Introduction
Radiation-induced skin injury ranges from acute dermatitis to chronic skin changes.1, 2 Acute skin injury occurs during the course of radiation treatment and may take 1 to 3 months after the completion of radiation therapy to completely heal. Patients with mild acute dermatitis present with mild erythema, dry desquamation, pruritus, hyperpigmentation, and hair loss. Severe acute dermatitis is characterized by confluent moist desquamation, ulcers, hemorrhage, and necrosis.1, 3 Chronic radiation-induced skin injury includes chronic fibrosis with associated skin breakdown and infection.1, 4
The severity of radiation-induced skin toxicity depends on radiation factors (dose, fractionation, volume, and surface area) and patient-specific factors. Excessive skinfolds increase radiation-induced skin toxicity. Also, patients with poor nutrition status or a preexisting vascular condition or connective tissue disease have impaired wound healing and are at increased risk of skin toxicity.5 Actinic lichen planus is an autoimmune skin disease characterized by a violaceous papule rash.6 Here, we report the case of a patient with presumed latent actinic lichen planus treated with definitive radiation therapy who subsequently developed overt lichen planus and severe radiation dermatitis in the treatment fields.
Case report
A 55-year-old African American woman presented with intermittent hoarseness over several years, eventually limiting her voice to a whisper. She denied dysphagia, odynophagia, otalgia, dyspnea, or stridor. Suspension microlaryngoscopy revealed a bulky left vocal cord lesion. Bilateral vocal fold biopsies were obtained, and pathology revealed a superficially invasive, well-differentiated squamous cell carcinoma of the left true vocal cord without invasion of the right true vocal cord. She was staged as cT1bN0M0.
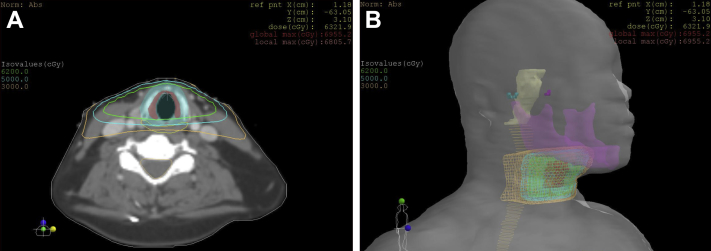
The patient was treated with definitive radiation therapy to the larynx and received a total dose of 62 Gy in 31 fractions of 2 Gy each delivered once daily using 6-MV photons, per our departmental policy. The total treatment duration was 50 days, including breaks and weekends. After she received 14 Gy, she had a treatment break of 10 days because she was hospitalized at an outside facility for a gastrointestinal bleed. She then completed treatment as planned. Right and left posterior oblique fields were used, with gantry angles of 260° and 100°, respectively. Thirty-degree wedges were used to reduce anterior hotspots. The field sizes were 5.7 cm superior to inferior and 4.6 cm anterior to posterior. No bolus was used throughout the course of radiation therapy. The dosimetry is shown in Fig 1.
Figure 1.
Dosimetry. (A) Representative axial sections showing isodose lines. Green = 62 Gy, cyan = 50 Gy, orange = 30 Gy. (B) Three-dimensional rendering of isodose lines. Mandible is purple, spinal cord is light orange, and brain stem is yellow.
During treatment she experienced grade 3 radiation dermatitis, grade 1 hoarseness, and grade 2 pharyngeal mucositis. Table 1 lists the toxicity grades for radiation dermatitis. Her skin toxicity became most severe after receiving 52 Gy. The radiation plan was reviewed, and optically stimulated luminescent dosimeters were used to verify that the skin dose did not exceed 2.0 Gy per fraction. She was prescribed silver sulfadiazine 1% for management of her dermatitis and hydrocodone with acetaminophen 7.5 to 500 mg/15 mL oral solution for pain control; however, the radiation dermatitis persisted through the end of treatment. On her final day of treatment, she had developed skin hyperpigmentation in the anterior neck, including the superior border of nodal station 2 through the inferior border of nodal station 3. She developed moist desquamation in the areas where the oblique fields entered through the skin, correlating with the 50-Gy isodose line. Our patient's dermatitis healed after 2 months, as depicted in Fig 2.
Table 1.
Toxicity grades for radiation dermatitis, defined as a finding of cutaneous inflammatory reaction occurring as a result of exposure to biologically effective levels of ionizing radiation
| Radiation dermatitis grade | |
|---|---|
| 1 | Faint erythema or dry desquamation |
| 2 | Moderate to brisk erythema; patchy moist desquamation, mostly confined to skin folds and creases; moderate edema |
| 3 | Moist desquamation in areas other than skin folds and creases; bleeding induced by minor trauma or abrasion |
| 4 | Life-threatening consequences; skin necrosis or ulceration of full thickness dermis; spontaneous bleeding from involved site; skin graft indicated |
| 5 | Death |
Adapted from Common Terminology Criteria for Adverse Events (CTCAE) version 4.03, June 14, 2010, U.S. Department of Health and Human Services.
