Abstract
Previous reports of altered grey and white matter structure in Major Depressive Disorder (MDD) have been inconsistent. Recent meta-analyses have, however, reported reduced hippocampal grey matter volume in MDD and reduced white matter integrity in several brain regions. The use of different diagnostic criteria, scanners and imaging sequences may, however, obscure further anatomical differences. In this study, we tested for differences in subcortical grey matter volume (n = 1157) and white matter integrity (n = 1089) between depressed individuals and controls in the subset of 8590 UK Biobank Imaging study participants who had undergone depression assessments. Whilst we found no significant differences in subcortical volumes, significant reductions were found in depressed individuals versus controls in global white matter integrity, as measured by fractional anisotropy (FA) (β = −0.182, p = 0.005). We also found reductions in FA in association/commissural fibres (β = −0.184, pcorrected = 0.010) and thalamic radiations (β = −0.159, pcorrected = 0.020). Tract-specific FA reductions were also found in the left superior longitudinal fasciculus (β = −0.194, pcorrected = 0.025), superior thalamic radiation (β = −0.224, pcorrected = 0.009) and forceps major (β = −0.193, pcorrected = 0.025) in depression (all betas standardised). Our findings provide further evidence for disrupted white matter integrity in MDD.
Introduction
Major Depressive Disorder (MDD) is a common psychiatric illness, affecting between 5 and 30% of the population which accounts for around 10% of all days lived with disability1. There is therefore an urgent need to identify the mechanisms underlying MDD and human in vivo MRI has been widely applied in this search2.
Many brain imaging studies have measured grey matter volume differences between healthy individuals and, predominantly clinically ascertained, individuals with MDD. Prefrontal cortex and limbic areas are fundamental to emotion processing and mood regulation3, and these areas have also been consistently implicated in imaging studies of MDD4–6. As the use of automated methods such as voxel-based morphometry7, 8 and Freesurfer9 have increased, this has expanded the search across the whole brain. In general, structural abnormalities have been reported across diverse brain networks in MDD. Regions including the thalamus10, amygdala4, insula8, caudate9, anterior cingulate cortex4, along with prefrontal areas such as orbital prefrontal cortex (OFC)11 and dorsal lateral prefrontal cortex (PFC)12 have been reported to be smaller in MDD versus healthy controls. However, other studies have found conflicting results9, 13, or have reported null findings7. This inconsistency may be due to limited sample sizes and other sources of heterogeneity such as sample characteristics, recruitment criteria, data acquisition and image processing14.
The lack of a single anatomically circumscribed abnormality in MDD has led many to suggest that the disorder might be due to abnormalities of brain networks affecting connections between several regions. In support of this, findings from individual studies of white matter structure in MDD have shown patterns of alteration using diffusion tensor imaging (DTI). Proxy measures of white matter integrity, including fractional anisotropy (FA) and mean diffusivity (MD), have been used to infer connectivity differences between groups. Decreased FA indicates lower directionality of water molecule diffusion along fibre pathways and is a proxy of decreased tract integrity, whilst increased MD indicates less constrained water molecule diffusion and a proxy for lower integrity.
White matter integrity of frontal-limbic tracts have been suggested to underlie clinical features in MDD due to a lack of frontal cortical control over brain regions that involve in emotion processing15. Studies have reported altered water diffusivity of white matter tracts in MDD compared to healthy controls, but the tracts identified are often inconsistent. Some studies reported decreased white matter integrity in tracts that connect prefrontal areas (e.g. fronto-occipital fasciculus, superior longitudinal fasciculus)16. While some studies using similar sample sizes also found consistent results17, other groups reported FA deficits in limbic areas (e.g. posterior thalamic radiation, posterior corona radiata)15. Similar to the studies of subcortical volumes described above, DTI investigations of MDD have often used relatively small sample sizes17, 18.
Meta-analytic methods may help to overcome issues related to small sample sizes and are also able to quantify and test for between-study heterogeneity. A recent meta-analysis of subcortical structures by Schmaal et al. tested over 1650 MDD patients and around 7000 healthy controls across 15 studies, and reported hippocampal grey matter volume reductions in MDD. No other case-control differences were found19. Meta-analyses of white matter integrity measures in MDD have also reported FA reductions in superior longitudinal fasciculus, fronto-occipital fasciculus, and thalamic radiations17, 18. These studies, however, often require the combination of imaging data from different scanners, using different ascertainment criteria and methodology, different clinical instruments and have differing levels of phenotypic data to pursue further research questions. Meta-analytic findings therefore highlight the pressing need to measure brain structural abnormalities in MDD using larger single-scanner samples where robust conclusions can be made in the absence of differing study methodologies.
In the current study, we examined the volumetric structural imaging data of subcortical brain structures and tract-specific white matter integrity measures from the UK Biobank imaging study. UK Biobank is a study of 500,000 subjects recruited from across the United Kingdom20. The dataset used in the current study is the latest release of imaging data on 8590 participants who participated in the brain imaging assessment21. For our current purposes this included 354/342 MDD and 803/762 controls respectively who provided usable for T1-weighted/DTI data from a single scanner, along with available data regarding diagnostic and phenotypic information. The scanning protocol and pre-processing pipelines were devised by UK Biobank, with consistent, compatible setting of scanner parameters and participant-friendly experimental procedures. This data therefore allowed us to explore structural changes associated with depression in a single large population-based sample using data from an individual study source with unified depression classification, and with scanning sequences and image processing procedures applied consistently across all subjects, all of whom were imaged on a single MRI scanner.
Method
Participants
In the latest release of imaging data from UK Biobank, 5797 people completed the subcortical brain structural MRI measurements and 5171 completed DTI assessment (Fig. S1). The study has been approved by the National Health Service (NHS) Research Ethics Service (approval letter dated 17th June 2011, reference 11/NW/0382), and by the UKB Access Committee (Project #4844). Written informed-consent was obtained from each subject. All assessments were performed in accordance with the regulations and protocols from the committees.
