Abstract
Objective
Our objective was to report safety and efficacy of stereotactic radiosurgery (SRS) to the surgical bed following resection of brain metastases.
Methods
Eighty-seven consecutive patients who underwent cavity-directed SRS to the operative bed for the treatment of brain metastases between 2002 and 2010 were evaluated. SRS required a gadolinium-enhanced, high-resolution, T1-weighted magnetic resonance imaging for tumor targeting and delivered a median dose of 18 Gy (14-22 Gy) prescribed to encompass the entire resection cavity. Whole brain irradiation was reserved for salvage. Patients were followed every 3 months with clinical examination and magnetic resonance imaging. Overall survival, local and regional recurrence, and factors affecting these outcomes were evaluated using Kaplan-Meier and log-rank analyses.
Results
The median imaging follow-up was 7.1 months, with >40% of patients having imaging for ≥1 year. Local control at 1 and 2 years was 82% and 75%, respectively. Cavity recurrence was more common with a tumor diameter >3 cm (P < .020) or resection cavity volume >14 mL (P < .050). One-year local control for tumors <2 cm, 2 cm to 3 cm, and >3 cm were 100%, 86%, and 72%, respectively. Neither subtotal resection nor target margins >2 mm to 3 mm affected local control. The median overall survival was 14.3 months with actuarial 5-year survival of 20%. Actuarial regional central nervous system recurrence was 44% at 1 year. On univariate analysis, only the presence of extracranial disease was associated with survival (P < .001) and central nervous system failure (P < .030).
Conclusions
Excellent local control is achievable with cavity-directed SRS in well-selected patients, particularly for lesions with diameter <3 cm and resection cavity volumes <14 mL. Long-term survival is possible for select patients.
Summary.
This is a retrospective evaluation of patients treated at a single institution with stereotactic radiosurgery following surgical resection of brain metastases over a 10-year period. These were large symptomatic tumors, many incompletely resected, and represent patients requiring surgical intervention for symptom palliation. We demonstrate excellent cavity control and show recurrence is directly related to initial tumor size and resection cavity volume, but not extent of resection. We recommend cavity-directed radiosurgery in appropriate patients.
Introduction
Forty percent of all cancers eventually metastasize to the brain, with patients ultimately developing neurologic symptoms that negatively impact their quality of life.1 In up to one-third of patients, neurologic death can occur. There is a limited, if any, role for systemic treatment to address central nervous system (CNS) disease at present. The standard management has historically consisted of either whole brain radiation therapy (WBRT) or surgery followed by adjuvant WBRT depending on the clinical scenario.2 Stereotactic radiosurgery (SRS) evolved as an alternative to surgical resection for the local treatment of brain metastases. SRS delivers an accurate and highly conformal ablative dose of radiation to a target while sparing the adjacent normal tissue.3, 4 Studies demonstrating its efficacy reported local control rates between 70% and 90%, exceeding that of surgery alone.3 WBRT has been associated with neurocognitive sequelae not commonly observed following SRS.5 The role of SRS continues to expand and currently is a well-established therapeutic option as definitive therapy or as salvage treatment when WBRT has been exhausted.6, 7 An ongoing phase III multicenter trial is currently evaluating cavity-directed SRS directly comparing outcomes with WBRT following surgical resection.8 Here we report our single-institution outcomes using this approach and discuss its merits.
Materials and Method
A retrospective chart review of patients who developed brain metastases from any primary malignancy and had undergone surgery followed by SRS to the operative bed from 2002 to 2010 at Rhode Island Hospital was performed in compliance with Institutional Review Board approval (#224940). In total, 87 consecutive patients with a Karnofsky Performance Score >70 were identified. Patient characteristics including age, sex, histology, number of brain metastases, extent of extracranial disease, tumor size, resection cavity volume, duration from surgery to SRS, and SRS dose were obtained.
