh et al., 2001). Thus, actin cytoskeletal organization likely functions as a physiological regulator of ROS release from mitochondria and as a key element in the upstream activation of cell death pathways (Breitenbach et al., 2006).
In summary, our study demonstrates that TaARPC3 likely participates in the regulation of actin cytoskeleton, and moreover, plays a key role in the host response to pathogen infection. In support of this hypothesis, our data show that TaARPC3 was able to contribute to the stable of cytoskeleton to enhance wheat resistance via the management of ROS accumulation and cell death during the incompatible interaction of wheat and Pst.
Author Contributions
JG and QM designed the experiment. TQ, JW, and QS performed the experiments and analyzed the data. TQ, JW, JG, BD, and QM wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the reviewers for helpful comments and valuable suggestions during the revision of the early version of the manuscript.
Funding. This work was supported by the National Natural Science Foundation of China (Grant nos. 31272024, 31571960 and 31371889), the 111 Project from the Ministry of Education of China (B07049) and the Natural Science Foundation of Shaanxi Province (2017JM3007).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01245/full#supplementary-material
(A) Aliment and VIGS site of TaARPC3 of three copies of TaARPC3 located on chromosomes 7A, 7B, and 7D. (B) Aliment of TaARPC3 with ARC18 in yeast.
Effects of expression of TaARPC3 in yeast cells. 0.1 mM H2O2, solid medium containing 0.1 mM H2O2; 2 M Sorbitol, solid medium containing 2 M Sorbitol.
References
- Apel K., Hirt H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Ann. Rev. Plant Biol. 55 373–399. 10.1146/annurev.arplant.55.031903.141701 [DOI] [PubMed] [Google Scholar]
- Bereiterhahn J., Kajstura J. (1988). Scanning microfluorometric measurement of TRITC-phalloidin labelled F-actin. Dependence of F-actin content on density of normal and transformed cells. Histochemistry 90 271–276. 10.1007/BF00495970 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee S. (2005). Reactive oxygen species and oxidative burst: roles in stress, senescence and signal. Curr. Sci. 89 1113–1121. 10.1080/15216540252774694 [DOI] [Google Scholar]
- Boldogh I. R., Yang H. C., Nowakowski W. D., Karmon S. L., Hays L. G., Yates J. R., et al. (2001). Arp2/3 complex and actin dynamics are required for actin-based mitochondrial motility in yeast. Proc. Natl. Acad. Sci. U.S.A. 98 3162–3167. 10.1073/pnas.051494698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenbach M., Laun P., Gimona M. (2006). The actin cytoskeleton, RAS-cAMP signaling and mitochondrial ROS in yeast apoptosis. Trends Cell Biol. 15 637–639. 10.1016/j.tcb.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Bruun-Rasmussen M., Madsen C. T., Jessing S., Albrechtsen M. (2007). Stability of barley stripe mosaic virus-induced gene silencing in barley. Mol. Plant Microbe Interact. 20 1323–1331. 10.1094/MPMI-20-11-1323 [DOI] [PubMed] [Google Scholar]
- Cabrera R., Suo J., Young E., Chang E. (2011). Schizosaccharomyces pombe Arc3 is a conserved subunit of the Arp2/3 complex required for polarity, actin organization, and endocytosis. Yeast 28 495–503. 10.1093/nar/gkg500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone K. G., Welch M. D. (2010). A nucleator arms race: cellular control of actin assembly. Nat. Rev. Mol. Cell Biol. 11 237–251. 10.1038/nrm2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Jin S., Jin M., Jia Q., Li J. (2003). Identification of wheat varieties (lines) for resistance to stripe rust in 1994-2002. J. Plant Gen. Res. 2 6. [Google Scholar]