Individuals from the initial pilot phase of imaging using different acquisition parameters were excluded from the current study, as were those that did not complete pre-processing quality checks conducted by UK Biobank. In addition, scans from individuals that were identified by our internal quality check as having a structural measure that lay more than three standard deviations from the sample mean were excluded (Figs S2, S3 and Table S1). Any participants that had a diagnosis of Parkinson’s Disease, bipolar disorder, multiple personality disorder, schizophrenia, autism or intellectual disability were also excluded from the current analysis (ICD-10/9 or self-report). This resulted in data from 5397 participants with T1-weighted subcortical volumes and 4590 participants with DTI measures. Mean ages were 55.47 +/− 7.49 years for those with T1-weighted, grey matter data and 55.46 +/− 7.41 years for those with DTI, white matter integrity. The proportions of male participants are were similar in both datasets (45.78% for those providing T1-weighted data and 47.12% for those with DTI measures). Details of data exclusions are detailed within supplementary materials (Method, Participants; Fig. S1).
MDD definitions
The definition of MDD used in the current study was generated based on the putative MDD category summarized previously by Smith et al., as presented in supplementary materials (Fig. S4)22. They generated the criteria of single episode major depression, recurrent major depression (moderate), recurrent major depression (severe) and those who were absent of depression. This category was benchmarked by testing its prevalence in the sample, and by testing for association with a number of traits, such as neuroticism23, that have previously been associated with MDD24. However, since the category is based on hospital admission data and depressive symptoms, which were both self-reported, rather than more formal ICD/SCID criteria, cases should be considered ‘probable’ MDD rather than operationally defined on the basis of an interview.
We generated two definitions of probable MDD. One was the principal MDD definition that compared all MDD patients (recurrent and single episode) with healthy controls, while the other was based on recurrence and compared recurrent MDD patients with non-recurrent and non-MDD individuals.
The principal MDD definition therefore included those who were categorised in single and multiple episode major depression as cases. The corresponding control group contained participants that were absent of depression according to the putative MDD category described by Smith et al.22. For the recurrent MDD definition, the case group only included recurrent major depression. The corresponding control group therefore referred to the participants without recurrent MDD, which included single episode major depression, those who were absent of depression and those who reported depressive symptoms but not enough to be specified as MDD. Participants who did not answer one or more of the questions necessary for classification were excluded from this analysis.
For each definition of probable MDD, the participants with subcortical volume data consisted of 354 MDD cases and 803 controls and 261 MDD cases and 1196 controls respectively for principal and recurrent definitions. Participants with DTI data consisted of 335 MDD cases and 754 controls and 242 MDD cases and 1113 controls for principal and recurrent definitions respectively. Method used to derive the samples into analyses were presented in supplementary materials, Fig. S1.
The descriptions and demographic characteristics of each MDD definition are shown in supplementary materials (Tables S2 and S3). For the purposes of the current analysis, we used the principal definition of depression as the main definition as it most closely resembles the general application of typical clinical criteria. We also report results of the recurrent definition of MDD to highlight differences associated with a more severe recurrent MDD diagnosis (Supplementary materials, Table S3).
MRI acquisition and analyses
We used the imaging-derived phenotypes (IDPs) generated by UK Biobank. The MRI acquisition, pre-processing and imaging analysis for subcortical volumes and FA values of white matter tracts were all conducted by UK Biobank using standard protocols21, see supplementary material. Briefly, all imaging data was collected on a Siemens Skyra 3T scanner (https://www.healthcare.siemens.com/magnetic-resonance-imaging) and was preprocessed using FSL packages. For T1-weighted data, segmentation of brain was conducted in two steps: firstly, a tissue-type segmentation using FAST (FMRIB’s Automated Segmentation Tool)25 was applied to extract cerebrospinal fluid, grey matter and white matter; then subcortical structures are extracted using FIRST (FMRIB’s Integrated Registration and Segmentation Tool)26. For DTI data, parcellation of tracts were conducted using AutoPtx27.
The summary data contained volumes of grey matter, white matter, cerebrospinal fluid, thalamus, putamen, pallidum, hippocampus, caudate, brain stem, amygdala and accumbens (Fig. S2). DTI data provided tract-averaged FA for 27 major tracts (12 bilateral tracts in both hemispheres and 3 tracts that pass across brain): (a) association and commissural fibres: forceps major and minor, inferior fronto-occipital fasciculus, uncinate fasciculus, cingulum bundle and superior longitudinal fasciculus; (b) thalamic radiations: anterior, superior and posterior thalamic radiations; (c) projection fibres: corticospinal tract, acoustic radiation, medial lemniscus, middle cerebellar peduncle.
Scans with severe and obvious normalization problems were excluded by UK Biobank. In addition we also excluded observations that were more than three standard deviation from the sample mean for the analysis of subcortical volumes. For DTI measures, participants with at least one outlier of tract-averaged FA from the sample mean were excluded for that measure. Descriptions of the sample were reported in supplementary materials (Method, MRI preprocessing; Figs S1–3). For transparency, the results without excluding outliers are also presented in the supplementary materials.
Statistical methods
Subcortical volumes
First, differences in global intracranial volume (ICV) associated with a probable MDD diagnosis were examined by modelling ICV as dependent variable, controlling for age, age2, sex and assessment centre. ICV was measured by adding up volumes of white matter (WM), grey matter (GM) and cerebrospinal fluid (CSF). For bilateral subcortical volumes, age, age2, sex, hemisphere, assessment centre and ICV were set as covariates in a repeated-effect linear model to test for an association between both probable MDD definitions on subcortical volumes, adjusted for whole brain size. For unilateral structures, a general linear model was applied as above, without controlling for hemisphere. We also examined the interaction of hemisphere and MDD definitions on bilateral structures. Where there was a significant MDD by hemisphere interaction, analyses on both lateralised structures were conducted separately. All subcortical volumes were rescaled into zero mean and unitary standard deviation in order that effect sizes represent standardized scores. False Discovery Rate (FDR) multiple comparison correction was applied for tests of the 8 subcortical volumes plus additional tests on ICV, conducted separately for the two probable MDD definitions (Fig. 1, Tables 1 and S5).