SRS treatment
On the day of SRS, a stereotactic head frame was positioned by the treating neurosurgeon. A planning magnetic resonance imaging (MRI) scan consisting of a gadolinium-enhanced (0.1 mmol/kg), high-resolution T1-weighted with 1- to 2-mm image slice reconstruction was obtained. The imaging was reviewed by the treating radiation oncologist, neurosurgeon, neuroradiologist, and medical physicist. The target was defined as the surgical cavity in addition to any residual gadolinium enhancement with a 1- to 2-mm margin (Fig 1). Planning target volume margin was dependent on physician assessment and the sum of the setup error in addition to uncertainty of defining the cavity border. The target contour did not extend along the surgical tract when deep-seated lesions were resected. SRS was delivered with a median dose of 18 Gy (range, 14-22 Gy) prescribed to the 40% to 60% isodose line using a Leksell Model C (Elekta, Inc., Stockholm, Sweden). Lesser doses were prescribed for larger target volumes or when the target was located near sensitive normal structures in order to maintain dose to organs at risk below accepted tissue tolerance. If present, additional intact metastases were treated to the gadolinium enhanced lesion and a 1-mm margin with prescription doses dependent on size as described in the Radiation Therapy Oncology Group 90-05 study.9
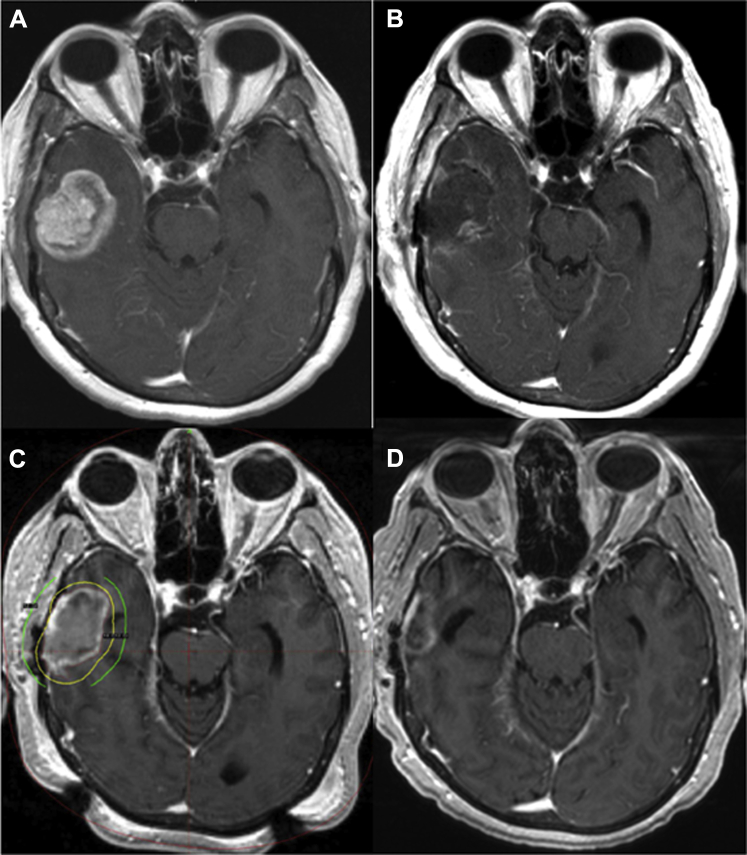
Figure 1.
Cavity-directed stereotactic radiosurgery (SRS) treatment. Illustrated are diagnostic magnetic resonance imaging (MRI) scans of an intact brain metastasis (A) and the cavity 24 hours following resection (B). The MRI performed on the day of SRS planning with 18 Gy (yellow: prescription) and 12 Gy isodose lines (green) is shown (C), as is the 6 month post-SRS scan (D).
Local recurrence
Patients were followed clinically and with high resolution MRI imaging every 3 months for the first year, and 6 months from that time unless symptomatic changes developed. If symptoms occurred, earlier imaging was obtained. When increasing enhancement within or near (<1 cm) the surgical bed was identified, a local recurrence was considered; however, changes consistent with tumor recurrence by magnetic resonance spectroscopy, magnetic resonance perfusion, or, when performed, surgical resection, were necessary to confirm the event as a local treatment failure. Tumor and treatment variables were assessed for influence on local control. To judge the impact of treatment margin on local recurrence, conformality index, and approximated target margins were calculated for each patient and the effect on local recurrence evaluated. Target margin approximation was performed by comparing the difference in spherical radii of the resection cavity volume and the volume encompassed by the prescription dose.
