Abstract
Patients with heterozygous familial hypercholesterolemia (HeFH) have been reported to be less vulnerable to type 2 diabetes mellitus (T2DM), although the mechanism is unknown. The aims of the present study were to assess the effects of low density lipoprotein (LDL) cholesterol concentration and the presence of FH-causing mutations on T2DM prevalence in HeFH. Data were collected from the Dyslipidemia Registry of the Spanish Arteriosclerosis Society. Inclusion criteria were definite or probable HeFH in patients aged ≥18 years. T2DM prevalence in HeFH patients was compared with data of the general population. 1732 patients were included. The prevalence of T2DM was lower in patients with HeFH compared with the general population (5.94% vs 9.44%; OR: 0.606, 95% CI 0.486–0.755, p < 0.001). Risk factors for developing T2DM were male sex, age, body mass index, hypertension, baseline triglyceride levels and years on statin therapy. The prevalence of T2DM in HeFH patients was 40% lower than that observed in the general population. Gene mutations and LDL cholesterol concentrations were not risk factors associated with the prevalence of T2DM in patients with HeFH. The prevalence of T2DM in patients with HeFH was 40% lower than in the general population matched for age and sex.
Introduction
Heterozygous familial hypercholesterolemia (HeFH), the most frequent monogenic disorder of human metabolism caused by some mutations in the genes that encode for the low-density lipoprotein (LDL) receptor, apolipoprotein (apo) B, proprotein convertase subtilisin/kexin-type 9 (PCSK9) or apo E1, 2, entails an increased risk of premature cardiovascular disease2.
Patients with HeFH had been reported to be less vulnerable to type 2 diabetes mellitus (T2DM) compared to the general population3. More recently, Besseling et al. in a cross-sectional study found the prevalence of T2DM to be 50% lower in HeFH patients compared to unaffected relatives4. One of the mechanisms suggested to explain this finding lies in potential decreased cholesterol uptake by pancreatic β cells in HeFH5. This lower T2DM risk contrasts with the fact that most HeFH patients are treated with high doses of potent statins which exert a dose-dependent diabetogenic effect6, 7. The molecular pathways that may be involved in the resistance to T2DM in HeFH and the statin-related T2DM have not been studied in depth in humans. Suggested mechanisms include defects in insulin signaling, pancreatic β -cell function and reduced glucose uptake in adipocytes throughout the glucose transporter-4 (GLUT4) pathway8. The potential effect of LDLR mutations or LDL cholesterol concentration on these anti-diabetogenic and pro-diabetogenic effects of HeFH and statins, respectively, are unknown.
The cholesterol-lowering effect of statin therapy is mainly due to the inhibition of 3-hydroxy-3-methylglutaryl-CoA reductase, a key enzyme in cholesterol synthesis that induces a reduction in intracellular cholesterol content and a subsequent increase in LDL receptor expression in different tissues and the promotion of transmembrane cholesterol transport9, the primary altered mechanism in FH10. If intracellular cholesterol uptake via the LDL receptor is involved in the pathogenesis of T2DM, then it would explain the hypothetical protection from diabetes in HeFH patients, but only in those with genetic defects affecting LDL receptor uptake, in contrast to the hypothesis that the protection would be dependent on the high plasma LDL-cholesterol concentrations observed in HeFH. Hence, the aims of the present study were to assess the prevalence of T2DM in HeFH patients of the Dyslipidemia Registry of the Spanish Arteriosclerosis Society, and evaluate the impact of mutations in the LDLR, APOB and PCSK9 genes, baseline LDL cholesterol concentration and duration of statin treatment on T2DM prevalence in this population.