- Cheng Y., Wu K., Yao J., Li S., Wang X., Huang L., et al. (2016). PSTha5a23, a candidate effector from the obligate biotrophic pathogen Puccinia striiformis f. sp. tritici, is involved in plant defense suppression and rust pathogenicity. Environ. Microbiol. 19 1717–1729. 10.1111/1462-2920.13610 [DOI] [PubMed] [Google Scholar]
- Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., et al. (2003). Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 31 3497–3500. 10.1093/nar/gkg500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesarone M. A., Goode B. L. (2009). Actin nucleation and elongation factors: mechanisms and interplay. Curr. Opin. Cell Biol. 21 28–37. 10.1016/j.ceb.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrčková F., Rivero F., Bavlnka B. (2004). Evolutionarily conserved modules in actin nucleation: lessons from Dictyostelium discoideum and plants. Protoplasma 224 15–31. 10.1007/s00709-004-0058-2 [DOI] [PubMed] [Google Scholar]
- Del S. G., Manfioletti G., Schneider C. (1989). The CTAB-DNA precipitation method: a common mini-scale preparation of template DNA from phagemids, phages or plasmids suitable for sequencing. Biotechniques 7 514–520. [PubMed] [Google Scholar]
- Eun S., Bae S., Lee Y. (2001). Cortical actin filaments in guard cells respond differently to abscisic acid in wild-type and abi1-1 mutant Arabidopsis. Planta 212 466–469. 10.1007/s004250000489 [DOI] [PubMed] [Google Scholar]
- Fehrenbacher K. L., Boldogh I. R., Pon L. A. (2005). A role for Jsn1p in recruiting the Arp2/3 complex to mitochondria in budding yeast. Mol. Biol. Cell 16 5094–5102. 10.1091/mbc.E05-06-0590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flor H. H. (1971). Current status of the gene-for-gene concept. Ann. Rev. Phytopathol. 9 275–296. 10.1007/s004250000489 [DOI] [Google Scholar]
- Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415 806–809. 10.1038/415806 [DOI] [PubMed] [Google Scholar]
- Fu Y., Duan X., Tang C., Li X., Voegele R. T., Wang X., et al. (2014). TaADF7, an actin-depolymerizing factor, contributes to wheat resistance against Puccinia striiformis f. sp. tritici. Plant J. 78 16–30. 10.1111/tpj.12457 [DOI] [PubMed] [Google Scholar]
- Garcia-Brugger A., Lamotte O., Vandelle E., Bourque S., Lecourieux D., Poinssot B., et al. (2006). Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe Interact. 19 711–724. 10.1094/MPMI-19-0711 [DOI] [PubMed] [Google Scholar]
- Gourlay C. W., Ayscough K. R. (2005). The actin cytoskeleton: a key regulator of apoptosis and ageing? Nat. Rev. Mol. Cell Biol. 6 583–589. 10.1083/jcb.200310148 [DOI] [PubMed] [Google Scholar]
- Gourlay C. W., Ayscough K. R. (2006). Actin-induced hyper-activation of the Ras signaling pathway leads to apoptosis in Saccharomyces cerevisiae. Mol. Cell Biol. 26 6487–6501. 10.1128/MCB.00117-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourlay C. W., Carpp L. N., Timpson P., Winder S. J., Ayscough K. R. (2004). A role for the actin cytoskeleton in cell death and aging in yeast. J. Cell Biol. 164 803–809. 10.1083/jcb.200310148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. (2002). Purification of intact plant protoplasts by flotation at 1g. Sci. World J. 2 1397–1399. 10.1100/tsw.2002.835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardham A. R., Jones D. A., Takemoto D. (2007). Cytoskeleton and cell wall function in penetration resistance. Curr. Opin. Plant Biol. 10 342–348. 10.1016/j.pbi.