Regional recurrence, survival, and necrosis
When a new area of enhancement, not present on the treatment planning MRI, was identified on follow-up scans, it was classified as a regional recurrence. Survival was measured as the time from SRS until the time of death or the last documented follow-up, at which point patients were censored. When imaging changes on magnetic resonance spectroscopy or magnetic resonance perfusion were suggestive of necrosis, patients were followed closely with an MRI every 1 to 2 months until progression, stabilization, or resolution. A choline to N-acetylaspartate peak ≥2:1 ratio was considered consistent with tumor on spectroscopic analysis. For perfusion studies, higher cerebral blood volume compared with contralateral normal brain was also considered to be consistent with tumor recurrence. Patients were prescribed oral steroids if symptomatic; otherwise, no further treatment was administered. Only individuals who developed symptoms and had either imaging consistent with necrosis or those requiring surgical resection with pathologic evidence of necrosis in the absence of active tumor met criteria for this endpoint.
Analysis
Statistical analyses were performed with SPSS Statistics, version 19. Survival and recurrence rates were evaluated using the Kaplan-Meier method, and factors considered to influence outcomes were compared by univariate log-rank test. A P value <.05 was considered statistically significant.
Results
Patient characteristics
For the 87 patients included in this report, the median follow-up was 7.1 months (range, 1-102 months) (Table 1). The primary cancer types included non-small cell lung cancer (NSCLC; 51%), breast (10%), and melanoma (9%). Solitary lesions were treated in 58% of patients, whereas 31% and 10% had 2 to 4 and ≥5 metastases, respectively. Only 29% had metastatic disease outside the CNS. The median preoperative tumor diameter was 3.3 cm (range, 1-6.2 cm), resulting in a median postoperative cavity volume of 13.4 mL (range, 3-40.8 mL). Tumor location was primarily supratentorial (77%), with 62% of patients undergoing a gross total resection.
Table 1.
Patient characteristics (mean or median with ranges are shown)
| Characteristic | |
|---|---|
| Age, y | 59 (37-84) |
| Gender | |
| Male | 41% |
| Female | 59% |
| Histology | |
| NSCLC | 51% |
| Melanoma | 9% |
| Breast | 10% |
| Ovarian | 8% |
| Other | 22% |
| Number of brain metastases | |
| 1 | 58% |
| ≥2 | 42% |
| Extracranial metastatic disease | 29% |
| Extracranial definitive treatment | 67% |
| Chemotherapy | 72% |
| Synchronous presentation with brain metastases | 44% |
| Gross total resection | 62% |
| Preoperative size | 3.3 cm (1-6.2 cm) |
| Cavity volume | 13.4 mL (3-40.8 mL) |
| SRS dose | 18 Gy (14-22 Gy) |
| Interval between diagnosis and SRS | 0.7 years |
| Interval between surgery and SRS | 30 days (5-56 days) |
NSCLC, non-small cell lung cancer; SRS, stereotactic radiosurgery.