Research Design and Methods
Study population
The Dyslipidemia Registry of the Spanish Arteriosclerosis Society was created at the end of 2013 as an active on-line registry in which 50 certified Lipid Units distributed throughout Spain enter cases with different primary hyperlipidemias. These Lipid Units are the centers in the Spanish National Health Service to which most cases of primary hyperlipidemias are referred for diagnosis and treatment. The registry was approved by a central ethics committee to include anonymous clinical data (Comité Ético de Investigación Clínica de Aragón, Zaragoza, Spain) in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. Patients did not have to complete an informed consent form as the data were obtained from an official national registry. Minimum data for the inclusion of cases in the registry are: age, sex, smoking status, personal history of hypertension, diabetes and cardiovascular disease with age at the first event, body mass index (BMI), waist circumference, total cholesterol, LDL cholesterol, triglycerides and high-density lipoprotein (HDL) cholesterol without lipid-lowering treatment, and clinical diagnosis. Cardiovascular disease in the registry is defined as: coronary heart disease (myocardial infarction, acute coronary syndrome with stenosis >50% of a main coronary artery and coronary revascularization), stroke (ischemic and hemorrhagic), aortic aneurysm and lower limb ischemia (intermittent claudication with ankle/brachial index <0.90 or revascularization of lower limb arteries). T2DM is defined in the presence of fasting blood glucose >125 mg/dL or use of blood glucose-lowering drugs.
Inclusion criteria in the present study were: complete minimum data for the registry, plus information on family history of hypercholesterolemia and premature cardiovascular disease; personal history of tendon xanthomas and presence of arcus cornealis before the age of 45; genetic study of LDLR, APOB and PCSK9 genes; age at statin onset; and age at T2DM diagnosis. Only subjects with probable (6–8 points) or definite (>8 points) HeFH according to the Dutch Lipid Clinic Network criteria10 and age ≥18 years were finally included. The diagnosis of homozygous FH (HoFH) was an exclusion criterion.
Of the 2,475 cases with the clinical diagnosis of genetic hypercholesterolemia included in the registry, 1,732 diagnosed of HeFH with Dutch Lipid Clinic Network (DLCN) criteria ≥6 points and complete data were finally included. Reasons for exclusion from the analysis were HoFH or DLCN <6 points and lack of data on genetic analysis, among others.
The prevalence of T2DM was analyzed according to a probable or definite diagnosis of HeFH, the presence of a genetic mutation or the gene involved. Furthermore, T2DM prevalence in HeFH patients was compared with data of the general population matched for age and sex from the Di@bet.es Study. The Di@bet.es Study is a national, cross-sectional, population-based survey conducted in 2009–10 in a representative random sample of the Spanish population11.
Blood specimens were collected after an overnight (>10 h) fast and processed for laboratory analyses the same day. Serum total cholesterol, triglycerides and HDL cholesterol levels were measured locally using enzymatic methods. Serum LDL cholesterol concentration was calculated using the Friedewald formula.
DNA was isolated from whole blood using standard methods and screening for LDLR, APOB and PCSK9 mutations was carried out using the Lipochip Platform (Progenika Biopharma S. A., Bilbao, Spain). The platform consists of two consecutive steps: the first is LIPOchip® microarray analysis for the detection of the most frequent Spanish point mutations in the LDLR gene and in the APOB exon 26, as well as CNVs in LDLR. When the LIPOchip® microarray yielded a negative result (no mutation is found), the LDLR, APOB (binding domain) and PCSK9 gene coding sequences, exon-intron boundaries, and short proximal intronic sequences were sequenced with a GS Junior system (Roche Diagnostics Corporation, Basel, Switzerland)12.
Statistical analysis
Data were expressed as mean ± standard deviation for parametric variables and as median ± interquartile range for non-parametric quantitative variables. The Mann-Whitney U test was used for comparison between groups; quantitative variables were shown as % (n) and chi-square test was applied for qualitative variables between groups. Logistic regression analysis including sex, age, BMI, smoking status, hypertension, tendon xanthomas, baseline LDL and HDL cholesterol and triglyceride concentrations, genetic diagnosis and statin therapy duration was applied to evaluate factors independently associated with the presence of T2DM in HeFH patients. A two-sided p value < 0.05 was considered statistically significant. Statistical analysis was calculated with SPSS (version 19.0 for Windows; SPSS, Chicago, IL).
