Abstract
Proton pump inhibitors (PPIs) are widely used to treat gastric acid-related disorders. Concerns have been raised about potential fracture risk, especially at the hip, spine and wrist. However, fracture risk at other bone sites has not been as well studied. We investigated the association between PPIs and specific fracture sites using an aggregated knowledge-enhanced database, the Food and Drug Administration Adverse Event Reporting System Data Mining Set (AERS-DM). Proportional reporting ratio (PRR) was used to detect statistically significant associations (signals) between PPIs and fractures. We analyzed both high level terms (HLT) and preferred terms (PT) for fracture sites, defined by MedDRA (Medical Dictionary for Regulatory Activities). Of PPI users reporting fractures, the mean age was 65.3 years and the female to male ratio was 3.4:1. Results revealed signals at multiple HLT and PT fracture sites, consistent for both sexes. These included fracture sites with predominant trabecular bone, not previously reported as being associated with PPIs, such as ‘rib fractures’, where signals were detected for overall PPIs as well as for each of 5 generic ingredients (insufficient data for dexlansoprazole). Based on data mining from AERS-DM, PPI use appears to be associated with an increased risk for fractures at multiple sites.
Introduction
Proton pump inhibitors (PPIs) are acid suppressive agents used for managing gastric acid-related disorders, such as gastroesophageal reflux disease and peptic ulcers1–3. PPIs are among the most widely prescribed drugs; in the United States (US), PPIs were the third largest-selling therapeutic class and the 6th most widely dispensed retail prescription medications in 20084.
The first PPI introduced, omeprazole, has been on the pharmaceutical market since 1989. Subsequently, lansoprazole, rabeprazole and pantoprazole successively entered into clinical practice5. In 2001, esomeprazole, a left-handed (S)-isomer of omeprazole, was introduced and then was widely used, ranking 4th in the top 20 drug list by sales in the global market in 20126, 7. The newest PPI, dexlansoprazole, a right-handed (R)-isomer of lansoprazole, was approved in the US in 20097.
In recent years, concerns have been raised about potential adverse drug events (ADEs) associated with chronic PPI use, including fractures, hypomagnesaemia, interstitial nephritis, iron and vitamin B12 malabsorption, and infections8. Among these ADEs, fractures have received increasing attention since 2006 when Vestergaard et al.9 and Yang et al.10 reported that PPI use was associated with an increased risk of fractures, specifically at the proximal femur (hip) 9, 10 and spine9. Since then, several studies have explored the association between PPIs and fracture risk10–16. The most recent meta-analysis has suggested that there is an increased risk of fractures at the hip, spine and overall fractures with PPI use3. However, results from some studies do not support an association between PPI and bone fragility17. In May 2010, the US Food and Drug Administration (FDA) issued the warning that PPI use had a possible increased risk of fractures at the hip, spine and distal forearm (wrist)18.
However, the risk of chronic PPI use for fractures at other specific sites has not been as well determined. Studies that examined the association between PPIs and fractures other than at the hip, spine, and wrist have reported only on overall fracture risk, i.e., overall (any) fracture9, any-site fractures19–25. One paper focusing on fractures in hepatitis patients using PPIs did list specific fracture sites, however, only the association with overall fracture risk was reported26.
Spontaneous reporting systems (SRSs), primarily used for postmarketing drug safety surveillance before epidemiological investigation, can provide additional information about ADEs, including fractures. Data mining algorithms have been developed for signal detection in SRSs for ADEs, where a ‘signal’ means a possible association between a drug and an ADE27. One of the popular signal detection methods is proportional reporting ratio (PRR)28, typically calculated to summarize the extent to which a particular adverse event is associated with a specific drug, compared to the background rate at which the same adverse event is associated with other drugs29. PRR is computed by building a 2×2 contingency table28, 30. The number of reports with the drug of interest and the event is defined as a. The number of reports with the drug of interest and without the event is assigned as b. The number of reports with drugs other than the drug of interest and with the event is defined as c. The number of reports with drugs other than the drug of interest and without the event is defined as d. A signal is detected when the reports of the drug associated with ADEs are 3 or more, and the PRR is at least 2 with the chi-squared of 4 or more28
| 1 |
AERS-DM (Adverse Event Reporting System Data Mining Set) is a normalized knowledge-enhanced data mining set31 of FDA’s Adverse Event Reporting System (FAERS), that is one of the commonly used databases to detect signals through data mining algorithms32, 33. Compared to FAERS, AERS-DM is more conducive to data mining with normalization and aggregation34. Based on this large-scale data set, we previously detected 668 drugs used in the 20 most frequent treatment regimens for the most common conditions in the US, and revealed sex differences in ADEs among 307 drugs corresponding to 736 statistically significant signals, some of which have been reported in drug labels and verified35. We also built a knowledge base of severe adverse events leveraging AERS-DM and semantic web technologies36. In another study, we have also demonstrated the feasibility to integrate AERS-DM with electronic health records (EHRs), and profiled ADEs of cancer drug ingredients using information from the Mayo Clinic EHRs37.
In this study, we aim to leverage the unique resources of AERS-DM to investigate the association between PPIs and ADEs reported at specific fracture sites using the data mining method PRR.
Results
Among 2,459,001 reports in AERS-DM, 169,563 included at least one generic ingredient of PPIs, of which 3,782 are associated with fractures. Omeprazole, among PPIs, was the most frequently reported generic ingredient associated with all ADEs, including fractures, accounting for 33.7% and 34.0% of total reports and fracture reports, respectively, followed by esomeprazole (Table 1).
Table 1.