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Hardham A. R., Takemoto D., White R. G. (2008). Rapid and dynamic subcellular reorganization following mechanical stimulation of Arabidopsis epidermal cells mimics responses to fungal and oomycete attack. BMC Plant Biol. 8:63 10.1186/1471-2229-8-63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havelková L., Nanda G., Martinek J., Bellinvia E., Sikorová L., Šlajcherová K., et al. (2015). Arp2/3 complex subunit ARPC2 binds to microtubules. Plant Sci. 241 96–108. 10.1016/j.plantsci.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Henty-Ridilla J. L., Shimono M., Li J., Chang J., Day B., Staiger C. J. (2013). The plant actin cytoskeleton responds to signals from microbe-associated molecular patterns. PLoS Pathog. 9:e1003290 10.1371/journal.ppat.1003290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepler P. K., Vidali L., Cheung A. Y. (2001). Polarized cell growth in higher plants. Annu. Rev. Cell Dev. Biol. 17 159–187. 10.1146/annurev.cellbio.17.1.159 [DOI] [PubMed] [Google Scholar]
- Higgs H. N., Pollard T. D. (2001). Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins 1. Annu. Rev. Biochem. 70 649–676. 10.1371/journal.pone.0021895 [DOI] [PubMed] [Google Scholar]
- Holzberg S., Brosio P., Gross C., Pogue G. P. (2002). Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 30 315–327. 10.1046/j.1365-313X.2002.01291 [DOI] [PubMed] [Google Scholar]
- Hussey P. J., Ketelaar T., Deeks M. J. (2006). Control of the actin cytoskeleton in plant cell growth. Annu. Rev. Plant Biol. 57 109–125. 10.1146/annurev.biochem.70.1.649 [DOI] [PubMed] [Google Scholar]
- Jones J. D., Dangl J. L. (2006). The plant immune system. Nature 444 323–329. 10.1038/nature05286 [DOI] [PubMed] [Google Scholar]
- Kang Z., Huang L., Buchenauer H. (2002). Ultrastructural changes and localization of lignin and callose in compatible and incompatible interactions between wheat and Puccinia striiformis. J. Plant Dis. Prot. 109 25–37. [Google Scholar]
- Kang Z., Li Z. (1984). Discovery of a normal T. type new pathogenic strain to Lovrin10. Acta Clle. Septent. Occident. Agric. 4 18–28. [Google Scholar]
- Kim H., Park M., Kim S. J., Hwang I. (2005). Actin filaments play a critical role in vacuolar trafficking at the Golgi complex in plant cells. Plant Cell 17 888–902. 10.1105/tpc.104.028829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi I., Kobayashi Y., Hardham A. R. (1994). Dynamic reorganization of microtubules and microfilaments in flax cells during the resistance response to flax rust infection. Planta 195 237–247. 10.1007/BF00199684 [DOI] [Google Scholar]
- Kotchoni S. O., Zakharova T., Mallery E. L., Le J., El-Assal S. E., Szymanski D. B. (2009). The association of the Arabidopsis actin-related protein2/3 complex with cell membranes is linked to its assembly status but not its activation. Plant Physiol. 151 2095–2109. 10.1104/pp.109.143859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Blanchoin L., Yang Z., Lord E. M. (2003). The putative Arabidopsis Arp2/3 complex controls leaf cell morphogenesis. Plant Physiol. 132 2034–2044. 10.1104/pp.103.028563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Li J. H., Wang W., Chen N. Z., Ma T. S., Xi Y. N., et al. (2014). ARP2/3 complex-mediated actin dynamics is required for hydrogen peroxide-induced stomatal closure in Arabidopsis. Plant Cell Environ. 37 1548–1560. 10.1111/pce.12259 [DOI] [PubMed] [Google Scholar]
- Liu J., Guan T., Zheng P., Chen L., Yang Y., Huai B., et al. (2016). An extracellular Zn-only superoxide dismutase from Puccinia striiformis confers enhanced resistance to host-derived oxidative stress. Environ. Microbiol. 18 4118–4135. 10.1111/1462-2920.13451 [DOI] [PubMed] [Google Scholar]
