Abstract
Childhood obesity is associated with a number of metabolic abnormalities leading to increased cardiovascular risk. Metabolites can be useful as early biomarkers and new targets to promote early intervention beginning in school age. Thus, we aimed to identify metabolomic profiles associated with obesity and obesity-related metabolic traits. We used data from the Obesity Research Study for Mexican children (ORSMEC) in Mexico City and included a case control (n = 1120), cross-sectional (n = 554) and a longitudinal study (n = 301) of 6–12-year-old children. Forty-two metabolites were measured using electrospray MS/MS and multivariate regression models were used to test associations of metabolomic profiles with anthropometric, clinical and biochemical parameters. Principal component analysis showed a serum amino acid signature composed of arginine, leucine/isoleucine, phenylalanine, tyrosine, valine and proline significantly associated with obesity (OR = 1.57; 95%CI 1.45–1.69, P = 3.84 × 10−31) and serum triglycerides (TG) (β = 0.067, P = 4.5 × 10−21). These associations were validated in the cross-sectional study (P < 0.0001). In the longitudinal cohort, the amino acid signature was associated with serum TG and with the risk of hypertriglyceridemia after 2 years (OR = 1.19; 95%CI 1.03-1.39, P = 0.016). This study shows that an amino acid signature significantly associated with childhood obesity, is an independent risk factor of future hypertriglyceridemia in children.
Introduction
Obesity represents a major global health problem in adults and children. In Mexico, according to the National Health and Nutrition survey 2012, the prevalence of overweight and obesity is 71.2% in adults and 34.3% in children1. Pediatric obesity is strongly associated with a number of metabolic abnormalities such as dyslipidemia, insulin resistance and hypertension. All the latter are well known risk factors for cardiovascular disease2, the leading worldwide cause of mortality3. Low serum high-density lipoprotein cholesterol (HDL-C) levels, elevated low-density lipoprotein cholesterol (LDL-C), high triglycerides (TG) levels, or a combination of the latter are frequent metabolic alterations in childhood obesity4. Indeed, it was recently reported that in Mexico almost 70% of obese and 50% of non-obese children have dyslipidemia, where hypertriglyceridemia is the most frequent5. Furthermore, lipid levels during childhood are predictive of dyslipidemia and subclinical atherosclerosis in adulthood6, 7.
Therefore there is an urgent need to identify risk factors during childhood to prevent future dyslipidemia and ultimately cardiovascular disease in adults8. Metabolomics has emerged as a powerful tool to identify novel risk factors for early detection of various health-related traits. Branched-chain (BCAA) and aromatic amino acid (AA) serum levels are increased in obesity in humans and animal models9, 10, and have been associated with current and future development of insulin resistance in adults and children11–13. Furthermore, a recent meta-analysis confirmed that higher levels of BCAA and AA increase the risk of type 2 diabetes14. In addition amino acid profiles have been associated with dyslipidemia in adults15, 16. Therefore, the objective of the present study was to identify metabolomic profiles associated with obesity and metabolic traits in Mexican children using both a case-control study and a longitudinal cohort-based approach.
Methodology
Study participants
This study was embedded in the “Obesity research study for Mexican children” (ORSMEC), which is a population based study, with the overall aim of identifying risk factors for obesity and metabolic abnormalities in school age children. ORSMEC includes 6–12-year-old children recruited from a summer camp of children of employees of the Mexican Health Ministry (Convivencia Infantil, Sindicato de la Secretaria de Salud) and Hospital Infantil de Mexico from 2008–2015. All children included were free of chronic medical illness. Parents or guardians of each child signed the informed consent form and children assented to participate. The study was approved by the Ethics Committee of participant institutions; Instituto Nacional de Medicina Genómica, Instituto Nacional de Ciencias Medicas y Nutrición and Hospital Infantil de Mexico and was in accordance with the Helsinki declaration II.
Three different study designs were tested. In the discovery phase, a case-control study for obesity was carried out to seek associations of metabolomic profiles with obesity. This study included 1120 healthy unrelated children [606 normal-weight and 514 obese, classified according to the Center for Disease Control and Prevention (CDC) criteria17]. Obesity status was defined as body mass index (BMI) percentile ≥95th for age and gender, while for the normal-weight group children with BMI between the 15th and 75th percentile for age and gender were selected. An independent cross-sectional study was performed including 554 children from ORSMEC recruited between 2013 and 2014, excluding those who were part of the initial case-control study. Finally, a longitudinal cohort was designed to test for metabolomic biomarkers predicting obesity and related metabolic traits. This included children, that attended two times to the summer camp and whose clinical data and biological samples, with a 2-year separation, were available (n = 301).