Characteristics of the 169,563 MedDRA Adverse Event Reports involving at least one proton pump inhibitor (PPI) ingredient, comparing reports with a fracture and all reports with ADEs.
| Characteristic | Reports with a fracture (%) N = 3,782 | All reports (%) N = 169,563 |
|---|---|---|
| Age (median), years | ||
| 0–19 (7) | 14 (0.4%) | 3,466 (2.0%) |
| 20–29 (24) | 24 (0.6%) | 3,953 (2.3%) |
| 30–39 (36) | 87 (2.3%) | 7,706 (4.5%) |
| 40–49 (46) | 271 (7.2%) | 16,783 (9.9%) |
| 50–59 (55) | 627 (16.6%) | 28,932 (17.1%) |
| 60–69 (64) | 809 (21.4%) | 33,086 (19.5%) |
| 70–79 (75) | 768 (20.3%) | 27,397 (16.2%) |
| 80 + (84) | 510 (13.5%) | 15,427 (9.1%) |
| missing | 672 (17.8%) | 32,813 (19.4%) |
| Self-report | 438 (11.58%) | 15,273 (9.01%) |
| Females (%) | 2,895 (76.5%) | 100,192 (59.1%) |
| Self-report by female | 361 | 10,652 |
| Males (%) | 851 (22.5%) | 65432 (38.6%) |
| Self-report by male | 77 | 4,553 |
| Active PPI Ingredient* | ||
| Omeprazole | 1,421 (34.0%) | 59,356 (33.7%) |
| Esomeprazole | 1,220 (29.2%) | 49,678 (28.2%) |
| Pantoprazole | 748 (17.9%) | 30,142 (17.1%) |
| Lansoprazole | 607 (14.5%) | 29,486 (16.7%) |
| Rabeprazole | 180 (4.3%) | 7,552 (4.3%) |
| Dexlansoprazole | 9 (<0.1%) | 59 (<0.1%) |
*More than one generic ingredient could be reported in the reports.
Information was available on sex in 3,742 (99%) PPI fracture reports and on age in 3,110 (82%). The mean age was 65.3 years (range: < 1 to ~99 years) and the ratio of females to males was 3.4:1. The age group of 60 to 69 years had the most ADE reports on fractures (21.4%) (Table 1). An increasing trend was observed for the reporting of fractures with PPI use and its percentage among all PPI-associated ADEs, especially after 2010 when the FDA black box warning was issued (Fig. 1).
Figure 1.
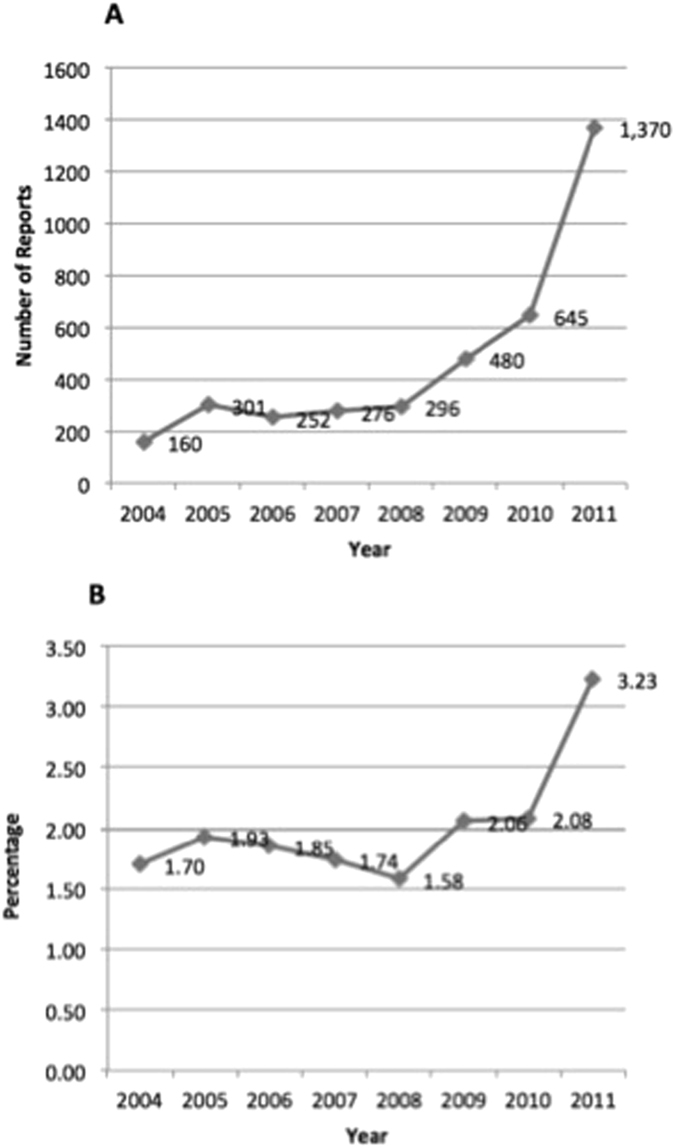
Increasing trend of proton pump inhibitor (PPI)-fracture reports from 2004 to 2011. (A) Reports of PPI with fractures. (B) Percentage of PPI-fracture reports among all PPI-AEs reports.
At the drug class-ADE class level, there were signals detected for 4 of the 8 HLT fracture sites ‘thoracic cage fractures non-spinal’, ‘pelvic fractures’, ‘pathological fractures and complications’ and ‘spinal fractures’ (Table 2), of which the first three HLT categories of fracture sites have not previously been specifically reported. When analyses were stratified by age group, these signals were consistently observed in the 50–69 years and ≥ 70 years age groups but not in the age group ≤ 49 years.
Table 2.
Signal detection between any proton pump inhibitor (PPI) and reported fracture adverse events as classified by MedDRA’s 8 High Level Terms (HLT) and corresponding 61 Preferred Terms (PT), by overall and age groups.