Figure 1.
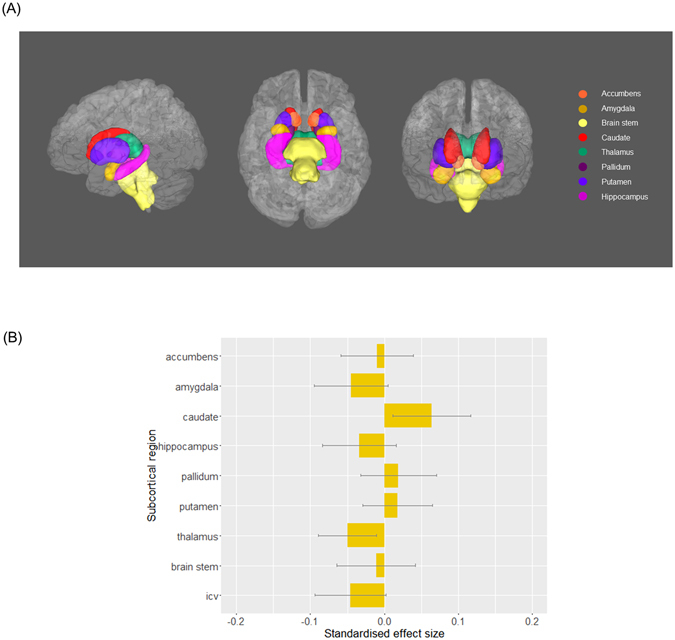
(A) Subcortical structures of interest in left, inferior and anterior view. (B) The effect of principal definition of probable MDD on subcortical volumes. Linear models were conducted, controlling the effect of age, age2, sex, assessment centre and intracranial volume (and hemisphere for the regions that have bilateral values). The x-axis shows the standardised effect size of MDD definition, and y-axis is the layout of the subcortical structures. The error bar represents standard deviation of mean.
Table 1.
The effect of MDD definition on the volumes of subcortical regions and brain matters.
| Subcortical regions | Principal definition | Recurrent definition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect size | Standard deviation | t value | p value | pcorrected | Effect size | Standard deviation | t value | p value | pcorrected | |
| Accumbens | −0.010 | 0.049 | −0.211 | 0.833 | 0.838 | −0.018 | 0.052 | −0.348 | 0.728 | 0.819 |
| Amygdala | −0.045 | 0.050 | −0.896 | 0.371 | 0.834 | 0.038 | 0.053 | 0.711 | 0.477 | 0.819 |
| Caudate | 0.064 | 0.053 | 1.198 | 0.231 | 0.834 | 0.025 | 0.056 | 0.453 | 0.650 | 0.819 |
| Hippocampus | −0.034 | 0.050 | −0.682 | 0.495 | 0.838 | −0.040 | 0.053 | −0.758 | 0.449 | 0.819 |
| Pallidum | 0.019 | 0.051 | 0.372 | 0.710 | 0.838 | −0.022 | 0.054 | −0.414 | 0.679 | 0.819 |
| Putamen | 0.018 | 0.047 | 0.386 | 0.700 | 0.838 | −0.008 | 0.049 | −0.162 | 0.871 | 0.871 |
| Thalamus | −0.050 | 0.039 | −1.284 | 0.199 | 0.834 | −0.059 | 0.041 | −1.428 | 0.154 | 0.819 |
| Brain stem | −0.011 | 0.053 | −0.205 | 0.838 | 0.838 | 0.045 | 0.056 | 0.794 | 0.428 | 0.819 |
| ICV | −0.046 | 0.048 | −0.953 | 0.341 | 0.834 | −0.049 | 0.051 | −0.959 | 0.338 | 0.819 |
White matter integrity
In order to test for an association between probable MDD and FA, as above we used a general linear model with age, age2, sex and assessment centre as covariates and the definition of MDD as a fixed factor. First we examined for the effects of diagnosis on global whole brain white matter integrity. The brain’s white matter tracts have been shown to share a considerable proportion of variance in their microstructural properties in this28 and other samples29, 30. Global integrity was determined using standardised approaches by applying principal component analysis (PCA) on the 27 tracts to extract a latent measure31. Scores of the first un-rotated component of FA were extracted and set as the dependent variable of the general linear model to test the effect of probable MDD diagnosis (variance explained = 36.5%). Then we separately examined three subsets of white matter tracts: (a) association and commissural fibres which include tracts connecting cortex to cortex, (b) projection fibres which consist of tracts connecting cortex to spinal cord and brainstem, as well as sensory tracts that connect cortex to thalamus and (c) thalamic radiations that connect thalamus with cortical areas28. Scores of the principal un-rotated component for each subset was extracted (variance explained = 44.1%, 60.1% and 38.1% respectively for A/CF, TR and PF) for further general linear modelling as with the global latent measure. Loadings and scree plot of PCA analyses are in supplementary materials (Table S10 and Fig. S5). Finally, we examined the effects of depression on each tract individually. Repeated-effect linear models were used for the measures of bilateral white matter tracts correcting for hemisphere as above, while random-effect general linear models were used for the unilateral midline tracts. Both the main effect of MDD definition and its interaction with hemisphere were tested. Where the interaction was significant, tests were applied individually for left and right sides separately. FDR correction was individually applied over the three subsets of white matter tracts as well as individual tracts32.