Survival
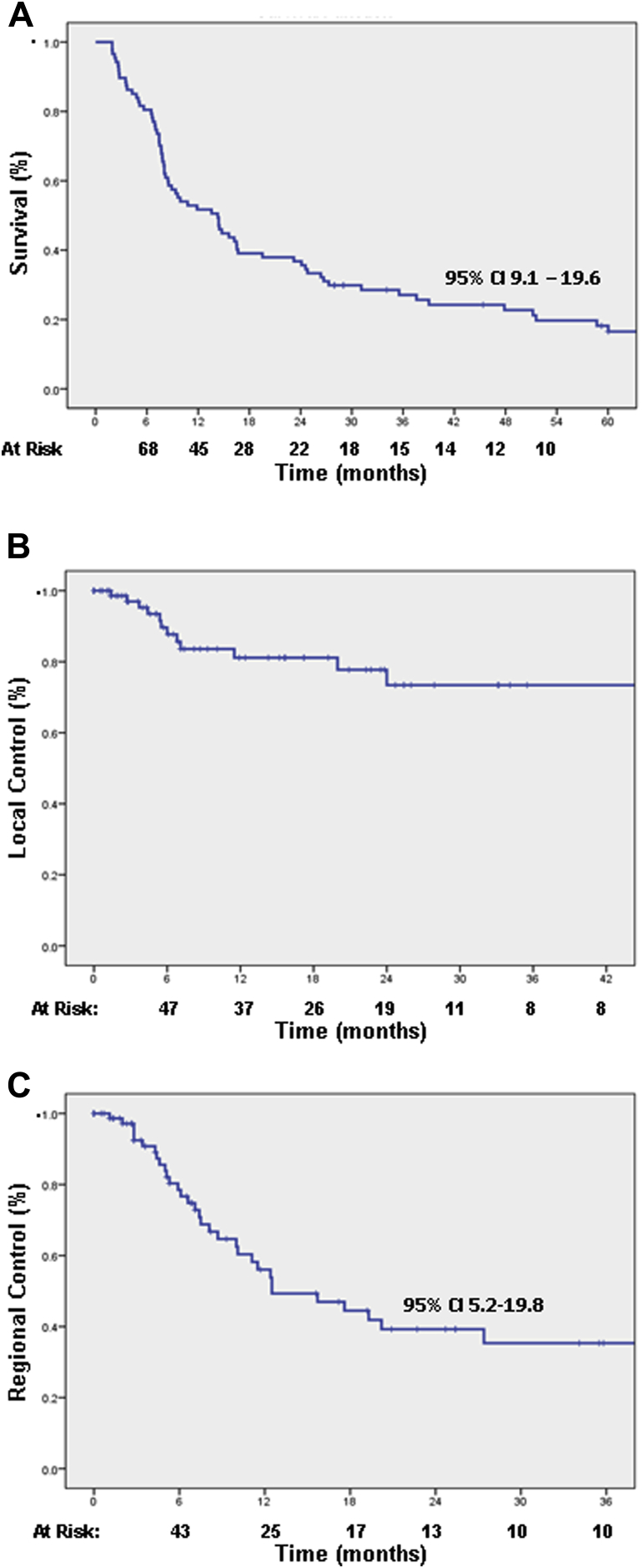
The median overall survival was 14.3 months with actuarial survival rates at 1, 2, and 5 years of 54%, 39%, and 20%, respectively (Fig 2A). Of the 87 patients, 19 (21.8%) were alive with follow-up ≥3 years (NSCLC [n = 9], ovarian [n = 2], renal cell carcinoma [n = 2], breast [n = 2], melanoma [n = 2], and endometrial [n = 2]). Overall survival was improved for patients without evidence of metastatic disease at the time of treatment (Table 2). Although not statistically significant, patients with a single metastasis also survived longer than those with ≥2 lesions (P = .09).
Figure 2.
Overall survival (A), local control (B), and regional control (C). (Time is represented in months.)
Table 2.