| Fracture Site | Proportional Rate Ratio (PRR) by Age Groups (years) | |||||||
|---|---|---|---|---|---|---|---|---|
| Overall | ≤49 | 50–69 | ≥70 | |||||
| N | PRR(χ2) | N | PRR(χ2) | N | PRR(χ2) | N | PRR(χ2) | |
| Skull and face fractures | 113 | 1.7(29.5) | 22 | 1.2(0.6) | 35 | 1.9(12.1) | 39 | 2.3(25.5) |
| Skull fractured base | 8 | 2.8(7.9) | 2 | 2.1(1.0) | 3 | 5.1(7.1) | 3 | 3.7(4.6) |
| Skull fracture | 24 | 1.2(0.7) | 8 | 1.2(0.2) | 5 | 0.9(<0.1) | 7 | 1.5(1.1) |
| Facial bones fracture | 81 | 1.8(25.7) | 12 | 1.0(<0.1) | 27 | 2.0(12.2) | 29 | 2.5(22.5) |
| Fractured skull depressed | 0 | — | 0 | — | 0 | — | 0 | — |
| Thoracic cage fractures non-spinal (excl pathological) | 437 | 2.5(323.5) | 52 | 1.6(11.8) | 165 | 2.6(139.0) | 137 | 2.9(143.0) |
| Sternal fracture | 22 | 1.9(9.5) | 6 | 2.8(5.7) | 11 | 3.1(12.7) | 3 | 1.0(<0.1) |
| Rib fracture | 423 | 2.5(325.4) | 47 | 1.6(8.9) | 160 | 2.6(136.9) | 135 | 3.0(151.3) |
| Flail chest | 2 | 2.5(1.5) | 0 | — | 1 | 13.5(5.8) | 1 | 2.3(0.6) |
| Spinal fractures (excl pathological) | 517 | 2.2(290.7) | 52 | 1.6 (9.3) | 181 | 2.6(155.4) | 188 | 2.4(136.5) |
| Spinal fracture | 323 | 2.3(209.9) | 30 | 1.6(5.4) | 108 | 2.9(112.0) | 117 | 2.5(91.4) |
| Cervical vertebral fracture | 50 | 1.8(16.3) | 7 | 1.3(0.5) | 14 | 1.9(5.3) | 17 | 2.5(12.6) |
| Thoracic vertebral fracture | 58 | 2.6(47.7) | 4 | 1.1(0.1) | 26 | 3.2(31.7) | 21 | 2.9(21.0) |
| Lumbar vertebral fracture | 68 | 1.9(27.3) | 7 | 1.7(1.9) | 29 | 2.6(23.5) | 24 | 1.9(8.7) |
| Fractured sacrum | 43 | 3.6(64.0) | 5 | 3.1(5.7) | 15 | 3.4(20.1) | 18 | 5.0(41.6) |
| Fractured coccyx | 13 | 1.2(0.6) | 3 | 1.2(0.1) | 4 | 1.1(<0.1) | 3 | 1.1(<0.1) |
| Pelvic fractures | 200 | 2.1(98.4) | 11 | 1.0(<0.1) | 54 | 2.2(30.3) | 90 | 2.3(55.1) |
| Pelvic fracture | 160 | 1.9(62.6) | 10 | 1.2(0.2) | 40 | 2.0(18.0) | 70 | 1.9(29.0) |
| Ilium fracture | 4 | 1.4(0.3) | 0 | — | 2 | 1.8(0.6) | 1 | 1.4(0.1) |
| Pubis fracture | 26 | 3.8(41.5) | 0 | — | 11 | 4.4(21.6) | 13 | 4.2(24.0) |
| Acetabulum fracture | 14 | 3.6(21.1) | 1 | 0.9(0.01) | 4 | 2.8(3.9) | 7 | 7.9(26.6) |
| Fractured ischium | 2 | 1.0(<0.1) | 0 | — | 1 | 1.2(<0.1) | 1 | 1.5(0.2) |
| Upper limb fractures | 768 | 1.8(249.3) | 87 | 1.2(3.4) | 287 | 1.9(123.5) | 270 | 2.4(179.5) |
| Upper Limb fracture | 282 | 1.7(69.6) | 22 | 0.9(0.3) | 106 | 1.8(34.2) | 108 | 2.5(81.9) |
| Clavicle fracture | 57 | 1.7(15.1) | 11 | 1.3(0.9) | 20 | 1.9(8.2) | 15 | 2.1(7.8) |
| Scapula fracture | 14 | 2.7 (12.5) | 3 | 2.5(2.3) | 8 | 5.4(20.5) | 2 | 1.6(0.4) |
| Humerus fracture | 114 | 2.2(61.4) | 10 | 1.3(0.6) | 44 | 2.5(32.7) | 41 | 2.3(25.7) |
| Forearm fracture | 10 | 1.8(3.3) | 2 | 2.1(1.0) | 6 | 4.3(11.4) | 2 | 1.1(<0.1) |
| Radius fracture | 65 | 2.6(51.6) | 10 | 1.9(3.9) | 23 | 2.4(15.8) | 26 | 3.6(38.3) |
| Ulna fracture | 30 | 3.0(32.3) | 5 | 2.3(3.2) | 17 | 5.3(43.0) | 4 | 1.3(0.3) |
| Wrist fracture | 205 | 1.9(78.7) | 21 | 1.5(3.2) | 75 | 1.9(28.4) | 82 | 2.6(66.4) |
| Hand fracture | 100 | 1.9(38.9) | 24 | 1.8(7.3) | 34 | 1.9(11.7) | 20 | 2.3(12.9) |
| Scapulothoracic dissociation | 0 | — | 0 | — | 0 | — | 0 | — |
| Lower limb fractures | 1714 | 1.5(270.0) | 161 | 1.1(1.6) | 662 | 1.7(177.0) | 609 | 1.9(237.7) |
| Lower limb fracture | 202 | 1.4(24.0) | 25 | 0.9(0.1) | 94 | 1.8(30.7) | 41 | 1.6(7.8) |
| Hip fracture | 497 | 1.7(136.7) | 23 | 1.4(2.4) | 121 | 1.6(24.4) | 264 | 2.3(159.9) |
| Femoral neck fracture | 81 | 1.5(12.5) | 4 | 1.0(0.0) | 25 | 2.1(13.1) | 43 | 1.5(5.8) |
| Femur fracture | 454 | 1.3(24.8) | 32 | 1.4(2.9) | 203 | 1.6(40.2) | 177 | 1.7(44.1) |
| Patella fracture | 35 | 1.7(8.2) | 3 | 0.9(0.1) | 17 | 2.1(8.3) | 10 | 1.9(3.4) |
| Tibia fracture | 92 | 1.9(37.8) | 9 | 0.8(0.6) | 42 | 2.1(21.8) | 29 | 3.6(42.3) |
| Fibula fracture | 71 | 2.2(39.7) | 11 | 1.4(0.9) | 34 | 2.4(23.1) | 14 | 2.5(10.8) |
| Ankle fracture | 223 | 1.5(31.1) | 22 | 0.6(4.7) | 103 | 1.6(23.3) | 42 | 1.8(12.4) |
| Foot fracture | 291 | 1.8(97.1) | 56 | 1.5(8.4) | 149 | 2.2(79.3) | 39 | 1.9(15.8) |
| Fractures NEC (excl pathological) | 638 | 1.9(277.9) | 80 | 1.7(18.8) | 269 | 2.6(225.7) | 185 | 2.3(115.8) |
| Periprosthetic fracture | 4 | 4.9(9.1) | 0 | — | 2 | 3.9(3.3) | 2 | 13.5(11.6) |
| Fracture displacement | 18 | 4.2(33.4) | 1 | 2.7(0.9) | 8 | 3.6(11.9) | 8 | 5.4(20.5) |
| Compression fracture | 169 | 3.7(256.9) | 17 | 4.2(31.4) | 73 | 5.4(185.3) | 53 | 2.9(54.3) |
| Bone fragmentation | 28 | 3.6(41.5) | 2 | 1.7(0.5) | 18 | 5.5(47.3) | 4 | 1.9(1.4) |
| Avulsion fracture | 5 | 2.7(4.5) | 1 | 1.4(0.1) | 2 | 3.9(3.3) | 2 | 4.5(4.1) |
| Jaw fracture | 77 | 2.5(61.0) | 12 | 1.7(3.2) | 37 | 2.9(38.5) | 17 | 3.1(20.2) |
| Bone fissure | 6 | 1.3(0.3) | 1 | 2.3(0.6) | 1 | 0.4(0.7) | 3 | 2.5(2.3) |
| Complicated fracture | 3 | 3.5(0.1) | 0 | — | 2 | 13.5(11.6) | 1 | 3.4(1.3) |
| Epiphyseal fracture | 1 | 3.4(1.3) | 1 | 3.4(1.3) | 0 | — | 0 | — |
| Fracture | 168 | 1.3(13.9) | 12 | 1.0(0.0) | 64 | 2.3(38.1) | 51 | 1.6(11.4) |