Results
One thousand, seven hundred and thirty-two patients were included (354 with probable and 1,378 with definite HeFH) (Fig. 1). No differences were found between groups in terms of gender, cardiovascular disease prevalence and HDL cholesterol concentration. As expected because of the DLCN definition, total cholesterol and LDL cholesterol concentrations were higher, and the presence of tendon xanthomas more common in definite than in probable HeFH. In contrast, the prevalence of T2DM and variables associated with the metabolic syndrome and diabetes i.e. age, BMI, waist circumference, triglycerides, glucose and prevalence of hypertension were higher in the probable HeFH group than in definite HeFH. Subjects with definite HeFH had begun statin therapy 7 years earlier than probable HeFH (Table 1). A total of 103 (5.9%) HeFH were diagnosed of T2DM. The characteristics of subjects with and without T2DM are presented in Table 2. Diabetic patients were older and presented higher BMI, waist circumference, triglycerides, glycosylated hemoglobin, cardiovascular disease and hypertension prevalence than non-diabetics. HDL cholesterol was higher and a positive genetic diagnostic test was more common in non-diabetics than in diabetics. The number of years under statin treatment was higher in diabetics than in non-diabetics (Table 2).
Figure 1.
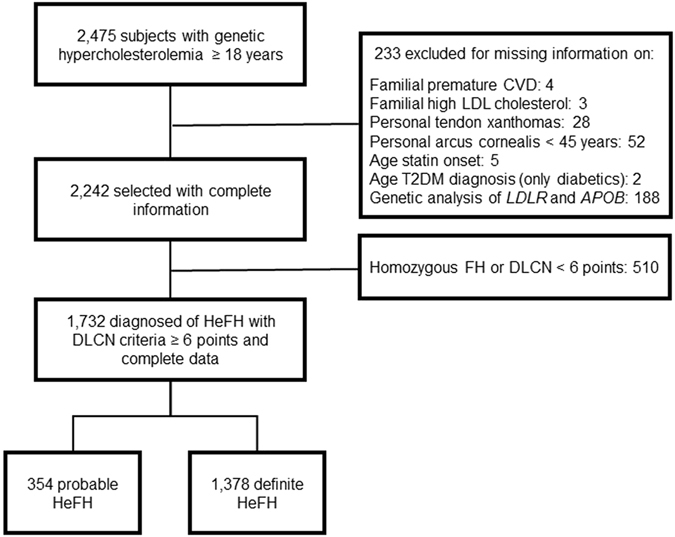
Study flow diagram. HeFH, heterozygous familial hypercholesterolemia; HoFH, homozygous familial hypercholesterolemia; DLCN, Dutch Lipid Clinic Network.
Table 1.
Demographic and clinical characteristics of heterozygous familial hypercholesterolemia patients.
| Characteristics | Probable FH N = 354 | Definite FH N = 1,378 | P |
|---|---|---|---|
| Men, % (n) | 50.0 (177) | 48.9 (674) | 0.715 |
| Age, years | 56.0 (49.0–64.5) | 50.0 (38.0–60.0) | <0.001 |
| Body mass index, kg/m2 | 26.7 (22.5–28.6) | 26.2 (24.0–28.9) | 0.006 |
| Waist circumference, cm | 93.0 (84.5–99.0) | 90.0 (78.0–97.0) | <0.001 |
| Total cholesterol, mg/dL | 300 (272–333) | 340 (298–395) | <0.001 |
| Triglycerides, mg/dL | 143 (99.5–194) | 105 (75.0–143) | <0.001 |
| HDL cholesterol, mg/dL | 54.0 (46.5–64.5) | 53.0 (44.0–64.0) | 0.753 |
| LDL cholesterol, mg/dL | 213 (190–251) | 260 (216–316) | <0.001 |
| Glucose, mg/dL | 91.0 (84.5–97.5) | 89.0 (81.0–98.0) | 0.051 |
| Tendon xanthomas, % (n) | 3.3 (12) | 35.2 (485) | <0.001 |
| Cardiovascular disease, % (n) | 11.9 (42) | 13.4 (185) | 0.489 |
| Hypertension, % (n) | 26.4 (93) | 17.7 (244) | <0.001 |
| Diabetes, % (n) | 9.1 (32) | 5.2 (71) | 0.006 |
| Age at diabetes diagnosis, years | 54.0 (50.8–60.0) | 52.5 (46.5–60.3) | 0.563 |
| Age at statin onset, years | 48.3 (37.0–56.0) | 41.0 (31.0–51.0) | <0.001 |
| Statin therapy, years | 5.0 (2.0–11.0) | 7.0 (3.0–13.0) | <0.001 |
Values are expressed as median (interquartile range) for continuous variables and percentage (n) for categorical variables.