Anthropometric and clinical parameters
Anthropometric measurements were determined following the procedures recommended by Lohman et al.18 and included weight, height, waist and hip circumferences. All instruments were calibrated following the standard methods of the manufacturers. Blood pressure (BP; mmHg) was measured twice at sitting position using an automatic manometer (Microlife), and the average of both measurements was used for the analyses. Body fat mass percentage was obtained by bioelectrical impedance analysis (Quantum X Body Composition Analyzer, RJL Systems). Center for Disease Control 2000 growth charts were used as reference to determine BMI percentiles17. As previously mentioned, obesity status was defined as body mass index (BMI) percentile ≥95th, whereas overweight was defined between 85th and 95th percentile, normal-weight between the 5th and 85th percentile and underweight < 5th BMI percentile for age and gender. National Heart, Lung, and Blood Institute reference data were used to determine BP percentiles based on height, age and gender19. Hypertriglyceridemia was defined as serum triglyceride levels ≥130 mg/dL as suggested by the Expert Panel on Integrated Guidelines for Cardiovascular Health Risk Reduction in Children and Adolescents20.
Blood sampling and biochemical analyses
Five mL blood samples were drawn after 8–12 hours of fasting. For serum samples a 30-min period was allowed for clotting before serum separation and then stored at −80 °C until further analysis. Serum levels of glucose, creatinine, uric acid, total cholesterol, HDL cholesterol, LDL cholesterol, TG, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma glutamyl transpeptidase (GGT), Apo B and C-reactive protein (CRP) were measured with commercially available standardized methods (UNICEL DxC600, Beckman coulter). Insulin was determined using an Access 2 Immunoassay System (Beckman coulter). Insulin sensitivity was indirectly estimated with the homeostasis model assessment for insulin resistance (HOMA-IR).
Metabolomics analysis
We used a targeted metabolomics approach by electrospray tandem mass spectrometry (Quattro Micro API tandem MS) that includes metabolites previously associated with obesity and obesity-related traits and that is also useful to assess amino acid and fatty acid metabolism in a high number of samples9. Serum concentrations of 11 amino acids, free carnitine and 30 acylcarnitines were measured with a commercial kit (NeoBase Non-derivatized MS/MS kit; PerkinElmer Waltham Massachusetts) using multiple reaction monitoring (MRM) mode. The assay was performed according to the manufacturer’s instructions. Briefly, fasting serums samples (previously stored at −80 °C) were applied to filter paper cards (Whatman 903TM, GE Healthcare Bio-Sciences Corp. Westborough, MA) and dried for 3 hours. For every sample a 3mm-diameter disk was punched with an automatic device into a 96-well sample plate where 190 µL of extraction solution containing a mixture of 22 stable isotope-labeled internal standards were added (Supplemental Table 1). The plate was covered with aluminum foil, incubated with agitation (30 °C at 650 × g for 30 min) and placed in a 2777 C Waters auto-sampler (Waters Corp., Milford, MA). An HPLC pump (Waters 1525μ) was used for flow injection analysis (FIA). Thirty μL of sample extracts were directly injected at a flow rate of 1.5 mL/min and in an analysis time of 1.5 min. A blank sample (extraction solution with internal standards) was included in each plate. Low and high analytical controls as well as a quality control sample (pool of all serum samples) were included in each plate in triplicate. Metabolites were quantified by reference to appropriate internal standards. Intra- and inter-plate variation coefficients were calculated based on repetitive measurements of the quality control sample. Leucine and isoleucine are reported as a single analyte because they are not resolved by the MS/MS method. Inter and intra-assay variation coefficients for arginine, leucine/isoleucine, valine, phenylalanine, tyrosine and proline ranged from 5 to 9%.
Statistical Analysis
In the case-control study, differences between groups were assessed using Mann-Whitney-Wilcoxon Test. In the discovery phase, a principal component analysis (PCA) was performed on the 42 metabolites using Facto-MineR in R package. This package performs unit variance scaling of metabolites as data pre-treatment prior to PCA21. Components with eigenvalues >1.0 were retained and metabolites with component loading values higher than >0.5 were retained for a given component. Logistic regression models were used to test the effect of the principal components (PCs) on the risk for obesity, while linear regression was used to test the association with clinical and biochemical variables. The degree of multicollinearity among metabolites was estimated with the Variance Inflation Factor (VIF).
To test the findings in the cross-sectional and longitudinal studies a combined index of the amino acids represented in principal component 2 (PC2) was generated. Cohort Z-score values were calculated for each normalized metabolite in all subjects. The sum of amino acid Z-scores represented in PC2 (PC2-Z) was tested for associations with metabolic traits by linear regression models. In the longitudinal cohort, paired sample Wilcoxon signed-rank test was used to compare clinical and biochemical variables between baseline (T = 0) and 2 year follow up measurements (T = 1). The association of the PC2-Z with hypertriglyceridemia after 2 years was assessed with multivariate logistic regression analysis, using stepwise modeling to determine the effect of other risk factors or potential confounders (gender and baseline age, BMI percentile, TG and HOMA-IR). C statistics was used to test the ability of several models to predict hypertriglyceridemia, by calculating the area under the receiver operating characteristic curve (AUC). Furthermore, the contribution of the amino acid component was assessed by the integrated discrimination index (IDI) and the net reclassification improvement (NRI) method, which evaluates the proportion of subjects moving accurately or inaccurately from one risk category to another, after adding the predictor of interest into the model22. For NRI calculation, participants were assigned to one of four categories reflecting their risk of 2-year hypertriglyceridemia based on each model (<5, 5 to <10, 10–20, and >20%). Model calibration was tested by the Hosmer-Lemeshow χ2 test. All non-normally distributed clinical variables were transformed using the Box-Cox method before testing associations. This algorithm transforms data into a normal shape by using the parameter λ. Logistic and linear regression models were adjusted for age, gender and sampling calendar year, while associations with biochemical variables were also adjusted for BMI percentile. The resulting P-value was corrected for multiple comparisons with false discovery rate (FDR) method23. Statistical significance was set at P < 0.05.