Results
The effect of MDD definitions on subcortical volumes
We found no significant group effect for ICV based on the principal definition of MDD (β = −0.046, puncorrected = 0.341). There were also no significant differences between groups based on the principal definition of MDD for any of the subcortical brain regions, including the hippocampus (βs = −0.050~0.064, psuncorrected > 0.199, pscorrected > 0.834); see Fig. 1 and Table 1. No region demonstrated significant interaction of hemisphere, therefore no region was examined separately on different hemispheres.
The same models were also applied to compare recurrent MDD and controls, see above. No subcortical regions reached significance in this definition of recurrent cases versus controls. The largest nonsignificant effect size was observed for the caudate (β = 0.064, puncorrected = 0.231).
The effect of probable MDD on measures of white matter integrity
Firstly we tested the effect of probable MDD on general white matter FA (gFA). For both the principal and recurrent definitions, gFA was lower in probable MDD cases versus controls (β = −0.182, p = 0.005; β = −0.160, p = 0.022 respectively).
We then examined tracts categorised into association/commissural fibres (gAF), thalamic radiations (gTR) and projection fibres (gPF). We found effects of probable MDD on measures of FA in two of the three groups of tracts. Probable MDD at principal and recurrent definitions showed smaller values in gAF (Probable MDD: β = −0.184, pcorrected = 0.010; Recurrent MDD: β = −0.170, pcorrected = 0.045) and gTR (Probable MDD: β = −0.159, pcorrected = 0.020; Recurrent MDD: β = −0.141, pcorrected = 0.068). No effect was found for gPF (Probable MDD: β = −0.115, pcorrected = 0.073; Recurrent MDD: β = −0.057, pcorrected = 0.401). The above findings were checked in self-declare depression, and the results were found to be similar (see supplementary materials, MDD definitions).
We then proceeded to compare FA values in the individual tracts between cases and controls. Initially, we tested the tracts controlling for hemisphere effects. Then we tested the interaction of hemisphere and probable MDD definitions on bilateral tracts to identify any lateralised effects. There was a significant interaction of hemisphere in superior longitudinal fasciculus for recurrent definition of probable MDD (β = 0.117, pcorrected = 0.026). The left and right superior longitudinal fasciculi were therefore tested separately.
We found reduced FA in the left superior longitudinal fasciculus for both definitions of MDD versus controls (Probable MDD: β = −0.194, pcorrected = 0.025; Recurrent MDD: β = −0.221, pcorrected = 0.025) (Fig. 2 and Table 2). No significant association was found with right superior longitudinal fasciculus (Principal MDD: Probable MDD: β = −0.057, pcorrected = 0.379; Recurrent MDD: β = −0.029, pcorrected = 0.684). Significant FA decrease was found in superior thalamic radiation and forceps major, but only for principal MDD definition (Probable MDD: β = −0.224, pcorrected = 0.009; β = −0.193, pcorrected = 0.025. Recurrent MDD: β = −0.179, pcorrected = 0.080; β = −0.133, pcorrected = 0.150 respectively for the two tracts). In order to check whether the decreased FA in the above tracts was due to global changes in gFA, the effect of MDD definitions was tested again with gFA included as a covariate (Table S6). Left superior longitudinal fasciculus remained significant in both definitions (Probable MDD: β = −0.194, pcorrected = 0.038; Recurrent MDD: β = −0.221, pcorrected = 0.025). Forceps major showed decreased FA in probable MDD definition (β = −0.193, pcorrected = 0.038) but not in recurrent MDD (β = −0.133, pcorrected = 0.350). The effect MDD definitions on superior thalamic radiation didn’t reach significance after correcting for gFA (Probable MDD: β = −0.110, pcorrected = 0.162; Recurrent MDD: β = −0.077, pcorrected = 0.568). The above results of individual tracts turned null if outliers weren’t excluded, but the standard effect sizes were in similar trend (Table S7).
Figure 2.
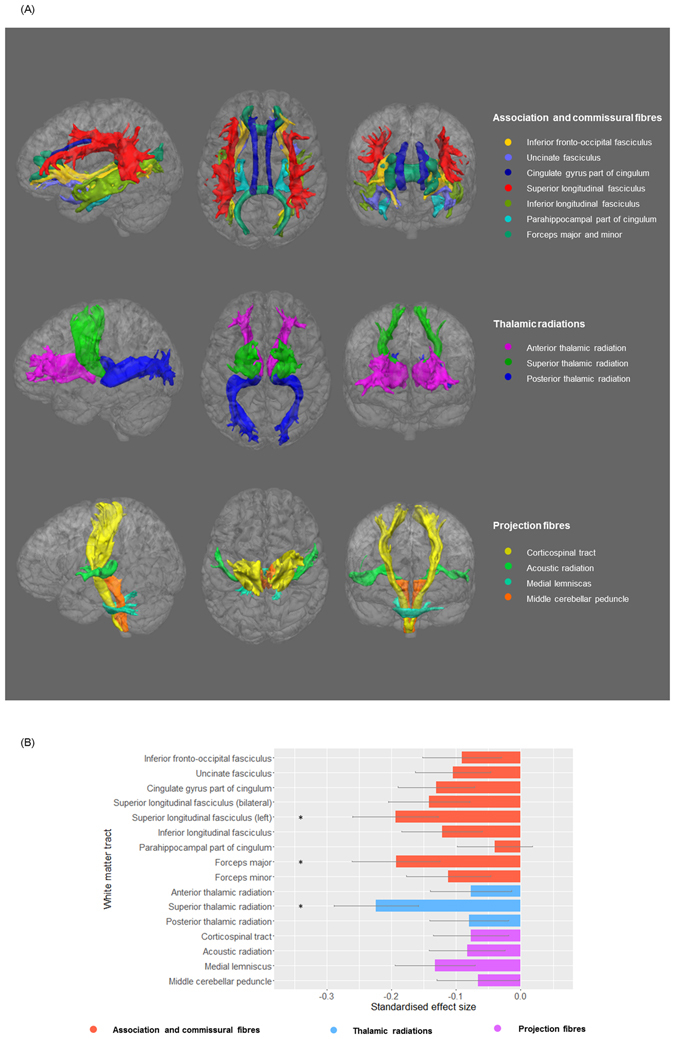
(A) White matter tracts in each anatomical subset in left, posterior and anterior view. (B) The effect of principal definition of probable MDD on FA value of tracts. Linear models were conducted, controlling the effect of age, age2, sex and assessment centre (and hemisphere for the tracts that have bilateral values). Left superior longitudinal fasciculus was presented because there was a significant interaction between recurrent MDD definition and hemisphere. Follow-up analysis showed a lateral effect of probable MDD definition on left superior longitudinal fasciculus. The x-axis shows the standardised effect size of MDD definition, and y-axis is the layout of the white matter tracts. The error bar represents standard deviation of mean.