Figure 2.
Grade 3 radiation dermatitis. (A) Before radiation treatment. (B) On the final day of radiation treatment. (C) Three weeks after radiation treatment. (D) Two months after radiation treatment.
One month after our patient completed radiation therapy, she presented to dermatology and was noted to have gray to violaceous patches in the buccal mucosa and tongue. She also had many scattered flat-topped hyperpigmented to violaceous patches in the extremities and buttocks. She reported an intermittent pruritic rash of the arms, legs, and trunk for the previous 10 years. She was using triamcinolone cream and hydroxyzine with minimal improvement in this rash. A shave biopsy was obtained from a representative lesion in the right volar forearm. Pathology showed patchy interface dermatitis with pigment incontinence, compact orthokeratosis with hypergranulosis, focal basal layer squamatization, and occasional apoptotic keratinocytes limited to the lower portion of the epidermis. Based on the clinical and histological findings, she was diagnosed with actinic lichen planus.
Discussion
Actinic lichen planus is an inflammatory skin reaction thought to be caused by an autoimmune reaction that typically develops on sun-exposed areas in dark-skinned individuals. Only 7 cases of radiation-induced cutaneous lichen planus have been reported, shown in Table 2.7, 8, 9, 10, 11, 12, 13 However, no report to our knowledge discusses radiation-induced skin toxicity in a patient with lichen planus. The case presented here is the first case of severe radiation dermatitis in the treatment field of a patient with presumed occult actinic lichen planus.
Table 2.
Case reports of postradiation cutaneous lichen planus
| Author | Year | Site | Localized/diffuse actinic lichen planus |
|---|---|---|---|
| Boyd et al7 | 1991 | Breast | Diffuse |
| Eichbaum et al8 | 2006 | Breast | Diffuse |
| Morar et al10 | 2009 | Plasmacytoma of skull | Diffuse |
| Kim et al9 | 2002 | Thyroid | Localized |
| Shurman et al12 | 2004 | Penis | Localized |
| Vergilis-Kalner et al13 | 2008 | Metastatic breast and thigh | Localized |
| Pretel et al11 | 2007 | Breast | Localized |
At our institution, T1 glottis squamous cell carcinomas are treated with doses of 60 to 66 Gy in fractions of 2 Gy, with a local control exceeding 90% (unpublished data). The generally accepted radiation treatment for T1 glottic cancers is a hypofractionated course of 63 Gy in 2.25 Gy per fraction based on the study by Yamazaki et al that showed improved local control in the hypofractionation arm versus the conventional fractionation arm (60-66 Gy in 2 Gy per fraction).14 However, in the aforementioned study, local control in the conventional fractionation arm was only 77% at 5 years.14 Previous studies have shown the 5-year local control of T1 squamous cell carcinomas of the glottis treated with conventional fractionation to be 88%15; therefore, we have adopted a conventional fractionation treatment regimen in the management of T1 squamous cell carcinomas of the glottis.
Common acute toxicities following radiation therapy for laryngeal cancer include hoarseness, sore throat, odynophagia, and skin irritation. A concerning late toxicity is laryngeal edema.16 Regarding skin toxicity, Emami et al showed that, when a 30 cm2 area of skin receives a dose of 60 Gy or a 10 cm2 area of skin receives a dose of 70 Gy, there is a 5% risk of developing necrosis and ulceration within 5 years from radiation therapy.17 In the presenting case, each treatment field was 26.2 cm2, and the maximum point dose was 69.5 Gy. Additionally, our patient developed grade 3 dermatitis after receiving 52 Gy and with an unexpected 10-day treatment break after 7 fractions. In the Yamazaki et al hypofractionation study, only 9% of patients in the hypofractionation arm developed dry desquamation, and no patient in that study developed moist desquamation.14 Thus, given the treatment factors for our patient, grade 3 radiation dermatitis is well beyond the realm of expected toxicities. Our patient had symptoms of lichen planus before radiation therapy to the larynx; however, at the time of treatment, she had not yet been diagnosed with actinic lichen planus. Our report represents the first case of a patient with occult actinic lichen planus to develop grade 3 radiation-induced dermatitis in the treatment field following external beam radiation therapy for squamous cell carcinoma of the vocal cord. Although previous case reports showed that radiation therapy may induce or exacerbate localized or diffuse lichen planus, we show that the severity of radiation dermatitis was increased in an irradiated patient with presumed latent actinic lichen planus.