Results
Case control study
Anthropometric and biochemical characteristics in normal weight and obese children are described in Supplemental Table 2. Body fat percentage and all clinical parameters excluding lean mass percentage and HDL-C levels were significantly higher in obese than in normal weight children. To analyze the metabolomics profile, we used PCA that cluster metabolites into highly correlated species. Seven components including 37 metabolites showed eigenvalues >1, which explained 69% of the variance in metabolites. Component 7 was excluded from the analysis since no metabolites had loading values greater that 0.5 (results not shown). Six components explaining the 66.6% of variance in metabolites were retained for further analysis. The constituents of each component are shown in Table 1.
Table 1.
Principal component analysis of 42 metabolites and associations of clusters with obesity in a case-control cohort of Mexican school-age children.
| Description | Component metabolites | Eigenvalue | Percentage of variance | Cumulative percentage of variance | Obesity | Percentage of variance explaining obesity | ||
|---|---|---|---|---|---|---|---|---|
| OR | P value | |||||||
| PC1 | Miscellaneus | Citrulline, Glycine, Alanine, Leucine + Isoleucine, Methionine, Phenylalanine, Valine, Succinylacetone, Propionylcarnitine C3, Butyryl + isobutyrylcarnitine C4, Tiglylcarnitine C5:1, Hexanoylcarnitine C6, Octanoylcarnitine C8, Octenoylcarnitine C8:1, Hexadecenoylcarnitine C16:1, Hydroxyhexadecenoylcarnitine C16:1-OH, Hydroxypalmitoylcarnitine C16-OH, Decanoylcarnitine C10, Decenoylcarnitine C10:1, Decadienoylcarnitine C10:2, Dodecanoylcarnitine C12, Dodecenoylcarnitine C12:1, Tetradecanoylcarnitine C14, Tetradecenoylcarnitine C14:1, Tetradecadienoylcarnitine C14:2, 3-hydroxytetradecenoylcarnitine C14:OH, Stearylcarnitine C18, Linoleylcarnitine C18:2, Hydroxybutyrylcarnitine C4OH + Malonylcarnitine C3DC, Hydroxyisovalerylcarnitine C5OH + Succinyl + methylmalonylcarnitine C4DC | 14.39 | 33.46 | 33.46 | 1.04 (1.00–1.07) | 0.03 | 1.03 |
| PC2 | Amino acids | Arginine, Leucine + Isoleucine, Phenylalanine, Tyrosine, Valine, Proline | 5.20 | 12.09 | 45.55 | 1.57 (1.45–1.69) | 3.84 × 10 −31 | 15.28 |
| PC3 | Acycarnitines | Acetylcarnitine C2, Palmitoylcarnitine C16, Stearylcarnitine C18 | 3.23 | 7.51 | 53.06 | 1.21 (1.11–1.33) | 4.46 × 10 −5 | 0.88 |
| PC4 | Shortchain acylcarnitines | Acetylcarnitine C2, Propionylcarnitine C3 | 2.49 | 5.78 | 58.84 | 0.97 (0.89–1.04) | 0.409 | 0.03 |
| PC5 | Medium chain acylcarnitines | Methylglutarylcarnitine C6DC, Dodecanoylacylcarnitine C12 | 2.13 | 4.95 | 63.79 | 0.53 (0.47–0.60) | 7.63 × 10 −25 | 6.52 |
| PC6 | Miscellaneus | Ornithine, Octenoylcarnitine C8:1 | 1.37 | 3.18 | 66.98 | 0.93 (0.84–1.03) | 0.19 | 0.30 |
The logistic regression model was adjusted for gender and age.Significant P-values are shown in bold.
Using logistic regression models, we found three components significantly associated with a higher risk of obesity [PC1 (OR = 1.04; 95%CI 1.0–1.07, P = 0.037), PC2 (OR = 1.57; 95%CI 1.46–1.70, P = 3.8 × 10−31) and PC3 (OR = 1.21; 95%CI 1.11–1.33, P = 4.46 × 10−5)], while PC5 (OR = 0.53; 95%CI 0.47–0.60, P = 7.63 × 10−25) was associated with a lower obesity risk. PC2, represented by high serum levels of arginine, leucine/isoleucine, phenylalanine, tyrosine, valine and proline showed the strongest association with obesity and explained 15.3% of the variance (Table 1). Multicollinearity among the amino acids present in PC2 was low to modest (VIF 1.5–6.7), and arginine and proline showed the lowest VIF values (1.5 and 1.9 respectively).