| Impacted fracture | 1 | 2.7(0.9) | 0 | — | 0 | — | 0 | — |
| Multiple fractures | 59 | 1.7(15.0) | 8 | 1.0(0.0) | 23 | 2.2(13.0) | 18 | 2.5(14.1) |
| Open fracture | 13 | 1.9(5.1) | 3 | 1.6(0.5) | 3 | 1.3(0.2) | 3 | 2.5(2.3) |
| Stress fracture | 116 | 1.9(40.8) | 17 | 3.1(19.7) | 55 | 2.0(24.0) | 30 | 2.5(23.7) |
| Torus fracture | 1 | 3.4(1.3) | 1 | 4.5(2.0) | 0 | — | 0 | — |
| Traumatic fracture | 11 | 1.5(1.9) | 0 | — | 4 | 1.5(0.6) | 4 | 2.2(2.2) |
| Greenstick fracture | 0 | — | 0 | — | 0 | — | 0 | — |
| Pathological fractures and complications | 277 | 2.6(223.3) | 32 | 1.8(10.3) | 119 | 2.7(103.8) | 94 | 3.6(137.4) |
| Pathological fracture | 188 | 3.1(218.6) | 20 | 2.5(14.9) | 78 | 3.2(93.5) | 72 | 4.3(137.6) |
| Pseudarthrosis | 8 | 2.3(5.3) | 3 | 4.1(5.3) | 4 | 3.2(4.8) | 1 | 1.1(<0.1) |
| Osteoporotic fracture | 31 | 2.3(19.7) | 5 | 1.2(0.1) | 7 | 2.3(4.4) | 12 | 3.7(18.4) |
| Fracture nonunion | 39 | 2.2(21.8) | 4 | 1.6(0.9) | 24 | 2.3(15.8) | 6 | 2.2(3.3) |
| Fracture malunion | 2 | 0.8(0.1) | 0 | — | 1 | 0.8(<0.1) | 0 | — |
| Fracture delayed union | 20 | 1.7(5.1) | 1 | 0.9(0.01) | 11 | 1.7(3.1) | 7 | 2.3(4.2) |
| Synostosis | 1 | 0.5(0.6) | 0 | — | 0 | — | 1 | — |
Around 82% of reports have age information. Bold indicates statistically significant signals.
Analyses at the drug class-ADEs level yielded signals at 22 of the 61 PT fracture sites, which were represented under all 8 HLTs (Table 2). Drug class-ADEs signals for the PT ‘rib fracture’ primarily contributed to the drug class-ADE class signal of the HLT ‘thoracic cage fractures non-spinal’. While the signal for the drug class-ADE class HLT ‘upper limb fractures’ did not reach statistical significance (PRR = 1.8), several PT fracture sites under this category either did show a signal or had a PRR that approached a statistically significant signal, including for the PT ‘wrist fracture’ (PRR = 1.9). Furthermore, a signal was more likely to be observed at more PT sites within the HLT ‘upper limb fractures’, including for ‘wrist fracture’ and ‘humerus fracture’ when considering the two older age groups (Table 2). Similarly, within the HLT ‘lower limb fractures’, signals were more likely at PT sites in the two older age groups. Specifically, a signal was observed for ‘hip fracture’ in the ≥ 70 years group (PRR = 2.3) and for ‘femoral neck fracture’ (PRR = 2.1) in the 50–69 years group. Lastly, within the HLT ‘fractures NEC’ there was a signal in all age groups for the PT ‘compression fracture’, while there was also a trend for signals in the PT ‘stress fracture’. These two PTs were the most common fracture sites within this HLT.
Supplementary Table S1 provides additional details on signals detected in females and males, separately, for the 8 HLTs and corresponding 61 PTs. The majority of signals observed for the PT fracture sites tended to be consistent between females and males.
For the drugs-ADEs level, dexlansoprazole showed no signals (results not shown) most likely due to the late launch into the market relative to the time period covered in our analyses with fewer corresponding data available, as noted in Table 1. For the remaining five PPIs, there were a total 112 signals detected corresponding to 42 PT sites of fractures. Among these PT fracture sites, ‘rib fracture’, ‘pathological fracture’ and ‘compression fracture’ each showed signals for all five PPIs (Supplementary Table S2). There was also a trend for signals at the overall HLTs ‘upper limb fractures’ and ‘spinal fractures’, as well as the PTs ‘hip fracture’ and ‘stress fracture’ for all five PPIs.
Discussion
To the best of our knowledge, this is the first study to explore associations between PPI use and fracture risk at all specific bone sites through data mining in a large spontaneous reporting database. Although statistically significant signals between PPI and fractures at multiple specific sites were detected in this study, it should be noted that the number of fracture reports was a small proportion (~2–3%) of the ADEs reported with PPIs.
We identified signals between the drug class PPI and three HLT fracture categories, as classified by MedDRA, ‘thoracic cage fractures non-spinal’, ‘pelvic fractures’ and ‘pathological fractures and complications’. Within the HLT ‘thoracic cage fractures non-spinal’, rib fractures were the most relevant. Our findings of a signal with the HLT ‘spinal fractures’ are also consistent with prior reports of an increased risk for spine fractures with PPI use. Similarly, when analyses were stratified by age groups, we identified signals in the older age groups between PPI use and several more specific PT fracture sites, including at the hip, wrist and humerus, typical sites for fragility fractures in this age range. Overall, the signals detected between the use of PPIs and risk for fracture at different sites appeared to be primarily driven by a greater risk in those over age 50 years.