Conclusions
We present a case in which radiation dermatitis was worsened in a patient with presumed latent actinic lichen planus. In addition to discussing possible increased risks of radiation toxicity in patients with collagen vascular disease, our case indicates that patients with lichen planus should also be informed of possible increased risks of radiation toxicity. It is uncertain how to properly treat lichen planus patients requiring external radiation for malignancies. With an understanding of the inflammatory mechanisms and factors in lichen planus patients following external beam radiation therapy, we can better manage these patients and limit radiation toxicity.
Footnotes
Conflicts of interest: None.
References
- 1.Hymes S.R., Strom E.A., Fife C. Radiation dermatitis: Clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28–46. doi: 10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 2.Wickline M.M. Prevention and treatment of acute radiation dermatitis: A literature review. Oncol Nurs Forum. 2004;31:237–247. doi: 10.1188/04.ONF.237-247. [DOI] [PubMed] [Google Scholar]
- 3.Malkinson F.D., Keane J.T. Radiobiology of the skin: Review of some effects on epidermis and hair. J Invest Dermatol. 1981;77:133–138. doi: 10.1111/1523-1747.ep12479347. [DOI] [PubMed] [Google Scholar]
- 4.Delanian S., Balla-Mekias S., Lefaix J.L. Striking regression of chronic radiotherapy damage in a clinical trial of combined pentoxifylline and tocopherol. J Clin Oncol. 1999;17:3283–3290. doi: 10.1200/JCO.1999.17.10.3283. [DOI] [PubMed] [Google Scholar]
- 5.Harper J.L., Franklin L.E., Jenrette J.M., Aguero E.G. Skin toxicity during breast irradiation: Pathophysiology and management. South Med J. 2004;97:989–993. doi: 10.1097/01.SMJ.0000140866.97278.87. [DOI] [PubMed] [Google Scholar]
- 6.Meads S.B., Kunishige J., Ramos-Caro F.A., Hassanein A.M. Lichen planus actinicus. Cutis. 2003;72:377–381. [PubMed] [Google Scholar]
- 7.Boyd A.S., Neldner K.H. Lichen planus. J Am Acad Dermatol. 1991;25:593–619. doi: 10.1016/0190-9622(91)70241-s. [DOI] [PubMed] [Google Scholar]
- 8.Eichbaum M., Harms W., Bolz S., Schneeweiss A., Sohn C. Generalized lichen ruber planus—induced by radiotherapy of the breast? Onkologie. 2006;29:521–523. doi: 10.1159/000096048. [DOI] [PubMed] [Google Scholar]
- 9.Kim J.H., Krivda S.J. Lichen planus confined to a radiation therapy site. J Am Acad Dermatol. 2002;46:604–605. doi: 10.1067/mjd.2002.119654. [DOI] [PubMed] [Google Scholar]
- 10.Morar N., Francis N.D. Generalized lichen planus induced by radiotherapy: Shared molecular mechanisms? Clin Exp Dermatol. 2009;34:e434–e435. doi: 10.1111/j.1365-2230.2009.03434.x. [DOI] [PubMed] [Google Scholar]
- 11.Pretel M., Espana A. Lichen planus induced by radiotherapy. Clin Exp Dermatol. 2007;32:582–583. doi: 10.1111/j.1365-2230.2007.02449.x. [DOI] [PubMed] [Google Scholar]
- 12.Shurman D., Reich H.L., James W.D. Lichen planus confined to a radiation field: The “isoradiotopic” response. J Am Acad Dermatol. 2004;50:482–483. doi: 10.1016/s0190-9622(03)02144-3. [DOI] [PubMed] [Google Scholar]
- 13.Vergilis-Kalner I.J., Sharma V., Sethi A. Lichen planus arising in radiation therapy treatment sites. Cutis. 2008;82:353–355. [PubMed] [Google Scholar]
- 14.Yamazaki H., Nishiyama K., Tanaka E., Koizumi M., Chatani M. Radiotherapy for early glottic carcinoma (T1N0M0): Results of prospective randomized study of radiation fraction size and overall treatment time. Int J Radiat Oncol Biol Phys. 2006;64:77–82. doi: 10.1016/j.ijrobp.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Mendenhall W.M., Parsons J.T., Million R.R., Fletcher G.H. T1-T2 squamous cell carcinoma of the glottic larynx treated with radiation therapy: Relationship of dose-fractionation factors to local control and complications. Int J Radiat Oncol Biol Phys. 1988;15:1267–1273. doi: 10.1016/0360-3016(88)90220-9. [DOI] [PubMed] [Google Scholar]
- 16.Sanguineti G., Adapala P., Endres E.J. Dosimetric predictors of laryngeal edema. Int J Radiat Oncol Biol Phys. 2007;68:741–749. doi: 10.1016/j.ijrobp.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Emami B., Lyman J., Brown A. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]