We then tested whether these PCs were associated with other clinical variables (Supplemental Table 3). The amino acid component (PC2) showed the strongest associations with HOMA-IR, serum insulin, glucose, TG and total cholesterol levels, but not with ApoB (Fig. 1). PC2 was also significantly associated with higher systolic BP percentile. Moreover, PC3, represented by C2 and C16 carnitines, showed positive associations with systolic BP percentile, creatinine, uric acid, AST and GGT levels. Component PC5, negatively associated with obesity, showed no significant associations with biochemical variables (Supplemental Table 3). Because the amino acid component (PC2) explained the highest percentage in obesity variance and was also associated with biochemical variables, we focused on this component to test the findings in a cross-sectional and a longitudinal study
Figure 1.
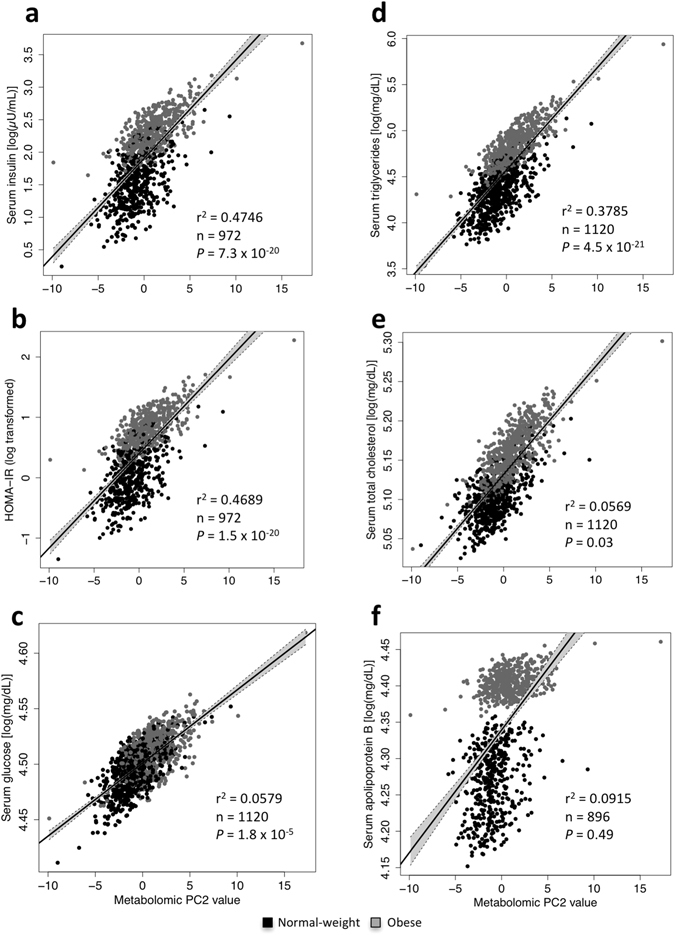
Case-control study. Correlation between PC2 amino acids (arginine, leucine/isoleucine, phenylalanine, tyrosine, valine and proline) and serum insulin (a), HOMA-IR (b), serum glucose (c), serum triglycerides (d), serum total cholesterol levels (e) and serum ApoB (f). The linear regression model was adjusted by age, gender, BMI percentile and sampling calendar year. P-values were corrected by FDR.
Cross-sectional study
The cross-sectional study included 554 children with available fasting metabolomics data, and with the following weight status distribution: 7.4% underweight, 51.4% normal weight, 31.9% overweight and 9.2% obese. Their clinical characteristics are summarized in Supplemental Table 4. In consistency with the observations in the case-control study, in this study, PC2-Z was associated with obesity (OR = 1.18; 95%CI 1.05–1.34, P = 9.3 × 10−3), BMI percentile, fat mass percentage, insulin, and TG and levels (all P < 0.05; Supplemental Table 5). PC2-Z was also associated with HDL cholesterol and GGT levels (all P < 0.05; Supplemental Table 5). On stratifying by weight status, the association of PC2-Z with serum TG and GGT was nominally significant, and remained close to significance after FDR correction in all groups (P < 0.17), except for underweight children (Supplemental Table 6). The associations of PC2-Z with serum insulin and HDL-cholesterol levels did not reach significance in any weight status group after FDR correction (P > 0.16; Supplemental Table 6).
Longitudinal cohort
The longitudinal cohort included children followed for 2 years and with the following weight status distribution at baseline: 4.9% underweight, 53.8% normal weight, 16.6% overweight and 24.6% obese. Clinical characteristics measured at baseline and after 2 years are described in Supplemental Table 7. Height, body weight, BMI, fat mass percentage and several biochemical variables (insulin, HOMA-IR, uric acid, ALT and GGT) increased significantly over time. In contrast, systolic BP percentile, glucose, creatinine, total cholesterol and LDL cholesterol, showed a slight but significant decrease after the 2-year follow up (Supplemental Table 7). We used linear regression models to assess the associations of baseline amino acids (represented in PC2) with future metabolic traits. Consistently with the case-control and cross-sectional studies, PC2-Z was significantly associated with 2-year BMI percentile (P = 1 × 10−3), body fat percentage (P = 6 × 10−4; Supplemental Table 8) and after further adjustment for BMI percentile, with serum TG levels (Fig. 2a). The association with TG remained significant even after excluding children with hypertriglyceridemia at baseline (≥130 mg/dL) (P = 1.5 × 10−3; Fig. 2b). PC2-Z also showed a significant positive association with serum insulin levels, although lost significance after correcting for multiple tests (Supplemental Table 8).