Table 2.
The effect of MDD definition on FA values of DTI tracts.
| DTI tracts | Principal definition | Recurrent definition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Effect size | Standard deviation | t value | p value | pcorrected | Effect size | Standard deviation | t value | p value | pcorrected | |
| Acoustic radiation | −0.083 | 0.059 | −1.410 | 1.59E-001 | 0.231 | −0.094 | 0.063 | −1.485 | 1.38E-001 | 0.221 |
| Anterior thalamic radiation | −0.077 | 0.063 | −1.221 | 2.22E-001 | 0.254 | −0.065 | 0.067 | −0.973 | 3.31E-001 | 0.441 |
| Cingulate gyrus part of cingulum | −0.131 | 0.059 | −2.213 | 2.71E-002 | 0.085 | −0.102 | 0.064 | −1.601 | 1.10E-001 | 0.195 |
| Corticospinal tract | −0.077 | 0.058 | −1.321 | 1.87E-001 | 0.236 | −0.049 | 0.062 | −0.795 | 4.27E-001 | 0.488 |
| Inferior fronto-occipital fasciculus | −0.091 | 0.061 | −1.489 | 1.37E-001 | 0.219 | −0.059 | 0.065 | −0.901 | 3.68E-001 | 0.453 |
| Inferior longitudinal fasciculus | −0.122 | 0.062 | −1.983 | 4.76E-002 | 0.109 | −0.124 | 0.066 | −1.891 | 5.89E-002 | 0.150 |
| Medial lemniscus | −0.133 | 0.062 | −2.148 | 3.19E-002 | 0.085 | −0.141 | 0.066 | −2.155 | 3.14E-002 | 0.100 |
| Parahippocampal part of cingulum | −0.040 | 0.058 | −0.683 | 4.94E-001 | 0.494 | −0.018 | 0.060 | −0.304 | 7.61E-001 | 0.761 |
| Posterior thalamic radiation | −0.080 | 0.061 | −1.306 | 1.92E-001 | 0.236 | −0.089 | 0.065 | −1.373 | 1.70E-001 | 0.247 |
| Superior longitudinal fasciculus (bilateral) | −0.142 | 0.063 | −2.246 | 2.49E-002 | 0.085 | −0.151 | 0.068 | −2.229 | 2.60E-002 | 0.100 |
| Superior longitudinal fasciculus (left) | −0.194 | 0.066 | −2.951 | 3.23E-003 | 0.025 | −0.221 | 0.070 | −3.165 | 1.59E-003 | 0.025 |
| Superior thalamic radiation | −0.224 | 0.065 | −3.461 | 5.58E-004 | 0.009 | −0.179 | 0.069 | −2.580 | 9.99E-003 | 0.080 |
| Uncinate fasciculus | −0.105 | 0.058 | −1.810 | 7.06E-002 | 0.141 | −0.107 | 0.062 | −1.718 | 8.60E-002 | 0.172 |
| Forceps major | −0.193 | 0.068 | −2.834 | 4.69E-003 | 0.025 | −0.133 | 0.072 | −1.842 | 6.57E-002 | 0.150 |
| Forceps minor | −0.112 | 0.065 | −1.723 | 8.52E-002 | 0.152 | −0.159 | 0.070 | −2.266 | 2.36E-002 | 0.100 |
| Middle cerebellar peduncle | −0.066 | 0.064 | −1.024 | 3.06E-001 | 0.326 | 0.039 | 0.068 | 0.576 | 5.65E-001 | 0.602 |
Discussion
In the current study, we sought to determine whether MDD was associated with differences in subcortical grey matter volume or white matter integrity in a large imaging dataset from a single scanner of more than 8000 people, and among them over 1000 were included as cases and controls in the analyses for the present study. The sample sizes of MDD cases and controls included in the analysis of white matter integrity is by far the largest to our knowledge. Also, the present study considered two important brain structural modalities in two highly overlapping samples. Whilst we did not find any statistically significant subcortical volumetric differences between unaffected participants and individuals with probable MDD (using any of the definitions with increasing severity), we did find substantial evidence of reduced white matter integrity in MDD. This was seen globally, in two of the three categories of tracts (association/commissural fibres and thalamic radiation tracts), and in individual tracts (bilateral superior thalamic radiation, forceps major and left superior longitudinal fasciculus). Similar patterns of findings were seen for both principal and recurrent definition of depression with generally greater effect sizes in recurrent cases, with the exception of the localised differences in the superior thalamic radiation and forceps major.
Our study notably did not find evidence for bilateral hippocampal volume reduction as previously reported in the large collaborative meta-analysis of MDD19. We also did not find evidence of reductions in hippocampal volume when looking at recurrent MDD as published in the same study. The lack of subcortical volumetric differences associated with probable MDD diagnoses in the current study therefore does not support the widely held belief that there are subcortical volumetric changes associated with the disorder. There are several potential explanations for this. Firstly, the UK Biobank dataset included only community-dwelling, ambulant individuals who could independently complete the health and cognitive assessments, and attend the follow-up imaging assessments. This approach arguably selected MDD groups that were more well/better functioning but equally more representative of the general population than purely clinically ascertained samples. We also used a composite ‘probable’ MDD diagnosis that was based on self-report symptoms and hospital admission statistics, and the cases were selected based on self-report lifetime experience of probable depression. In contrast, many other studies previously used a structured clinical interview schedule, such as the Structured Clinical Interview for DSM-IV (SCID), to define MDD according to standard criteria. Some studies have specifically studied people who were certainly experiencing depression at the time of imaging assessment33. Whilst the probable MDD definitions used in the current paper were not based on an interview, they showed many of the same epidemiological and risk-factor associations as clinically defined cases22, 34.