A signal for the PT ‘pathological fractures’ was detected at all levels in this study. MedDRA has no clear definition for ‘pathological fractures’. In other studies, a pathologic fracture is a fracture caused by disease leading to weakness of the bone38, 39. The different processes leading to a pathological fracture may include osteitis, osteogenesis imperfecta, osteomalacia/rickets, Paget’s disease or primary bone tumors/ metastases, although ‘osteoporosis’ has sometimes been included in this list39. Indeed, the PT ‘osteoporotic fracture’ was included in the HLT ‘pathological fractures and complications’. It is not clear what exactly qualified as a pathologic fracture in these reports, but if PPIs have an adverse effect on bone metabolism, it may be exacerbating the risk of a fracture in bones already weakened by a localized malignancy.
The mechanism through which PPIs may increase the risk for fractures remains unclear. Studies have explored the potential effects of PPIs on calcium absorption40, bone mineral density (BMD)41–44, bone metabolism45, 46, increased histamine release47, or their association with fall risk16, 23. In addition, it has been hypothesized that PPI use may be associated with hyperparathyroidism48, which has been recently reported in a cohort of elderly subjects49. We further investigated the association between PPIs and hyperparathyroidism in AERS-DM, and did find a signal (PRR 3.1837, χ2 40.001). It is possible that there may be multiple factors contributing to the association observed between PPIs and increased fractures. That the signals detected in our study seemed to be strongest in bones that were primarily trabecular (e.g., ribs, spine) may be particularly relevant in understanding the apparent adverse effect of PPIs on bone health. Indeed, Maggio et al., did suggest that PPIs may have an adverse effect on trabecular bone in particular50 . We found fracture risk with PPI use was more likely in ages over 50 years. If PPI use is adversely affecting bone metabolism, an increase in fractures may not be detectable in younger ages, where bone strength may still be sufficient enough to protect against fracture from low-to-moderate trauma. Duration of PPI use was not available for our analyses.
With the increase in age of those reporting an ADE, the ratio between female and male reporting fractures also increased. A few existing studies have focused on sex differences between PPI use and risk for fractures, and found that the association was stronger in men10, 14. Other studies have focused on postmenopausal women12, 20, 24, 51–53, children or young adults54. It was found women are also more likely to have fractures than men with increasing age55. Here, we also looked into the self-report and gender distribution in FAERS for PPIs (Table 1). Overall, 15,273 out of all PPI reports, i.e., 9.01%, are self-reported with 438 (0.26%) self-reported fracture cases. 361 out of 438 fracture cases are females. The ratio of females to males is 4.69 for self-reported fracture cases. Among all PPI reports, 100,192 (59.09%) are females with 2,895 (1.71%) female fracture cases. The ratio of females to males is 3.26 for all PPI self-reports. Comparing with the ratio of females to males in FAERS, i.e., 1.58, our results showed more fracture cases in females than males. Nonetheless, the signals we identified at different fracture sites were mostly consistent between females and males. Confounding factors could still exist and further studies are warranted to determine if there are any sex differences between PPI use and risk for fractures.
For all studied fracture sites, we used terms from MedDRA. We found MedDRA still has areas to improve due to its lack of clear definitions for terms. For example, it would be more consistent with clinical practice if the PT term ‘compression fracture’ is included in the HLT ‘spinal fractures’ instead of ‘fractures NEC’, and the PT term ‘jaw fracture’ was included under the HLT ‘skull and face fractures’ instead of ‘fractures NEC’. Furthermore, we note that the PT ‘osteoporotic fracture’ was included under the HLT ‘pathologic fractures and complications’.
In this study, we used AERS-DM as the data source. The features of drug classification and ADE aggregation make it possible to detect signals at different levels35. However, the mined ADE signals do not indicate a causal relationship with PPIs and the incidence cannot be obtained from the spontaneous reporting system due to lacking the number of people exposed to PPIs. In addition, drug-drug interaction cannot be considered. Bias in reporting of ADEs, may also influence results. We did note an increase in reporting of fracture ADE with PPIs following the FDA black box warning in 2010. Confounding by concurrent comorbidities cannot be excluded when using AERS-DM. Therefore, data from randomized controlled trial studies or from other epidemiologic studies are needed to clarify the relationships observed when using AERS-DM. Nevertheless, data mining through such unique resources can provide valuable information on potential ADEs, including fractures, which would then require further investigation.
Conclusions
The current study reveals associations between PPI use and multiple different sites of fractures based on data mining from AERS-DM. Our findings revealed an increased fracture risk at three previously unreported categories of fracture sites including ‘thoracic cage fractures non-spinal’, ‘pelvic fractures’ and ‘pathological fractures and complications’, in addition to the more recognized site of ‘spinal fractures’. Furthermore, signals were identified for multiple different fracture sites beyond these broader categories, including at the hip and wrist, with the PRR for many other sites approaching a statistically significant signal. These findings were primarily observed in individuals who were older, at least over the age of 50 years. Signals identified tended to also be consistent between women and men. These results suggest that PPI use may contribute to an adverse effect on bone metabolism, which may particularly influence trabecular bone. Further work, however, is necessary to better understand how PPI may increase fracture risk. Our findings would generate hypotheses for future experimental studies to investigate potential association between PPIs and risk for fracture at multiple sites, as well as underlying mechanism. Our work also illustrates the advantages of data mining the AERS-DM to help identify drugs that may potentially be associated with fractures.
Methods
Data sources
As a database supporting the post-marketing safety surveillance for drug and therapeutic biologic products56, FAERS distributes seven datasets regarding the information of each report, including DEMO (demographic and administrative information), DRUG (drug information), REAC (reaction information), OUTC (patient outcome information), RPSR (information on the source of the reports), THER (drug therapy start dates and end dates), and INDI (“Medical Dictionary for Regulatory Activities” [MedDRA] terms coded for the indications for use [diagnoses] for the reported drugs)57.