Figure 2.
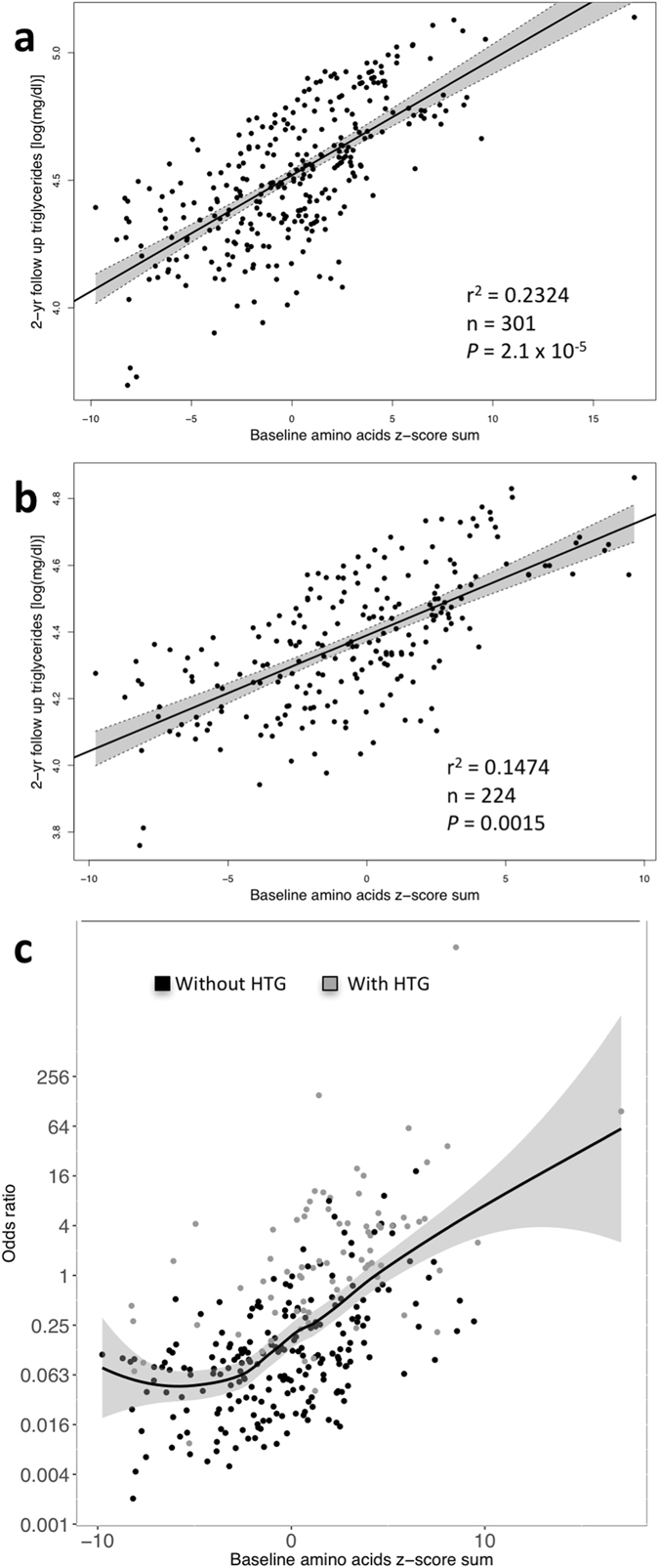
Longitudinal study. Association between z-score sum of baseline amino acids from PC2-Z (arginine, leucine/isoleucine, phenylalanine, tyrosine, valine and proline) and triglycerides after 2 years. (a) All individuals. (b) Individuals without hypertriglyceridemia (HTG) at baseline. (c) Logistic regression model reflecting risk of HTG (with 95% CI) according to PC2-Z and excluding children with HTG at baseline (n = 224). The multivariate model was adjusted by gender, and sampling calendar year as well as baseline age, BMI percentile and serum triglycerides.
Because PC2-Z was associated with TG levels in the three studies, we examined whether this component was associated with future development of hypertriglyceridemia. Seventy-seven children (25%) with hypertriglyceridemia at baseline were excluded from the analysis. After 2 years, 32 children developed hypertriglyceridemia. Figure 2c shows that increased levels of the amino acid signature (as represented by the amino acid Z-score sum) showed association with higher odds of hypertriglyceridemia after 2 years. This association remained significant after adjusting for gender and baseline age, as well as potential confounders such as baseline BMI percentile, HOMA-IR and TG (OR = 1.19; 95%CI 1.03–1.39, P = 0.016; Table 2). This suggests that PC2-Z is an independent risk factor for hypertriglyceridemia. The area under the receiver operating characteristic (ROC) curve (AUC) for PC2-Z was 0.7067 (95%CI 0.6046–0.8088, P < 0.0001), stronger than fat mass percentage, BMI percentile and HOMA-IR (data not shown). Using multivariate logistic regression models, we studied the utility of three different models in the prediction of the development of hypertriglyceridemia over a 2-year period which included: 1) clinical variables (gender, baseline age and BMIp) 2) clinical and biochemical variables (baseline HOMA-IR and TG) 3) clinical and biochemical variables plus the metabolomic signature (Table 3). The three models showed a good fit as evaluated by the Hosmer-Lemeshow test (All P > 0.5). The model including clinical and biochemical variables as well as the metabolomic signature showed the highest prediction value (AUC = 0.811 95%CI 0.726–0.897; Table 3). To evaluate the improvement in the prediction risk by adding the metabolomic signature, we calculated the integrated discrimination index (IDI) and the net reclassification improvement (NRI). The addition of the metabolomic signature to the model showed a 7% net reclassification improvement as well as significant IDI (P < 0.01: Table 3).