HDL denotes high-density lipoprotein; LDL, low-density lipoprotein.
Table 2.
Demographic and clinical characteristics of heterozygous familial hypercholesterolemia patients with or without diabetes.
| Characteristics | Diabetes N = 103 | No diabetes N = 1,629 | P |
|---|---|---|---|
| Men, % (n) | 58.3 (60) | 48.6 (792) | 0.057 |
| Age, years | 64 (55.0–69.0) | 51.0 (40.0–60.0) | <0.001 |
| Body mass index, kg/m2 | 28.9 (25.3–33.3) | 25.5 (22.8–28.4) | <0.001 |
| Waist circumference, cm | 100 (92.0–108) | 89.0 (80.0–96.0) | <0.001 |
| Total cholesterol, mg/dL | 348 (291–395) | 331 (292–384) | 0.318 |
| Triglycerides, mg/dL | 189 (129–256) | 106 (78.0–155) | <0.001 |
| HDL cholesterol, mg/dL | 47.5 (35.0–59.0) | 53.0 (45.0–64.0) | <0.001 |
| LDL cholesterol, mg/dL | 255 (202–312) | 253 (211–300) | 0.960 |
| HbA1c*, % | 6.7 (6.2–7.4) | 5.5 (5.3–5.9) | <0.001 |
| Tendon xanthomas, % (n) | 32.0 (33) | 28.7 (468) | 0.487 |
| Positive genetic diagnosis, % (n) | 46.6 (48) | 64.6 (1052) | 0.005 |
| Cardiovascular disease, % (n) | 40.8 (42) | 11.3 (184) | <0.001 |
| Hypertension, % (n) | 64.1 (66) | 16.7 (271) | <0.001 |
| Age at diabetes diagnosis, years | 53.0 (48.0–60–0) | — | |
| Age at statin onset, years | 51.5 (43.0–59.0) | 42.0 (31.5–52.0) | <0.001 |
| Statin therapy, years | 10.0 (6.0–17.0) | 7.0 (2.5–12.0) | <0.001 |
*Values refer to 87 (84.5%) diabetics and 410 (25.2%) non-diabetics.
The overall and divided-by-age group gender and genetic diagnosis prevalence of T2DM in HeFH with the odds ratios (OR) compared with the prevalence of the age- and gender-adjusted Spanish population are shown in Table 3. T2DM was more common in males than in females and increased with age in HeFH and in the general population. However, T2DM was significantly less frequent among HeFH, 5.94% versus 9.44% in the general population (OR: 0.606, 95% CI 486–0.755, p < 0.001). This difference was also present when only HeFH with a positive genetic diagnosis (OR: 0.438, 95% CI 0.323–0.593, p < 0.001) were considered.T2DM protection in HeFH was similar in all age groups and in both genders, but reached statistical significance only in older groups, owing to the low T2DM prevalence in subjects under 46 years of age (Table 3). Subjects with a functional mutation in candidate genes were older, had higher BMI, waist circumference, triglyceride and glucose concentrations, and a higher prevalence of hypertension and T2DM than HeFH without mutations (Supplementary Table). On the other hand, no differences were observed in the prevalence of T2DM when patients were separated into different subgroups according to LDL-cholesterol concentrations (Fig. 2).
Table 3.