Univariate analysis of overall survival, local control and regional control
| Variable | Median (months) | P value |
|---|---|---|
| Overall survival | ||
| Gender (M vs F) | 9.9 vs 14.5 | .07 |
| Histology (NSCLC vs others) | 14.4 vs 13.5 | .44 |
| Brain metastases at initial diagnosis (yes vs no) | 9.6 vs 14.3 | .67 |
| Number of brain metastases (1 vs ≥2) | 14.5 vs 8.0 | .09 |
| Time from initial diagnosis to SRS (<2 vs ≥2 y) | 9.9 vs 11.5 | .19 |
| Extent of resection (gross total vs subtotal) | 14.4 vs 10.8 | .42 |
| Extracranial metastatic disease (yes vs no) | 7.4 vs 24.1 | <.01 |
| Local control | ||
| Histology (NSCLC vs others) | NR | .26 |
| Dose (≤16 Gy vs >16 Gy) | NR | .38 |
| Preresection tumor size (≤3 cm3 vs >3 cm3) | NR | .04 |
| Extent of resection (GTR vs STR) | NR | .27 |
| Treatment volume (≤14 cm3 vs >14cm3) | NR | .02 |
| Target margin (≤2 mm vs >2 mm) | NR | .14 |
| Target margin (≤3 mm vs >3 mm) | NR | .24 |
| Regional control | ||
| Gender (M vs F) | 12.4 vs 17.6 | .73 |
| Histology (NSCLC vs others) | 20.2 vs 10.1 | .37 |
| Brain metastases at initial diagnosis (yes vs no) | 12.5 vs 15.7 | .63 |
| Number of brain metastases (1 vs ≥2) | 19.3 vs 11.1 | .07 |
| Time from initial diagnosis to SRS (<2 vs >2 y) | NR vs 12.5 | .23 |
| Extracranial metastatic disease (yes vs no) | 10.0 vs 27.4 | .03 |
F, female; GTR, gross total resection; M, male; NR, not reached; NSCLC, non-small cell lung cancer; SRS, stereotactic radiosurgery; STR, subtotal resection.
Local control
Overall, 13.7% (12 of 87) of patients experienced a cavity recurrence confirmed either by imaging (n = 8) or surgical resection (n = 4). The actuarial local control rate at 1 and 2 years was 82% and 75%, respectively (Fig 2). For those who did recur locally, the median time to recurrence was 5.8 months (range, 1.4-24 months). Only 2 local failures were identified >1 year from SRS treatment. Salvage therapy for the 12 patients included a combination of re-resection (n = 4), WBRT (n = 6), and repeat SRS (n = 4). Five of the 6 patients treated with WBRT at the time of local recurrence had synchronous regional CNS recurrences as well. Two patients did not undergo salvage treatment. Survival for patients who received salvage treatment ranged between 4.4 and 59 months. Local failure was associated with increased preoperative tumor size >3 cm (P = .02) and postoperative resection cavity volumes >14 cm3 (P = .04) (Table 2). Local control at 1 year for tumors <2 cm, 2 cm to 3 cm, and >3 cm were 100%, 86%, and 72%, respectively. Similarly, local control at 1 year for resection cavities ≤14 cm3 and >14 cm3 was 89% and 65%, respectively. No difference was identified when comparing tumor location, time from resection to SRS, radiation dose, or histology. There was also no difference in local control comparing subtotal resection and gross total resection. The median estimated target margin was 2.5 mm for this cohort. Using 2 mm or 3 mm as a cutoff, no effect on local recurrence rates was identified. Conformality index also did not correlate with local control.
Regional control
The median time to regional brain recurrence was 14.3 months with actuarial regional control rates at 1 and at 2 years of 56% and 39%, respectively (Fig 2). On average, 3 (range, 1-10) new lesions were identified at the time of recurrence. Nine patients received salvage SRS, whereas 24 patients were treated with WBRT. Leptomeningeal disease was diagnosed in 8 individuals (breast [n = 4], NSCLC [n = 2], ovarian [n = 1], and neuroendocrine [n = 1]). The median size of tumors in patients who eventually developed leptomeningeal spread was 4.5 cm (range, 2.5-5 cm) with 50% located in the posterior fossa. The presence of extracranial metastatic disease was the greatest predictor for regional recurrence (Table 2). Patients with increasing number of brain metastases also appeared to be more likely to recur regionally, but this did not reach statistical significance.
Toxicity
Radiation necrosis was noted in 9 patients (10.3%). Of these, 2 were treated conservatively and 7 required surgical resection for continued symptoms and/or to exclude recurrent disease.