Table 2.
Associations between baseline amino acid signature and odds of hypertriglyceridemia, adjusting for gender and baseline age, BMI percentile, HOMA and serum TG.
| Model | Adjustments | OR | 95% Confidence interval | ||
|---|---|---|---|---|---|
| Lower CI | Upper CI | P value | |||
| 1 | Age and gender | 1.25 | 1.09 | 1.44 | 0.0019 |
| 2 | Model 1 + BMI percentile | 1.23 | 1.07 | 1.42 | 0.0040 |
| 3 | Model 2 + Baseline HOMA | 1.22 | 1.07 | 1.42 | 0.0040 |
| 4 | Model 3 + Baseline serum TG | 1.19 | 1.03 | 1.39 | 0.0160 |
All models were adjusted by sampling calendar year. Significant P-values are shown in bold.
Table 3.
Comparison of models for prediction of development of hypertrigliceridemia over 2 years in children with normal TG levels at baseline.
| AUC (95% CI) | P value | NRI | P value | IDI | P value | H-L | P value | |
|---|---|---|---|---|---|---|---|---|
| Model 1: Gender, age and BMI percentile | 0.757 (0.669–0.845) | 1.7 × 10 −6 | 11.49 | 0.175 | ||||
| Model 2: Model 1 + Baseline HOMA-IR and serum TG | 0.796 (0.712–0.880) | 4.7 × 10 −8 | 20% | 0.00045 | 6.0% | 0.0058 | 10.35 | 0.242 |
| Model 3: Model 2 + Amino acids Z-score sum | 0.811 (0.726–0.897) | 9.4 × 10 −9 | 7% | 0.00356 | 4.5% | 0.0047 | 6.62 | 0.578 |
H-L: Hosmer-Lemeshow χ2 statistics, NRI: net reclassification improvement, IDI: integrated discrimination index.
When analyzing amino acids composing PC2-Z individually, phenylalanine showed the strongest association with hypertriglyceridemia risk after adjusting for potential confounders (OR = 2.12; 95%CI 1.24–3.81, P = 0.0094; Supplemental Table 9). Moreover, the AUC for phenylalanine was similar to that of the entire amino acid signature (AUC = 0.6901; 95%CI 0.5806–0.7960, P < 0.0001).
Discussion
In the last decades, the prevalence of obesity has increased significantly in children. This epidemic has resulted in a large population of children and adolescents with obesity-associated metabolic complications such as dyslipidemia4, an independent risk factor for atherosclerosis. Early identification of children with a high risk of developing dyslipidemia is crucial to establish preventive measures to decrease cardiovascular risk in adulthood. We used a targeted metabolomics approach to identify metabolomic profiles associated with obesity, metabolic complications and increased cardiovascular risk. In the case-control study, the major metabolomic amino acid signature (PC2: arginine, leucine/isoleucine, phenylalanine, tyrosine, valine and proline) was associated with obesity, insulin resistance and lipid levels. This is consistent with previous reports where BCAA (leucine, isoleucine and valine) and AA (phenylalanine, tyrosine, tryptophan) were associated with obesity and insulin resistance in both adults and children12, 15, 24, 25. Although less consistently, high serum levels of proline and arginine have been observed in obese and hyperlipidemic adults11, 26. In our study, because VIF values for the latter amino acids were low, collinearity is unlikely to explain their association with obesity risk. However, further studies are needed to confirm whether proline and arginine are in fact associated with a higher obesity risk.
In the cross-sectional study, PC2-Z was associated with a higher risk for obesity and with higher BMI percentile and TG levels. Interestingly, the amino acid signature was associated significantly or as a trend (P < 0.1) with hepatic enzyme levels (ALT, AST and GGT). Increased BCAA serum levels have been observed in adults with non-alcoholic fatty liver disease (NAFLD)27, possibly by contributing to hepatic mitochondrial dysfunction28. Given that accurate, noninvasive biomarkers of liver disease in children are still needed29, and although we did not assess NAFLD in our children, it would be of interest to test whether this PC2 amino acid signature could add to improve prediction scores for NAFLD and other liver diseases.