Although we do not report subcortical volume differences, we did find substantive evidence for robust deficits in both global and local white matter integrity. We found that MDD patients had global loss of FA which was also found to be reduced in association and commissural fibres as well as in thalamic radiations, but not in projection fibres. FA in these structures was also more severely reduced in the recurrent MDD patients. The above results indeed reflect findings from previous small-sample and meta-analytic studies17, 35, 36, while extending them to a more generalizable population-based cohort excluding potential methodological confounds as associated with the previous studies. A previous meta-analytic study that compared 231 MDD patients with 261 healthy participants found reduced FA in inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, posterior thalamic radiation and corpus callosum, which belong to the association/commissural fibres and thalamic radiations17. Following the above study, another two recent meta-analyses found integrity reductions in the same categories, i.e. dorsal lateral PFC area, commissural fibres35, 37. The global loss of FA in these regions could be the result of general neurodevelopmental alterations in MDD patients38, and findings within defined subsets of white matter tracts could reflect the neurological basis of MDD as a disconnection within an integrated network of cortex-cortex and cortical-limbic pathways39. The general FA reductions in groups of tracts is also consistent with findings from resting-state fMRI studies, which reported abnormalities in MDD populations in regional networks rather than just individual regions or structures6, 40. The networks that derive from prefrontal cortex and thalamus has been found largely contribute to emotional and social cognition processes38. The reduced integrity in these groups of tracts may therefore reflect the repeatedly found impairment of emotion regulation41, 42, reward processing43 and executive control44 in MDD populations.
In the tests of single white matter tracts, we found significantly altered integrity in left superior longitudinal fasciculus and superior thalamic radiation both in the overall MDD population and recurrent MDD patients. Reduction of left superior longitudinal fasciculus was notably larger in recurrent MDD patients. Reduction of integrity in forceps major was also found in MDD compared with healthy subjects, however showed no specific change of FA in recurrent MDD.
Superior longitudinal fasciculus, as a part of association fibres, connects prefrontal cortex and other lobes45. Small-sample studies have specifically reported reduced integrity in superior longitudinal fasciculus in various depressive samples, including elderly patients with depression38, 46, depressive adolescents47 and adolescents with familial risk for depression45, compared with controls. Meta-analytic studies35, 48 and a review36 also ascertained that the reduction of white matter integrity specifically in superior longitudinal fasciculus may be an important biomarker of the presence of depression. A recent study combined genetic and neuroimaging techniques found that people with higher polygenic risk of depression have greater loss of FA in superior longitudinal fasciculus49, suggesting that it may also therefore be a useful trait-related marker of risk. Loss of integrity in superior longitudinal fasciculus has also previously been reported to be associated with various cognitive dysfunctions, like working memory50 and attention48. Severity of depressive symptoms was also found correlate with FA loss in superior longitudinal fasciculus51. There is increasingly convincing evidence therefore that reduced integrity in superior longitudinal fasciculus might be an important feature of the neurobiology of MDD and may underlie impaired emotional process and cognitive abilities in MDD population18.
Another strength of the present study is that cross-modality assessment was conducted on both subcortical volumes and white matter integrity. Though the findings were largely found in white matter integrity instead of subcortical volumes, this is consistent with another cross-modality study by Sexton et al.16, which presented that no significant group difference was found between late-life depression and healthy control, whereas white matter integrity was reduced in many regions16. Another study on 358 people similarly found that depressive symptoms of elderly subjects also showed significant deficit in white matter, but not in grey matter measures52. The age range for the present study is from 40 to 70, which covers a notable range of elderly participants. This feature of our sample could be the reason why it showed similar contrast of findings between white matter and grey matter measurements.
Potential limitations of the current study should be considered, these include the absence of a face-to-face structured diagnostic interview schedule and the lack of hospital-based sampling. The large sample size may, however, overcome some of these difficulties and community based population sampling may yield more generalizable findings than those based on clinically ascertained samples alone8, 53. The current investigation, by avoiding the combination of clinically and methodologically diverse samples, may also have ameliorated several important confounds such as differences due to different healthcare systems and illness related conditions including age of onset and duration of illness. Another factor of interest for future studies is the effect of hospital treatment. As studies have reported changes of depressive symptoms caused by medication or cognitive treatment3, investigates on the neurological effect of treatment should be conducted. The prevalence of the present study is lower than 10%, which is less than the prevalence of ~20% in overall sample of the cohort in the study by Smith et al.22. This was mainly due to the difference of sizes between the two samples. There were ~5500 participants in the sample with T1-weighted/DTI data, whereas over 30 times of people were included in the full cohort (N = 172,751). This difference therefore supports the necessity of studying MDD in a large sample to minimise the bias of selecting study sample. A further potential limitation is that for the volumetric analysis we only focused on the subcortical volumes in the current study. We can therefore not exclude the possibility of cortical differences in MDD, including regional volume differences, as well as measures of cortical thickness and gyrification for example.
Our study presents a comprehensive comparison of brain structural changes related to MDD using the largest single sample available to date from a single scanner with uniform methodologies for clinical categorisation and scanning. We mainly report reductions of white matter FA in general latent measures of association and commissural fibres as well as thalamic radiations, and in left superior longitudinal fasciculus both in MDD and recurrent MDD. Future work would be potentially focusing on structural changes in cortical areas as well as richer stratification of MDD into informative biologically-based subgroups.