In FAERS, drugs can be registered by arbitrary names, including trade names, abbreviations, and even with typographical errors, further complicating downstream analysis. Previously, we standardized FAERS data through three steps: de-duplication, drug normalization, and data aggregation31. First, redundant reports were removed as suggested by the FDA. Second, FAERS drug names, along with administration route and dose information, were normalized using a natural language processing (NLP) tool MedEx58 to RxNorm, a standardized nomenclature for clinical drugs and drug delivery devices59. Meanwhile, adverse event terms were mapped to MedDRA’s preferred term (PT) code and classified into MedDRA System Organ Class (SOC)60. Third, adverse events were then aggregated according to MedDRA SOC and PT codes, and drugs aggregated based on National Drug File–Reference Terminology (NDF-RT) classification information through RxNorm61.
We processed FAERS data from 2004 through 2011 into AERS-DM, which contains 2,459,001 reports and 37,029,228 Drug-ADE records. In total, 74% of FAERS unique drug names were normalized to 14,489 unique RxNorm concepts, of which 10,221 (71%) were classified in NDF-RT. The datasets of AERS-DM can be downloaded from the website http://informatics.mayo.edu/adepedia/index.php/Download.
Data extraction
MedDRA has a 5-level hierarchical structure, including System Organ Class (SOC), High Level Group Term (HLGT), High Level Term (HLT), Preferred Term (PT), and Low Level Term (LLT). Each high-level term list includes all sub-level terms. In order to extract PTs in AERS-DM, version 14.1 of MedDRA was utilized to extract the HLGT ‘Fractures’ with the code ‘10017322’ first. Then, 8 HLTs and 61 PTs and corresponding codes associated with fractures were extracted. The 8 HLTs were: 1) ‘skull and face fractures’, 2) ‘thoracic cage fractures non-spinal (excl pathological)’, 3) ‘spinal fractures (excl pathological)’, 4) ‘pelvic fractures’, 5) ‘upper limb fractures’, 6) ‘lower limb fractures’, 7) ‘fractures NEC [not elsewhere classified] (excl pathological)’ and 8) ‘pathological fractures and complications’. These 8 HLTs and their corresponding 61 PTs were used to report our results that are presented in Table 2 and in additional Supplementary Tables S1 and S2.
NDF-RT code ‘N0000000147’ for PPIs class as one of Cellular or Molecular Interactions was mapped to RxNorm code ‘986356’, that was then used to extract 33 associated RxNorm codes for sub-class drugs, including 6 PPI generic ingredients (omeprazole, esomeprazole, pantoprazole, lansoprazole, rabeprazole and dexlansoprazole).
Then the 61 fracture PTs codes and 6 PPI RxNorm codes were used to extract records from AERS-DM.
Data mining
The data mining method PRR was used to detect associations between PPIs and specific fractures reported using the 61 PTs for fractures as well as the 8 HLTs. To build the contingency table for PRR, the number of reports with PPI and fracture is defined as a. The number of reports with PPI and without fracture is assigned as b. The number of reports with drugs other than PPI and with fracture is defined as c. The number of reports with drugs other than PPI and without fracture is defined as d. PRR was then calculated base on Formula 1.
Studies have shown that higher levels of MedDRA could strengthen the sensitivity of signal detection for revealing associations in groups62, 63. In this study, we evaluated the PRR for different levels to gain insight into associations between PPIs and specific fracture sites, including drug class-ADE class (between all PPIs and 8 fracture HLTs), drug class-ADEs (between all PPIs and 61 fracture PTs) and drugs-ADEs (between each PPI and 61 fracture PTs). As fracture risk is different between women and men, and with increasing age, we also stratified analyses for the drug class-ADE class and drug class-ADEs, by sex and by age groups ( ≤ 49 years, 50–69 years and ≥ 70 years).
Electronic supplementary material
Acknowledgements
This project was supported by the National Natural Science Foundation of China (NSFC), No. 81601574, China Jilin Province Department of Education ‘Twelveth Five-Year’ Social Science Funding 2014B20 and PO1 AG04875.
Author Contributions
All co-authors are justifiably credited with authorship, according to the authorship criteria. Final approval is given by each co-author. L.W., H.L. and S.A. designed the research, analyzed the data and revised the paper. M.L. collected data, analyzed the data, drafted the manuscript; Y.C., Z.H., X.W. and E.J.A. analyzed the data.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Liwei Wang and Mei Li contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05552-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Liwei Wang, Email: wang.liwei@mayo.edu.
Hongfang Liu, Email: liu.hongfang@mayo.edu.
Shreyasee Amin, Email: amin.shreyasee@mayo.edu.
References
- 1.Gomm, W. et al. Association of Proton Pump Inhibitors With Risk of Dementia: A Pharmacoepidemiological Claims Data Analysis. JAMA Neurol, doi:10.1001/jamaneurol.2015.4791 (2016). [DOI] [PubMed]
- 2.Ksiadzyna D, Szelag A, Paradowski L. Overuse of proton pump inhibitors. Polskie Archiwum Medycyny Wewnetrznej-Polish Archives of Internal Medicine. 2015;125:289–298. doi: 10.20452/pamw.2790. [DOI] [PubMed] [Google Scholar]
- 3.Zhou B, Huang Y, Li H, Sun W, Liu J. Proton-pump inhibitors and risk of fractures: an update meta-analysis. Osteoporosis International. 2016;27:339–347. doi: 10.1007/s00198-015-3365-x. [DOI] [PubMed] [Google Scholar]
- 4.How Safe Are Popular Reflux Drugs? http://health.usnews.com/health-news/family-health/bones-joints-and-muscles/articles/2009/11/04/how-safe-are-popular-reflux-drugs (accessed 2016).
- 5.McDonagh, M. S., Carson, S. & Thakurta, S. In Drug Class Review: Proton Pump Inhibitors: Final Report Update 5 Drug Class Reviews (2009). [PubMed]
- 6.Top 20 Global Products 2012, IMS Health MIDAS, http://www.imshealth.com/deployedfiles/ims/Global/Content/Corporate/Press Room/Top-Line Market Data & Trends/Top_20_Global_Products_2012_2.pdf (accessed 2016).