Serum BCAA and AA levels have also been associated with future insulin resistance and dyslipidemia13, 16, 30, 31. Our longitudinal cohort demonstrated that the amino acid signature was not only associated with future BMI percentile but also with future hypertriglyceridemia risk, independently of gender and baseline BMI percentile, age and TG levels. These results are consistent with a recent 7-year follow up study of 396 girls where serum leucine levels predicted hypertriglyceridemia in early adulthood32. Interestingly in our longitudinal study 14% of the children developed hypertriglyceridemia in just 2 years, in contrast to the 13% developed in Finnish girls in seven years, highlighting the need of early biomarkers to prevent this disease. Our results show that not only leucine but a cluster of amino acids can predict hypertriglyceridemia in a much shorter time span, in both boys and girls.
Although, functionally it has not been established whether higher serum amino acids are biologically involved in the development of hypertriglyceridemia in humans. A recent study in rodents suggests that increased serum amino acid levels may have a causal role33. This study demonstrated that hepatic amino acid signaling regulates systemic lipid metabolism via a neuronal pathway, decreasing adipose lipoprotein lipase expression, thus suppressing plasma TG-hydrolysis activity and raising circulating TG levels. In the present study, the lack of association of the amino acid signature with serum ApoB, the major apolipoprotein contained in VLDL particles, is consistent with decreased TG clearance by peripheral tissues, rather than with higher TG exportation from the liver. Although further studies are need to fully elucidate the mechanism behind this effect, these amino acids could represent new targets for the prevention of dyslipidemia in children and ultimately future cardiovascular disease.
Circulating BCAA concentrations have been previously associated with future insulin resistance in children12, 32. However, while we observed an association of amino acids (PC2-Z) with HOMA-IR in the case control and cross-sectional studies, in the longitudinal study the association with future HOMA-IR did not reach statistical significance. This result could be confounded by effects of pubertal growth, as Tanner staging in children aged 9–11 has been associated with a decrease in insulin sensitivity34. Indeed, a limitation of our study is that Tanner staging was not assessed, so this result should be interpreted with caution.
Our study has other limitations that should be acknowledged. The diet of most of the participants was not assessed, and thus it was not possible to evaluate whether certain nutrients contribute to the serum amino acid levels variation. Moreover, it has been suggested that gut microbiota could also affect serum amino acid levels35, and thus should be included in the design of future studies. Finally, our study used a targeted metabolomics approach, and non-targeted approaches are still required to identify new metabolites associated with obesity and obesity-related complications.
The primary strength of this study is that, to the best of our knowledge, it is the first to include a large sample of children using tree different study designs, including validation in a longitudinal cohort. Moreover, this approach identified a metabolomics signature associated with obesity, which also showed predictive value for hypertriglyceridemia in Mexican children. It is important to mention that before attempting to translate these results into precision or personalized medicine; certain points need to be addressed. In addition to replication studies, pre-analytical methods need to be standardized, and normal levels of individual metabolites should be established in different biofluids, including total blood, plasma and serum.
In conclusion, this study shows that the amino acid signature composed of arginine, leucine/isoleucine, phenylalanine, tyrosine, valine and proline is associated with obesity, and is an independent risk factor and predictor of future hypertriglyceridemia in children.
Electronic supplementary material
Acknowledgements
We thank Hugo Tovar for his assistance in figures and Luz E. Guillén for her technical assistance. This work was supported by CONACYT grants #248765 to SM-R, 113861 to SC-Q and Programa de Apoyo a Proyectos de Investigación Tecnológica (PAPIIT-UNAM IA202413) to SC-Q.
Author Contributions
S.-M.R. and S.-C.Q. conceived the experiments, E.-O.M., M.-V.A., A.-H.V. and I.-I.G. performed metabolomics analysis, E.-O.M., R.-G.A., L.-M.K. and S.-M.R. performed the data analysis and interpretation, H.-V.R., B.-L.C., P.-L.M., J.-V.B., R.-G.V., R.-V.V., E.-S.C., B.R. contributed to data collection and database generation, C.-A.S. performed biochemical measurements, S.-M.R., R.-G.A., L.-M.K., T.-V.M., S.-C.Q. wrote the manuscript. All authors read and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05765-4
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sofia Moran-Ramos, Email: smoran@inmegen.gob.mx.
Samuel Canizales-Quinteros, Email: cani@unam.mx.
References
- 1.Gutierrez, J. et al. Encuesta Nacional de Salud y Nutricion 2012, Resultados Nacionales. (2012).