Prevalence of type 2 diabetes in a reference population and in heterozygous familial hypercholesterolemia patients by sex and age.
| 18–45 years | 46–60 years | >60 years | Total | |
|---|---|---|---|---|
| Men | ||||
| General population, N/Total (%) | 14/909 (1.54) | 69/578 (11.9) | 163/687 (23.7) | 246/2174 (11.3) |
| HeFH, N/Total (%) | 2/337 (0.59) | 23/338 (6.80) | 35/178 (19.7) | 60/853 (7.03) |
| Odds ratio (95% CI) | 0.382 (0.086–1.688) | 0.538 (0.329–0.881) | 0.787 (0.523–1.185) | 0.593 (0.442–0.796) |
| P | 0.261 | 0.012 | 0.272 | <0.001 |
| HeFH mutation+, N/Total (%) | 2/246 (0.81) | 15/188 (7.98) | 13/103 (12.6) | 30/537 (5.59) |
| Odds ratio (95% CI) | 0.524 (0.118–2.231) | 0.639 (0.356–1.147) | 0.478 (0.263–0.870) | 0.470 (0.319–0.694) |
| P | 0.545 | 0.141 | 0.010 | <0.001 |
| Women | ||||
| General population, N/Total (%) | 9/1222 (0.74) | 54/818 (6.60) | 170/858 (19.8) | 233/2898 (8.04) |
| HeFH, N /Total (%) | 2/277 (0.72) | 18/318 (5.66) | 23/285 (8.07) | 43/880 (4.89) |
| Odds ratio (95% CI) | 0.980 (0.211–4.562) | 0.849 (0.489–1.471) | 0.355 (0.225–0.562) | 0.588 (0.421–0.821) |
| P | 0.979 | 0.684 | <0.001 | 0.002 |
| HeFH mutation+, N/Total (%) | 2/221 (0.90) | 6/185 (3.24) | 10/157 (6.37) | 18/563 (3.20) |
| Odds ratio (95% CI) | 1.455 (0.359–5.093) | 0.508 (0.222–1.164) | 0.287 (0.150–0.549) | 0.387 (0.239–0.627) |
| P | 0.597 | 0.087 | <0.001 | <0.001 |
| All | ||||
| General population, N/Total (%) | 23/2131 (1.08) | 123/1396 (8.81) | 333/1545 (21.6) | 479/5072 (9.44) |
| HeFH, N /Total (%) | 4/614 (0.65) | 41/656 (6.25) | 58/463 (12.5) | 103/1733 (5.94) |
| Odds ratio (95% CI) | 0.601 (0.207–1.744) | 0.689 (0.478–0.995) | 0.521 (0.386–0.704) | 0.606 (0.486–0.755) |
| P | 0.343 | 0.046 | <0.001 | <0.001 |
| HeFH mutation+, N/Total (%) | 4/467 (0.86) | 21/373 (5.63) | 23/260 (8.85) | 48/1100 (4.36) |
| Odds ratio (95% CI) | 0.791 (0.273–2.300) | 0.617 (0.383–0.995) | 0.353 (0.226–0.551) | 0.438 (0.323–0.593) |
| P | 0.667 | 0.046 | <0.001 | <0.001 |
Figure 2.
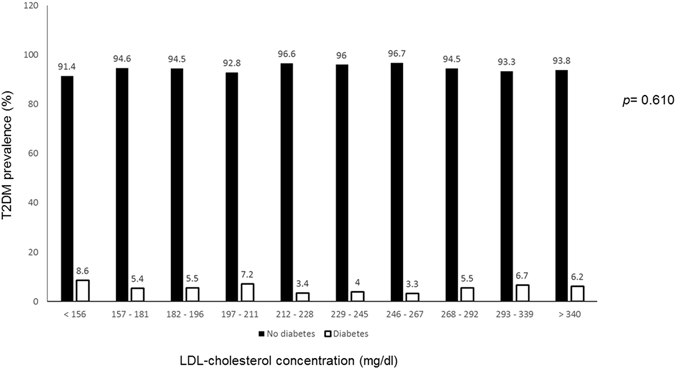
Prevalence of type 2 diabetes mellitus in heterozygous familial hypercholesterolemic patients according to LDL-cholesterol concentrations.
Risk factors for HeFH patients developing T2DM are presented in Table 4 for the whole HeFH group and in Table 5 for patients with a positive genetic diagnosis. Male sex, BMI, hypertension and years on statin therapy were independently associated with the presence of T2DM in both groups. These risk factors could account for up to 26% of the variability in presenting T2DM (Table 4). Other factors such as age, BMI, smoking status, xanthomas, baseline LDL, HDL cholesterol and triglyceride concentrations did not reach statistical significance.