Electronic supplementary material
Acknowledgements
This study is supported by a Wellcome Trust Strategic Award “Stratifying Resilience and Depression Longitudinally” (STRADL) (Reference 104036/Z/14/Z). We thank the UK Biobank participants for their participation, and the UK Biobank team for their work in collecting and providing these data for analysis. This research was conducted, using the UK Biobank Resource under approved project 10279, in The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology (CCACE) (http://www.ccace.ed.ac.uk), part of the cross-council Lifelong Health and Wellbeing Initiative (MR/K026992/1). XS receives support from China Scholarship Council. HCW is supported by a JMAS SIM fellowship from the Royal College of Physicians of Edinburgh and by an ESAT College Fellowship from the University of Edinburgh. SRC was supported by Medical Research Council grant MR/M013111/1. IJD and DCL are supported by the Medical Research Council award to CCACE (MR/K026992/1). IJD is additionally supported by the Dementias Platform UK (MR/L015382/1), and he and SRC by the Age UK-funded Disconnected Mind project (http://www.disconnectedmind.ed.ac.uk). DJS is supported by an Independent Investigator Award from the Brain and Behaviour Research Foundation (21930). Part of the work was undertaken in The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology (CCACE), funding from the Biotechnology and Biological Sciences Research Council (BBSRC) and Medical Research Council (MRC) is gratefully acknowledged. Age UK (The Disconnected Mind project) also provided support for the work undertaken at CCACE.
Author Contributions
A.M.M., H.C.W., S.R.C., L.M.R. and X.S. contributed to the design of the study, analysis of the data, and writing the manuscript. M.J.A. was involved in curating the data. I.J.D., D.C.L., D.J.S. and M.E.B. were involved in the conception of the study and overseeing analysis methodology. UK Biobank collected all data and was involved in the preprocessing of imaging data. All authors discussed and commented on the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05507-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marcus, M., Yasamy, M. T., Ommeren, M. van, Chisholm, D. & Saxena, S. Depression: A Global Public Health Concern at. http://www.who.int/mental_health/management/depression/who_paper_depression_wfmh_2012.pdf. (2012).
- 2.Gamazon ER, et al. A gene-based association method for mapping traits using reference transcriptome data. Nat. Genet. 2015;47:1091–1098. doi: 10.1038/ng.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat. Rev. Neurosci. 2008;9:788–796. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bora E, Fornito A, Pantelis C, Yucel M. Gray matter abnormalities in Major Depressive Disorder: A meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 5.Maller JJ, et al. Occipital bending in depression. Brain. 2014;137:1830–1837. doi: 10.1093/brain/awu072. [DOI] [PubMed] [Google Scholar]
- 6.Meng C, et al. Aberrant topology of striatum’s connectivity is associated with the number of episodes in depression. Brain. 2014;137:598–609. doi: 10.1093/brain/awt290. [DOI] [PubMed] [Google Scholar]
- 7.Wagner G, et al. Structural brain alterations in patients with major depressive disorder and high risk for suicide: Evidence for a distinct neurobiological entity? Neuroimage. 2011;54:1607–1614. doi: 10.1016/j.neuroimage.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 8.Soriano-Mas C, et al. Cross-sectional and longitudinal assessment of structural brain alterations in melancholic depression. Biol. Psychiatry. 2011;69:318–325. doi: 10.1016/j.biopsych.2010.07.029. [DOI] [PubMed] [Google Scholar]
- 9.Sacchet MD, Livermore EE, Iglesias JE, Glover GH, Gotlib IH. Subcortical volumes differentiate Major Depressive Disorder, Bipolar Disorder, and remitted Major Depressive Disorder. J. Psychiatr. Res. 2015;68:91–98. doi: 10.1016/j.jpsychires.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nugent AC, Davis RM, Zarate CA, Drevets WC. Reduced thalamic volumes in major depressive disorder. Psychiatry Res. - Neuroimaging. 2013;213:179–185. doi: 10.1016/j.pscychresns.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried EI, Kievit RA. The volumes of subcortical regions in depressed and healthy individuals are strikingly similar: a reinterpretation of the results by Schmaal et al. Mol. Psychiatry. 2015;1:1–2. doi: 10.1038/mp.2015.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amico F, et al. Structural MRI correlates for vulnerability and resilience to major depressive disorder. J. Psychiatry Neurosci. 2011;36:15–22. doi: 10.1503/jpn.090186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong L, et al. Frontal-subcortical volumetric deficits in single episode, medication-naïve depressed patients and the effects of 8 weeks fluoxetine treatment: A VBM-DARTEL study. PLoS One. 2014;9:e79055. doi: 10.1371/journal.pone.0079055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnone D, McIntosh AM, Ebmeier KP, Munafò MR, Anderson IM. Magnetic resonance imaging studies in unipolar depression: Systematic review and meta-regression analyses. Eur. Neuropsychopharmacol. 2012;22:1–16. doi: 10.1016/j.euroneuro.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Korgaonkar MS, Williams LM, Song YJ, Usherwood T, Grieve SM. Diffusion tensor imaging predictors of treatment outcomes in major depressive disorder. Br. J. Psychiatry. 2014;205:321–328. doi: 10.1192/bjp.bp.113.140376. [DOI] [PubMed] [Google Scholar]
- 16.Sexton CE, Allan CL, Masurier ML. Magnetic Resonance Imaging in Late-Life Depression. Arch. Gen. Psychiatry. 2012;69:680–689. doi: 10.1001/archgenpsychiatry.2011.1862. [DOI] [PubMed] [Google Scholar]
- 17.Liao Y, et al. Is depression a disconnection syndrome? Meta- analysis of diffusion tensor imaging studies in patients with MDD. J. Psychiatry Neurosci. 2013;38:49–56. doi: 10.1503/jpn.110180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murphy ML, Frodl T. Meta-analysis of diffusion tensor imaging studies shows altered fractional anisotropy occurring in distinct brain areas in association with depression. Biol. Mood Anxiety Disord. 2011;1:1–12. doi: 10.1186/2045-5380-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmaal L, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol. Psychiatry. 2016;21:806–812. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sudlow C, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12:1–10. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller, K. L. et al. Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat. Neurosci. doi:10.1038/nn.4393 (2016). [DOI] [PMC free article] [PubMed]
- 22.Smith DJ, et al. Prevalence and characteristics of probable major depression and bipolar disorder within UK biobank: cross-sectional study of 172,751 participants. PLoS One. 2013;8:e75362. doi: 10.1371/journal.pone.0075362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jylha P, Melartin T, Isometsa E. Relationships of neuroticism and extraversion with axis I and II comorbidity among patients with DSM-IV major depressive disorder. J. Affect. Disord. 2009;114:110–121. doi: 10.1016/j.jad.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex and stressful life events in the prediction of episodes of major depression. Am. J. Psychiatry. 2004;161:631–636. doi: 10.1176/appi.ajp.161.4.631. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. Med. Imaging, IEEE Trans. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 26.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Groot M, et al. Improving alignment in Tract-based spatial statistics: Evaluation and optimization of image registration. Neuroimage. 2013;76:400–411. doi: 10.1016/j.neuroimage.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox, S. R. et al. Age differences in brain white matter microstructure in UK Biobank (N = 3,513). bioRxiv 51771 (2016).