Discussion
Single fraction cavity-directed SRS following the resection of brain metastases provides excellent local control. Our reported institutional rate was 82% at 1 year, which compared favorably with what others have described.10, 11, 12, 13, 14 Smith et al11 reported a local control rate of 77% at 1 year in a cohort of 150 patients.12 The University of Pittsburgh group reported a cumulative local control of 86% in 120 patients.14 Similarly, Jensen et al reported on a cohort of 106 patients showing 1-year local control of 80.3%.13 The cohort we examined includes many individuals with larger tumors (80% >2 cm; 52% >3 cm) and who underwent subtotal resections (∼38%), implying that these outcomes are applicable to patients who commonly undergo palliative neurosurgical resection.
As others have described when reporting local control rates following SRS for intact brain metastases, preoperative tumor size was directly related to the risk of local failure following resection and cavity-directed SRS as well. Our 1-year recurrence rates for metastases <2 cm, 2 cm to 3 cm, and >3 cm were 0%, 14%, and 28%, respectively; thus, even with aggressive bimodality treatment that removed a bulk amount of tumor, the initial size continued to influence control. A recent phase II trial evaluating cavity-directed SRS also showed that tumors ≥3 cm in diameter had increased risk of local failure, supporting the effect of size on control.15 Of interest, subtotal resection did not correlate with inferior local control in the setting of cavity-directed SRS. This would suggest that aggressive removal of all tumor is not necessary, particularly if this would place the patient at risk for neurologic deficits and postoperative morbidity.
Several studies have assessed SRS target margins for intact metastases.16, 17 Kirkpatrick et al performed a single-institutional, randomized trial that showed no difference in local control using 1-mm compared with 3-mm margins with a trend for increased toxicity with larger margins.16 These studies, however, may not apply to the postoperative setting. Luther et al performed a patterns-of-failure analysis in 17 of 120 patients after cavity-directed SRS. They noted that local failure tended to occur at the SRS treatment margin and was more commonly seen with deep-seated tumors.14 From this limited experience, a recommended 2- to 3-mm target margin was suggested to improve control. Choi et al prospectively evaluated target margins for cavity-directed SRS showing a 1-year local failure rate of 16% when no margin was used and 3% using a 2-mm margin.10 Our cohort had a median estimated treatment margin of 2.5 mm, and analysis shows no difference in local control for margins >2 mm or >3 mm, suggesting that large margins are not necessary. It must be noted that few patients in our cohort had a target margin of less than 1 mm. Based on currently available data, a 2-mm target margin remains appropriate to achieve a high rate of local control.10 Further investigation into the effect, and optimal expansion, of target margins is necessary, especially with the increased risk of necrosis associated with this practice.17
Although the advantages of SRS relative to WBRT have been documented, SRS is certainly not without its limitations, the most clinically relevant being regional failure.3, 4, 18 In total, 38% of our patients eventually developed a recurrence elsewhere in the brain. Our median time to regional failure (14.3 months) is better than expected for all-comers with brain metastases.19 This is partially a reflection of the highly selected individuals to have undergone treatment at our institution and who were included in this study. More than 70% of patients had no evidence of extracranial metastatic disease, a feature consistently reported as the greatest predictor not only of survival, but early regional CNS failure following the local treatment of brain metastases.11, 20, 21 Of the 33 patients who recurred regionally, 8 developed leptomeningeal disease. Although this may represent small numbers, leptomeningeal spread is generally incurable and a real concern when treating the cavity alone while disregarding the possibility for tumor seeding during the procedure. Four of the 8 were patients who had breast cancer and whose tumors were located in the posterior fossa. Without sufficient evidence showing the safety of cavity-directed SRS with respect to leptomeningeal spread, recurrence, and necrosis, we caution its general use for large tumors located in the posterior fossa at this time. Further investigation, with emphasis on these concerns, is necessary.
Similar to regional failure, the median overall survival and 5-year survival compared favorably with those reported by other groups.10, 11, 22 Although this is promising, these results should be received with tempered enthusiasm because they are undoubtedly also influenced by patient selection. The majority of patients who received cavity-directed SRS had solitary lesions (59%) and no metastatic disease (71%), both of which are predictors of survival in the initial recursive partitioning analysis as well as the more recent disease-specific analyses.21, 23, 24 Although the cohort is not necessarily representative of all patients commonly treated in the clinic, these results demonstrate that in appropriately selected patients who present with large symptomatic brain metastases, good local control and long-term survival can be achieved with this approach. They also represent the best candidates for targeted treatment to limit long-term radiation associated neurologic sequelae.