- 7.Skrzydlo-Radomanska B, Radwan P. Dexlansoprazole - a new-generation proton pump inhibitor. Prz Gastroenterol. 2015;10:191–196. doi: 10.1007/s11377-015-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vakil N. Prescribing Proton Pump Inhibitors Is it Time To Pause and Rethink? Drugs. 2012;72:437–445. doi: 10.2165/11599320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Vestergaard P, Rejnmark L, Mosekilde L. Proton pump inhibitors, histamine H2 receptor antagonists, and other antacid medications, and the risk of fracture. Journal of Bone and Mineral Research. 2006;21:S174–S174. doi: 10.1007/s00223-006-0021-7. [DOI] [PubMed] [Google Scholar]
- 10.Yang YX, Lewis JD, Epstein S, Metz DC. Long-term proton pump inhibitor therapy and risk of hip fracture. Jama-Journal of the American Medical Association. 2006;296:2947–2953. doi: 10.1001/jama.296.24.2947. [DOI] [PubMed] [Google Scholar]
- 11.Fraser LA, et al. The effect of proton pump inhibitors on fracture risk: report from the Canadian Multicenter Osteoporosis Study. Osteoporosis International. 2013;24:1161–1168. doi: 10.1007/s00198-012-2112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Hoorn MMC, Tett SE, de Vries OJ, Dobson AJ, Peeters GMEE. The effect of dose and type of proton pump inhibitor use on risk of fractures and osteoporosis treatment in older Australian women: A prospective cohort study. Bone. 2015;81:675–682. doi: 10.1016/j.bone.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Corley DA, Kubo A, Zhao W, Quesenberry C. Proton Pump Inhibitors and Histamine-2 Receptor Antagonists Are Associated With Hip Fractures Among At-Risk Patients. Gastroenterology. 2010;139:93–101. doi: 10.1053/j.gastro.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pouwels S, et al. Use of proton pump inhibitors and risk of hip/femur fracture: a population-based case-control study. Osteoporosis International. 2011;22:903–910. doi: 10.1007/s00198-010-1337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes C, et al. Use of proton pump inhibitors and risk of fragility hip fracture in a Mediterranean region. Bone. 2013;52:557–561. doi: 10.1016/j.bone.2012.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Thaler HW, Sterke CS, van der Cammen TJM. Association of Proton Pump Inhibitor Use with Recurrent Falls and Risk of Fractures in Older Women: A Study of Medication Use in Older Fallers. The journal of nutrition, health & aging. 2016;20:77–81. doi: 10.1007/s12603-016-0679-0. [DOI] [PubMed] [Google Scholar]
- 17.Targownik, L. E., Goertzen, A. L., Luo, Y. & Leslie, W. D. Long-Term Proton Pump Inhibitor Use Is Not Associated With Changes in Bone Strength and Structure. The American Journal of Gastroenterology (2016). [DOI] [PubMed]
- 18.FDA Drug Safety Communication: Possible increased risk of fractures of the hip, wrist, and spine with the use of proton pump inhibitors, http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm213206.htm (accessed 2016).
- 19.Yu EW, Bauer SR, Bain PA, Bauer DC. Proton Pump Inhibitors and Risk of Fractures: A Meta-Analysis of 11 International Studies. American Journal of Medicine. 2011;124:519–526. doi: 10.1016/j.amjmed.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray SL, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: results from the Women’s Health Initiative. Archives of internal medicine. 2010;170:765–771. doi: 10.1001/archinternmed.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elaine WY, et al. Acid-suppressive medications and risk of bone loss and fracture in older adults. Calcified tissue international. 2008;83:251–259. doi: 10.1007/s00223-008-9170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fraser L, et al. The effect of proton pump inhibitors on fracture risk: report from the Canadian Multicenter Osteoporosis Study. Osteoporosis International. 2013;24:1161–1168. doi: 10.1007/s00198-012-2112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis JR, et al. Long-Term Proton Pump Inhibitor Therapy and Falls and Fractures in Elderly Women: A Prospective Cohort Study. Journal of Bone and Mineral Research. 2014;29:2489–2497. doi: 10.1002/jbmr.2279. [DOI] [PubMed] [Google Scholar]
- 24.Moberg LM, Nilsson PM, Samsioe G, Borgfeldt C. Use of proton pump inhibitors (PPI) and history of earlier fracture are independent risk factors for fracture in postmenopausal women. The WHILA study. Maturitas. 2014;78:310–315. doi: 10.1016/j.maturitas.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Ding J, Heller DA, Ahern FM, Brown TV. The relationship between proton pump inhibitor adherence and fracture risk in the elderly. Calcified tissue international. 2014;94:597–607. doi: 10.1007/s00223-014-9855-6. [DOI] [PubMed] [Google Scholar]
- 26.Mello M, et al. Proton pump inhibitors increase the incidence of bone fractures in hepatitis C patients. Digestive diseases and sciences. 2012;57:2416–2422. doi: 10.1007/s10620-012-2185-5. [DOI] [PubMed] [Google Scholar]
- 27.van Puijenbroek EP, et al. A comparison of measures of disproportionality for signal detection in spontaneous reporting systems for adverse drug reactions. Pharmacoepidemiology and Drug Safety. 2002;11:3–10. doi: 10.1002/pds.668. [DOI] [PubMed] [Google Scholar]
- 28.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiology and drug safety. 2001;10:483–486. doi: 10.1002/pds.677. [DOI] [PubMed] [Google Scholar]
- 29.Almenoff J, et al. Novel statistical tools for monitoring the safety of marketed drugs. Clinical Pharmacology & Therapeutics. 2007;82:157–166. doi: 10.1038/sj.clpt.6100258. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiology and Drug Safety. 2004;13:519–523. doi: 10.1002/pds.1001. [DOI] [PubMed] [Google Scholar]
- 31.Wang, L. W., Jiang, G. Q., Li, D. C. & Liu, H. F. Standardizing adverse drug event reporting data. Journal of Biomedical Semantics5 (2014). [DOI] [PMC free article] [PubMed]
- 32.Poluzzi E, Raschi E, Moretti U, De Ponti F. Drug-induced torsades de pointes: data mining of the public version of the FDA Adverse Event Reporting System (AERS) Pharmacoepidemiology and Drug Safety. 2009;18:512–518. doi: 10.1002/pds.1746. [DOI] [PubMed] [Google Scholar]
- 33.Sakaeda T, et al. Platinum Agent-Induced Hypersensitivity Reactions: Data Mining of the Public Version of the FDA Adverse Event Reporting System, AERS. International Journal of Medical Sciences. 2011;8:332–338. doi: 10.7150/ijms.8.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang, G. Q., Liu, H. F., Solbrig, H. R. & Chute, C. G. Mining severe drug-drug interaction adverse events using Semantic Web technologies: a case study. Biodata Mining8 (2015). [DOI] [PMC free article] [PubMed]
- 35.Yu Y, et al. Systematic Analysis of Adverse Event Reports for Sex Differences in Adverse Drug Events. Sci Rep. 2016;6:24955. doi: 10.1038/srep24955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang, G., Wang, L., Liu, H., Solbrig, H. R. & Chute, C. G. Building a knowledge base of severe adverse drug events based on AERS reporting data using semantic web technologies. MedInfo 2013, 496–500 (2013). [PubMed]
- 37.Wang, L. & Liu, H. In Bioinformatics and Biomedicine (BIBM), 2013 IEEE International Conference on. 46–52 (IEEE).