- 29.Penke L, et al. A General Factor of Brain White Matter Integrity Predicts Information Processing Speed in Healthy Older People. J. Neurosci. 2010;30:7569–7574. doi: 10.1523/JNEUROSCI.1553-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penke L, et al. Brain white matter tract integrity as a neural foundation for general intelligence. Mol. Psychiatry. 2012;17:1026–1030. doi: 10.1038/mp.2012.66. [DOI] [PubMed] [Google Scholar]
- 31.Cox SR, et al. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat. Commun. 2016;7:1–34. doi: 10.1038/ncomms13629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoav Benjamini YH. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple. Testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- 33.Turner AD, Furey ML, Drevets WC, Zarate C, Nugent AC. Association between subcortical volumes and verbal memory in unmedicated depressed patients and healthy controls. Neuropsychologia. 2012;50:2348–2355. doi: 10.1016/j.neuropsychologia.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okbay A, et al. Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat. Genet. 2016;48:624–633. doi: 10.1038/ng.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, et al. Disorganization of white matter architecture in major depressive disorder: a meta-analysis of diffusion tensor imaging with tract- based spatial statistics. Sci. Rep. 2016;6:1–11. doi: 10.1038/s41598-016-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sexton CE, Mackay CE, Ebmeier KP. A Systematic Review of Diffusion Tensor Imaging Studies in Affective Disorders. Biol. Psychiatry. 2009;66:814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 37.Wen MC, Steffens DC, Chen MK, Zainal NH. Diffusion tensor imaging studies in late-life depression: systematic review and meta-analysis. Int. J. Geriatr. Psychiatry. 2014;29:1173–1184. doi: 10.1002/gps.4129. [DOI] [PubMed] [Google Scholar]
- 38.Korgaonkar MS, et al. Loss of white matter integrity in major depressive disorder: Evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum. Brain Mapp. 2011;32:2161–2171. doi: 10.1002/hbm.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veer IM. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front. Syst. Neurosci. 2010;4:1–10. doi: 10.3389/fnsys.2010.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greicius MD, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol. Psychiatry. 2007;62:429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heller AS, et al. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA psychiatry. 2013;70:1181–9. doi: 10.1001/jamapsychiatry.2013.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanske P, Heissler J, Schönfelder S, Wessa M. Neural correlates of emotion regulation deficits in remitted depression: The influence of regulation strategy, habitual regulation use, and emotional valence. Neuroimage. 2012;61:686–693. doi: 10.1016/j.neuroimage.2012.03.089. [DOI] [PubMed] [Google Scholar]
- 43.Gradin VB, et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–1764. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- 44.Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol. Bull. 2013;139:81–132. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H, Fan X, Williamson DE, Rao U. White matter changes in healthy adolescents at familial risk for unipolar depression: a diffusion tensor imaging study. Neuropsychopharmacology. 2011;36:684–91. doi: 10.1038/npp.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheline YI, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am. J. Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cullen, K. R. et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J. Am. Acad. Child Adolesc. Psychiatry 49, 173–183.e1 (2010). [DOI] [PMC free article] [PubMed]
- 48.de Schotten MT, et al. A lateralized brain network for visuospatial attention. Nat. Neurosci. 2011;14:1245–1246. doi: 10.1038/nn.2905. [DOI] [PubMed] [Google Scholar]
- 49.Whalley HC, et al. Polygenic risk and white matter integrity in individuals at high risk of mood disorder. Biol. Psychiatry. 2013;74:280–286. doi: 10.1016/j.biopsych.2013.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karlsgodt KH, et al. Diffusion Tensor Imaging of the Superior Longitudinal Fasciculus and Working Memory in Recent-Onset Schizophrenia. Biol. Psychiatry. 2008;63:512–518. doi: 10.1016/j.biopsych.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 51.Lai CH, Wu YT. Alterations in white matter micro-integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychol. Med. 2014;44:2825–2832. doi: 10.1017/S0033291714000440. [DOI] [PubMed] [Google Scholar]
- 52.Allan CL, et al. Sub-threshold depressive symptoms and brain structure: A magnetic resonance imaging study within the Whitehall II cohort. J. Affect. Disord. 2016;204:219–225. doi: 10.1016/j.jad.2016.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benedetti F, et al. Disruption of white matter integrity in bipolar depression as a possible structural marker of illness. Biol. Psychiatry. 2011;69:309–317. doi: 10.1016/j.biopsych.2010.07.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