Our complication rate and toxicity profile are comparable to what other authors have reported following SRS.12, 25 In spite of a large tumor size and volume treated, only 10.3% (9 of 87 patients) of patients developed necrosis requiring surgical resection or medical management. Although alternative options for stereotactic treatment of brain metastases including hypofractionation or as neoadjuvant therapy may reduce necrosis rates, the merits of these approaches require further evaluation.25, 26, 27
As with all retrospective analyses, there exists bias inherent to patient selection limiting how results might be extrapolated to the general patient population. Although our cohort is highly selected, as demonstrated by superior survival and regional recurrence rates compared with general expectations, it underscores that well-selected postresection candidates can achieve excellent outcomes when considered for aggressive, local CNS treatment.
In summary, our study supports the efficacy of cavity-directed SRS for providing excellent local control following resection of brain metastases. Increasing tumor size or cavity volume negatively impacts control, whereas subtotal resection does not appear to influence local outcomes. Patient selection remains essential not only for survival, but also for limiting future local and regional CNS recurrence. Although further studies are necessary both to establish the relative value of cavity-directed treatment and to identify the most appropriate patient population, our findings support its use in experienced centers.
References
- 1.Tosoni A., Ermani M., Brandes A.A. The pathogenesis and treatment of brain metastases: A comprehensive review. Crit Rev Oncol Hematol. 2004;52:199–215. doi: 10.1016/j.critrevonc.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Soon Y.Y., Tham I.W., Lim K.H., Koh W.Y., Lu J.J. Surgery or Radiosurgery plus whole brain radiotherapy versus surgery or radiosurgery alone for brain metastases. Cochrane Database Syst Rev. 2014;3:1–41. doi: 10.1002/14651858.CD009454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nieder C., Grosu A.L., Gaspar L.E. Stereotactic radiosurgery (SRS) for brain metastases: A systematic review. Radiat Oncol. 2014;9:155–163. doi: 10.1186/1748-717X-9-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippitz B., Lindquist C., Paddick I., Peterson D., O'Neill K., Beaney R. Stereotactic radiosurgery in the treatment of brain metastases: The current evidence. Cancer Treat Rev. 2014;40:48–59. doi: 10.1016/j.ctrv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Chang E.L., Wefel J.J.S., Hess K.R. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 6.Abe E., Aoyama H. The role of whole brain radiation therapy for the management of brain metastases in the era of stereotactic radiosurgery. Curr Oncol Rep. 2012;14:79–84. doi: 10.1007/s11912-011-0201-0. [DOI] [PubMed] [Google Scholar]
- 7.Monaco E.A., 3rd, Bhatnagar J.P., Xu Y. Evaluation of tumor progression and detection of new tumors during repeat Gamma Knife(R) stereotactic radiosurgery utilizing the co-registration tool in Leksell Gamma Plan(R): Technical note. Stereotact Funct Neurosurg. 2014;92:300–305. doi: 10.1159/000365227. [DOI] [PubMed] [Google Scholar]
- 8.Stereotactic radiosurgery or whole-brain radiation therapy in treating patients with brain metastases that have been removed by surgery. Available at: https://clinicaltrials.gov/ct2/show/NCT01372774. Accessed July 5, 2016.