- Liu P., Myo T., Ma W., Lan D., Qi T., Guo J., et al. (2016). TaTypA, a ribosome-binding GTPase protein, positively regulates wheat resistance to the stripe rust fungus. Front. Plant Sci. 7:873 10.3389/fpls.2016.00873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25 402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Ma J., Huang X., Wang X., Chen X., Qu Z., Huang L., et al. (2009). Identification of expressed genes during compatible interaction between stripe rust (Puccinia striiformis) and wheat using a cDNA library. BMC Genomics 10:586 10.1186/1471-2164-10-586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Atkinson S. J., Ampe C., Vandekerckhove J., Pollard T. D. (1994). Purification of a cortical complex containing two unconventional actins from Acanthamoeba by affinity chromatography on profilin-agarose. J. Cell Biol. 127 107–116. 10.1083/jcb.127.1.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky L. M., Gould K. L. (1999). The Arp2/3 complex: a multifunctional actin organizer. Curr. Opin. Cell Biol. 11 117–121. 10.1016/S0955-0674(99)80014-3 [DOI] [PubMed] [Google Scholar]
- May R. C. (2001). The Arp2/3 complex: a central regulator of the actin cytoskeleton. Cell. Mol. Life Sci. 58 1607–1626. 10.1007/PL00000800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutinho A., Hussey P. J., Trewavas A. J., Malho R. (2001). cAMP acts as a second messenger in pollen tube growth and reorientation. Proc. Natl. Acad. Sci. U.S.A. 98 10481–10486. 10.1073/pnas.171104598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R. D., Heuser J. A., Pollard T. D. (1998). The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. U.S.A. 95 6181–6186. 10.1073/pnas.95.11.6181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty I., Jackson A. O. (1990). Mutational analysis of barley stripe mosaic virus RNA β. Virology 179 712–718. 10.1016/0042-6822(90)90138-H [DOI] [PubMed] [Google Scholar]
- Porter K., Shimono M., Tian M., Day B. (2012). Arabidopsis actin-depolymerizing Factor-4 links pathogen perception, defense activation and transcription to cytoskeletal dynamics. PLoS Pathog. 8:e1003006 10.1371/journal.ppat.1003006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield S. R., Huang L., Brandt A. S., Gill B. S. (2005). Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the Lr21-mediated leaf rust resistance pathway. Plant Physiol. 138 2165–2173. 10.1104/pp.105.061861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada C., Lipka V., O’Connell R., Okuno T., Schulze-Lefert P., Takano Y. (2006). Nonhost resistance in Arabidopsis-Colletotrichum interactions acts at the cell periphery and requires actin filament function. Mol. Plant Microbe Interact. 19 270–279. 10.1094/MPMI-19-0270 [DOI] [PubMed] [Google Scholar]
- Sparkes I., Runions J., Hawes C., Griffing L. (2009). Movement and remodeling of the endoplasmic reticulum in non-dividing cells of tobacco leaves. Plant Cell 21 3937–3949. 10.1105/tpc.109.072249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. (2007). MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24 1596–1599. 10.1093/molbev/msm092 [DOI] [PubMed] [Google Scholar]
- Tang C., Chen V. (2002). Nanofiltration of textile wastewater for water reuse. Desalination 143 11–20. 10.1016/S0011-9164(02)00216-3 [DOI] [Google Scholar]