- 2.Jellinger PS, et al. American Association of Clinical Endocrinologists’ Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocr Pract. 2012;18(Suppl 1):1–78. doi: 10.4158/EP.18.S1.1. [DOI] [PubMed] [Google Scholar]
- 3.WHO. Global Status report on noncommunicable diseases 2014. (2015). [DOI] [PubMed]
- 4.Cook, S. & Kavey, R. E. Dyslipidemia and pediatric obesity. Pediatr Clin North Am58, 1363-1373, ix, doi:10.1016/j.pcl.2011.09.003 (2011). [DOI] [PMC free article] [PubMed]
- 5.Bibiloni, Mdel, M. et al. Serum lipid levels and dyslipidaemia prevalence among 2-10 year-old Northern Mexican children. PLoS One10, e0119877, doi:10.1371/journal.pone.0119877 (2015) [DOI] [PMC free article] [PubMed]
- 6.Miller M, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123:2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 7.Juonala M, et al. Associations of dyslipidemias from childhood to adulthood with carotid intima-media thickness, elasticity, and brachial flow-mediated dilatation in adulthood: the Cardiovascular Risk in Young Finns Study. Arterioscler Thromb Vasc Biol. 2008;28:1012–1017. doi: 10.1161/ATVBAHA.108.163329. [DOI] [PubMed] [Google Scholar]
- 8.Pires A, Sena C, Seica R. Dyslipidemia and cardiovascular changes in children. Curr Opin Cardiol. 2016;31:95–100. doi: 10.1097/HCO.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 9.Lynch CJ, Adams SH. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol. 2014;10:723–736. doi: 10.1038/nrendo.2014.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott P, et al. Urinary metabolic signatures of human adiposity. Sci Transl Med. 2015;7:285ra262. doi: 10.1126/scitranslmed.aaa5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCormack SE, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8:52–61. doi: 10.1111/j.2047-6310.2012.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hellmuth C, et al. Tyrosine Is Associated with Insulin Resistance in Longitudinal Metabolomic Profiling of Obese Children. J Diabetes Res. 2016;2016:2108909. doi: 10.1155/2016/2108909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guasch-Ferre M, et al. Metabolomics in Prediabetes and Diabetes: A Systematic Review and Meta-analysis. Diabetes Care. 2016;39:833–846. doi: 10.2337/dc15-2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho JE, et al. Metabolomic Profiles of Body Mass Index in the Framingham Heart Study Reveal Distinct Cardiometabolic Phenotypes. PLoS One. 2016;11:e0148361. doi: 10.1371/journal.pone.0148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mook-Kanamori DO, et al. Increased amino acids levels and the risk of developing of hypertriglyceridemia in a 7-year follow-up. J Endocrinol Invest. 2014;37:369–374. doi: 10.1007/s40618-013-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuczmarski, R. J. et al. CDC growth charts: United States. Adv Data, 1-27 (2000). [PubMed]
- 18.Lohman, T. G., Roche, A. F. & Martorell, R. Anthropometric standardization reference manual. (1988).
- 19.National High Blood Pressure Education Program Working Group on High Blood Pressure in, C. & Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics114, 555-576 (2004). [PubMed]
- 20.Expert Panel on Integrated Guidelines for Cardiovascular, H., Risk Reduction in, C., Adolescents, National Heart, L. & Blood, I. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: summary report. Pediatrics128 Suppl 5, S213-256, doi:10.1542/peds.2009-2107C (2011). [DOI] [PMC free article] [PubMed]
- 21.Le S, Josse J, Husson F. FactoMineR: An R package for multivariate analysis. J Stat Softw. 2008;25:1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 22.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–1711. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- 24.Newbern D, et al. Sex differences in biomarkers associated with insulin resistance in obese adolescents: metabolomic profiling and principal components analysis. J Clin Endocrinol Metab. 2014;99:4730–4739. doi: 10.1210/jc.2014-2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butte NF, et al. Global metabolomic profiling targeting childhood obesity in the Hispanic population. Am J Clin Nutr. 2015;102:256–267. doi: 10.3945/ajcn.115.111872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, Q. et al. Amino Acid and Biogenic Amine Profile Deviations in an Oral Glucose Tolerance Test: A Comparison between Healthy and Hyperlipidaemia Individuals Based on Targeted Metabolomics. Nutrients8, doi:10.3390/nu8060379 (2016). [DOI] [PMC free article] [PubMed]
- 27.Cheng S, et al. Adipose Tissue Dysfunction and Altered Systemic Amino Acid Metabolism Are Associated with Non-Alcoholic Fatty Liver Disease. PLoS One. 2015;10:e0138889. doi: 10.1371/journal.pone.0138889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sunny NE, et al. Cross-talk between branched-chain amino acids and hepatic mitochondria is compromised in nonalcoholic fatty liver disease. Am J Physiol Endocrinol Metab. 2015;309:E311–319. doi: 10.1152/ajpendo.00161.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koot BG, et al. Accuracy of prediction scores and novel biomarkers for predicting nonalcoholic fatty liver disease in obese children. Obesity (Silver Spring) 2013;21:583–590. doi: 10.1002/oby.20173. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Canela M, et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin Chem. 2016;62:582–592. doi: 10.1373/clinchem.2015.251710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamakado M, et al. Plasma Free Amino Acid Profiles Predict Four-Year Risk of Developing Diabetes, Metabolic Syndrome, Dyslipidemia, and Hypertension in Japanese Population. Sci Rep. 2015;5:11918. doi: 10.1038/srep11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiklund P, et al. Serum Amino Acid Profiles in Childhood Predict Triglyceride Level in Adulthood: A 7-Year Longitudinal Study in Girls. J Clin Endocrinol Metab. 2016;101:2047–2055. doi: 10.1210/jc.2016-1053. [DOI] [PubMed] [Google Scholar]
- 33.Uno K, et al. A hepatic amino acid/mTOR/S6K-dependent signalling pathway modulates systemic lipid metabolism via neuronal signals. Nat Commun. 2015;6:7940. doi: 10.1038/ncomms8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen HK, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535:376–381. doi: 10.1038/nature18646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