- 9.Shaw E., Scott C., Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 10.Choi C.Y., Chang S.D., Gibbs I.C. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases: Prospective evaluation of target margin on tumor control. Int J Radiat Oncol Biol Phys. 2012;84:336–342. doi: 10.1016/j.ijrobp.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Smith T.R., Lall R.R., Lall R.R. Survival after surgery and stereotactic radiosurgery for patients with multiple intracranial metastases: Results of a single-center retrospective study. J Neurosurg. 2014;121:1–7. doi: 10.3171/2014.4.JNS13789. [DOI] [PubMed] [Google Scholar]
- 12.Zairi F., Ouammou Y., Le Rhun E. Relevance of gamma knife radiosurgery alone for the treatment of non-small cell lung cancer brain metastases. Clin Neurol Neurosurg. 2014;125:87–93. doi: 10.1016/j.clineuro.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 13.Jensen C.A., Chan M.D., McCoy T.P. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg. 2011;114:1585–1591. doi: 10.3171/2010.11.JNS10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luther N., Kondziolka D., Kano H. Predicting tumor control after resection bed radiosurgery of brain metastases. Neurosurgery. 2013;73:1001–1006. doi: 10.1227/NEU.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 15.Brennan C., Yang T.J., Hilden P. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys. 2014;88:130–136. doi: 10.1016/j.ijrobp.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkpatrick J.P., Wang Z., Sampson J.H. Defining the optimal planning target volume in image-guided stereotactic radiosurgery of brain metastases: Results of a randomized trial. Int J Radiat Oncol Biol Phys. 2015;91:100–108. doi: 10.1016/j.ijrobp.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Nataf F., Schlienger M., Liu Z. Radiosurgery with or without a 2-mm margin for 93 single brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:766–772. doi: 10.1016/j.ijrobp.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Patel K.R., Prabhu R.S., Kandula S. Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: Whole brain radiotherapy versus stereotactic radiosurgery alone. J Neurooncol. 2014;120:657–663. doi: 10.1007/s11060-014-1601-4. [DOI] [PubMed] [Google Scholar]
- 19.Zindler J.D., Slotman B.J., Lagerwaard F.J. Patterns of distant brain recurrences after radiosurgery alone for newly diagnosed brain metastases: Implications for salvage therapy. Radiother Oncol. 2014;112:212–216. doi: 10.1016/j.radonc.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Kress M.A., Oermann E., Ewend M.G., Hoffman R.B., Chaudhry H., Collins B. Stereotactic radiosurgery for single brain metastases from non-small cell lung cancer: Progression of extracranial disease correlates with distant intracranial failure. Radiat Oncol. 2013;8:64. doi: 10.1186/1748-717X-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Likhacheva A., Pinnix C.C., Parikh N.R. Predictors of survival in contemporary practice after initial radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2013;85:656–661. doi: 10.1016/j.ijrobp.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 22.Huttenlocher S., Dziggel L., Hornung D., Blanck O., Schild S.E., Rades D. A new prognostic instrument to predict the probability of developing new cerebral metastases after radiosurgery alone. Radiat Oncol. 2014;9:215. doi: 10.1186/1748-717X-9-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regine W.F., Huhn J.L., Patchell R.A. Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases: Results and implications. Int J Radiat Oncol Biol Phys. 2002;52:333–338. doi: 10.1016/s0360-3016(01)02645-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang T.J., Saad S., Qureshi Y.H. Outcomes of gamma knife radiosurgery, bi-modality & tri-modality treatment regimens for patients with one or multiple brain metastases: The Columbia University Medical Center experience. J Neurooncol. 2015;122:399–408. doi: 10.1007/s11060-015-1728-y. [DOI] [PubMed] [Google Scholar]
- 25.Rajakesari S., Arvold N.D., Jimenez R.B. Local control after fractionated stereotactic radiation therapy for brain metastases. J Neurooncol. 2014;120:L339–L346. doi: 10.1007/s11060-014-1556-5. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed KA, Sarangkasiri S, Chinnaiyan P, et al. Outcomes following hypofractionated stereotactic radiotherapy in the management of brain metastases [e-pub ahead of print]. Am J Clin Oncol. http://dx.doi.org/10.1097/coc.0000000000000076, accessed July 5, 2016. [DOI] [PubMed]
- 27.Asher A.L., Burri S.H., Wiggins W.F. A new treatment paradigm: Neoadjuvant radiosurgery before surgical resection of brain metastases with analysis of local tumor recurrence. Int J Radiat Oncol Biol Phys. 2014;88:899–906. doi: 10.1016/j.ijrobp.2013.12.013. [DOI] [PubMed] [Google Scholar]