- 38.Pathologic Fracture, http://orthopedics.about.com/cs/brokenbones/g/pathologic.htm (accessed 2016).
- 39.Pathological Fracture, http://www.ivyroses.com/HumanBody/Skeletal/Fractures/Pathological-Fracture.php (accessed 2016).
- 40.O’Connell MB, Madden DM, Murray AM, Heaney RP, Kerzner LJ. Effects of proton pump inhibitors on calcium carbonate absorption in women: A randomized crossover trial. American Journal of Medicine. 2005;118:778–781. doi: 10.1016/j.amjmed.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Kirkpantur A, Altun B, Arici M, Turgan C. Proton pump inhibitor omeprazole use is associated with low bone mineral density in maintenance haemodialysis patients. International journal of clinical practice. 2009;63:261–268. doi: 10.1111/j.1742-1241.2008.01883.x. [DOI] [PubMed] [Google Scholar]
- 42.Targownik LE, Lix LM, Leung S, Leslie WD. Proton-Pump Inhibitor Use Is Not Associated With Osteoporosis or Accelerated Bone Mineral Density Loss. Gastroenterology. 2010;138:896–904. doi: 10.1053/j.gastro.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 43.Amoako, A. O., Jafilan, L., Nasiri, P. & Pujalte, G. G. Correlation of Bone Mineral Density Scores and Proton Pump Inhibitors Use in the Elderly. Curr Rheumatol Rev (2016). [PubMed]
- 44.Targownik LE, et al. The relationship between proton pump inhibitor use and longitudinal change in bone mineral density: a population-based study [corrected] from the Canadian Multicentre Osteoporosis Study (CaMos) The American journal of gastroenterology. 2012;107:1361–1369. doi: 10.1038/ajg.2012.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa-Rodrigues J, Reis S, Teixeira S, Lopes S, Fernandes MH. Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. Febs Journal. 2013;280:5052–5064. doi: 10.1111/febs.12478. [DOI] [PubMed] [Google Scholar]
- 46.Histing T, et al. Pantoprazole, a proton pump inhibitor, delays fracture healing in mice. Calcif Tissue Int. 2012;90:507–514. doi: 10.1007/s00223-012-9601-x. [DOI] [PubMed] [Google Scholar]
- 47.Abrahamsen B, Vestergaard P. Proton pump inhibitor use and fracture risk - effect modification by histamine H-1 receptor blockade. Observational case-control study using National Prescription Data. Bone. 2013;57:269–271. doi: 10.1016/j.bone.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 48.Lau AN, Tomizza M, Wong-Pack M, Papaioannou A, Adachi JD. The relationship between long-term proton pump inhibitor therapy and skeletal frailty. Endocrine. 2015;49:606–610. doi: 10.1007/s12020-015-0576-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hinson AM, et al. Hyperparathyroidism Associated with Long-Term Proton Pump Inhibitors Independent of Concurrent Bisphosphonate Therapy in Elderly Adults. Journal of the American Geriatrics Society. 2015;63:2070–2073. doi: 10.1111/jgs.13661. [DOI] [PubMed] [Google Scholar]
- 50.Maggio M, et al. Use of proton pump inhibitors is associated with lower trabecular bone density in older individuals. Bone. 2013;57:437–442. doi: 10.1016/j.bone.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roux C, et al. Increase in vertebral fracture risk in postmenopausal women using omeprazole. Calcified tissue international. 2009;84:13–19. doi: 10.1007/s00223-008-9188-4. [DOI] [PubMed] [Google Scholar]
- 52.Khalili H, et al. Use of proton pump inhibitors and risk of hip fracture in relation to dietary and lifestyle factors: a prospective cohort study. BMj. 2012;344:e372. doi: 10.1136/bmj.e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thaler H, Sterke C, Van Der Cammen TJ. Association of proton pump inhibitor use with recurrent falls and risk of fractures in older women: A study of medication use in older fallers. The journal of nutrition, health & aging. 2016;20:77–81. doi: 10.1007/s12603-016-0679-0. [DOI] [PubMed] [Google Scholar]
- 54.Freedberg D, et al. Use of proton pump inhibitors is associated with fractures in young adults: a population-based study. Osteoporosis International. 2015;26:2501–2507. doi: 10.1007/s00198-015-3168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ. Trends in Fracture Incidence: A Population‐Based Study Over 20 Years. Journal of Bone and Mineral Research. 2014;29:581–589. doi: 10.1002/jbmr.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Questions and Answers on FDA’s Adverse Event Reporting System (FAERS), http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm (accessed 2016).
- 57.FDA Adverse Event Reporting System (FAERS): Latest Quarterly Data Files, http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm082193.htm (accessed 2016).
- 58.Xu H, et al. MedEx: a medication information extraction system for clinical narratives. Journal of the American Medical Informatics Association. 2010;17:19–24. doi: 10.1197/jamia.M3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson SJ, Zeng K, Kilbourne J, Powell T, Moore R. Normalized names for clinical drugs: RxNorm at 6 years. Journal of the American Medical Informatics Association. 2011;18:441–448. doi: 10.1136/amiajnl-2011-000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pearson RK, et al. Influence of the MedDRA® hierarchy on pharmacovigilance data mining results. International journal of medical informatics. 2009;78:e97–e103. doi: 10.1016/j.ijmedinf.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Pathak, J. et al. In AMIA Annual Symposium Proceedings. 1089 (American Medical Informatics Association).
- 62.Roberto G, Piccinni C, D’Alessandro R, Poluzzi E. Triptans and serious adverse vascular events: Data mining of the FDA Adverse Event Reporting System database. Cephalalgia. 2014;34:5–13. doi: 10.1177/0333102413499649. [DOI] [PubMed] [Google Scholar]
- 63.Pearson RK, et al. Influence of the MedDRA (R) hierarchy on pharmacovigilance data mining results. International Journal of Medical Informatics. 2009;78:E97–E103. doi: 10.1016/j.ijmedinf.2009.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