- Tang C., Deng L., Chang D., Chen S., Wang X., Kang Z. (2014). TaADF3, an actin-depolymerizing factor, negatively modulates wheat resistance against Puccinia striiformis. Front. Plant Sci. 6:1214 10.3389/fpls.2015.01214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian M., Chaudhry F., Ruzicka D. R., Meagher R. B., Staiger C. J., Day B. (2009). Arabidopsis actin-depolymerizing factor AtADF4 mediates defense signal transduction triggered by the Pseudomonas syringae effector AvrPphB. Plant Physiol. 150 815–824. 10.1104/pp.109.137604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantard M., Blanchoin L. (2002). Actin polymerization processes in plant cells. Curr. Opin. Plant Biol. 5 502–506. 10.1016/S1369-5266(02)00300-X [DOI] [PubMed] [Google Scholar]
- Wang C., Huang L., Buchenauer H., Han Q., Zhang H., Kang Z. (2007). Histochemical studies on the accumulation of reactive oxygen species (O2- and H2O2) in the incompatible and compatible interaction of wheat-Puccinia striiformis f. sp. tritici. Physiol. Mol. Plant Pathol. 71 230–239. 10.1016/j.pmpp.2008.02.006 [DOI] [Google Scholar]
- Wang X., Liu W., Chen X., Tang C., Dong Y., Ma J., et al. (2010). Differential gene expression in incompatible interaction between wheat and stripe rust fungus revealed by cDNA-AFLP and comparison to compatible interaction. BMC Plant Biol. 10:9 10.1186/1471-2229-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tang C., Zhang G., Li Y., Wang C., Liu B., et al. (2009). cDNA-AFLP analysis reveals differential gene expression in compatible interaction of wheat challenged with Puccinia striiformis f. sp. tritici. BMC Genomics 10:289 10.1186/1471-2229-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M. D., Mullins R. D. (2002). Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 18 247–288. 10.1146/annurev.cellbio.18.040202.112133 [DOI] [PubMed] [Google Scholar]
- Wellings C. R., Wright D. G., Keiper F., Loughman R. (2003). First detection of wheat stripe rust in Western Australia: evidence for a foreign incursion. Australas. Plant Pathol. 32 321–322. 10.1071/AP03023 [DOI] [Google Scholar]
- Winter D., Lechler T., Li R. (1999). Activation of the yeast Arp2/3 complex by Bee1p, a WASP-family protein. Curr. Biol. 9 501–505. 10.1016/S0960-9822(99)80218-8 [DOI] [PubMed] [Google Scholar]
- Yin C., Jurgenson J. E., Hulbert S. H. (2011). Development of a host-induced RNAi system in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Mol. Plant Microbe Interact. 24 554–561. 10.1094/MPMI-10-10-0229 [DOI] [PubMed] [Google Scholar]
- Yokota K., Fukai E., Madsen L. H., Jurkiewicz A., Rueda P., Radutoiu S., et al. (2009). Rearrangement of actin cytoskeleton mediates invasion of Lotus japonicus roots by Mesorhizobium loti. Plant Cell 21 267–284. 10.1105/tpc.108.063693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Wang X., Wang C., Chen X., Qu Z., Yu X., et al. (2010). Wheat defense genes in fungal (Puccinia striiformis) infection. Funct. Integr. Genomics 10 227–239. 10.1007/s10142-010-0161-8 [DOI] [PubMed] [Google Scholar]
- Zhang B., Hua Y., Wang J., Huo Y., Shimono M., Day B., et al. (2016). TaADF4, an actin-depolymerizing factor from wheat, is required for resistance to the stripe rust pathogen Puccinia striiformis f. sp. tritici. Plant J. 89 1210–1214. 10.1111/tpj.13459 [DOI] [PubMed] [Google Scholar]
- Zhang C., Mallery E. L., Szymanski D. (2013). ARP2/3 localization in Arabidopsis leaf pavement cells: a diversity of intracellular pools and cytoskeletal interactions. Front. Plant Sci. 4:238 10.3389/fpls.2013.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Aliment and VIGS site of TaARPC3 of three copies of TaARPC3 located on chromosomes 7A, 7B, and 7D. (B) Aliment of TaARPC3 with ARC18 in yeast.
Effects of expression of TaARPC3 in yeast cells. 0.1 mM H2O2, solid medium containing 0.1 mM H2O2; 2 M Sorbitol, solid medium containing 2 M Sorbitol.