Table 4.
Factors associated with the presence of type 2 diabetes mellitus in clinically-defined heterozygous familial hypercholesterolemia patients (≥6 DLCN points).
| Standard coeff. (β) | Exp (B) | 95% CI | P | R2 | |
|---|---|---|---|---|---|
| Sex, male | 0.649 | 1.913 | 1.204–3.040 | 0.006 | 0.261 |
| Age, years | 0.050 | 1.052 | 1.030–1.074 | <0.001 | |
| BMI, kg/m2 | 0.021 | 1.021 | 1.007–1.035 | 0.004 | |
| Hypertension, yes | 1.522 | 4.582 | 2.834–7.407 | <0.001 | |
| Triglycerides, mg/dL | 0.002 | 1.002 | 1.001–1.003 | 0.001 | |
| Statin therapy, years | 0.031 | 1.031 | 1.003–1.060 | 0.03 |
DLCN denotes Dutch Lipid Clinic Network; BMI, body mass index.
Independent variables included in the model: sex, age, BMI, smoking status, hypertension, tendon xanthomas, baseline LDL and HDL cholesterol and triglyceride concentrations, genetic diagnosis and statin therapy duration.
Table 5.
Factors associated with the presence of type 2 diabetes mellitus in genetically-defined heterozygous familial hypercholesterolemia patients (with a pathogenic mutation in LDLR, APOB or PCSK9 genes).
| Standard coeff. (β) | Exp (B) | 95% CI | p | R2 | |
|---|---|---|---|---|---|
| Sex, male | 0.713 | 2.041 | 1.081–3.853 | 0.028 | 0.195 |
| BMI, kg/m2 | 0.018 | 1.018 | 1.003–1.034 | 0.022 | |
| Hypertension, yes | 2.072 | 7.942 | 4.249–14.845 | <0.001 | |
| Statin therapy, years | 0.044 | 1.045 | 1.002–1.090 | 0.040 |
BMI denotes body mass index.
Independent variables included in the model: sex, age, BMI, smoking status, hypertension, tendon xanthomas, baseline LDL and HDL cholesterol and triglyceride concentrations, genetic diagnosis and statin therapy duration.
Finally, different regression models were applied to identify the association of a pathogenic mutation in LDLR, APOB or PCSK9 in the presence of T2DM. None of the models with different adjustments showed any significant association of a positive genetic diagnosis with T2DM (Table 6).
Table 6.
Effect of phenotype severity in heterozygous familial hypercholesterolemia on type 2 diabetes prevalence.
| Comparison | All | HeFH with LDL <250 mg/dL | HeFH with LDL ≥250 mg/dL | HeFH with >8 points |
|---|---|---|---|---|
| Odds ratio (95% CI) Unadjusted | 0.821 (0.429–1.572) | 0.736 (0.287–1.887) | 2.384 (0.557–10.207) | 0.707 (0.294–1.696) |
| Odds ratio (95% CI) Adjusted* | 0.954 (0.488–1.866) | 1.386 (0.498–3.852) | 2.395 (0.549–10.444) | 0.747 (0.303–1.839) |
| Odds ratio (95% CI) Adjusted† | 0.936 (0.478–1.835) | 1.388 (0.496–3.887) | 2.806 (0.631–12.478) | 0.735 (0.298–1.813) |
| Odds ratio (95% CI) Adjusted‡ | 1.072 (0.533–2.155) | 1.474 (0.512–4.243) | 3.056 (0.670–13.930) | 0.877 (0.341–2.250) |
*Adjusted for age, sex and years of statin use.
†Adjusted for age, sex, years of statin use and body mass index.
‡Adjusted for age, sex, years of statin use, body mass index, triglycerides, and hypertension.
Discussion
The prevalence of T2DM in HeFH patients in the present study was 5.9%, lower than the known 9.4% prevalence in the age and sex-adjusted Spanish population11. In a Dutch cohort, the prevalence of T2DM in HeFH patients was 1.75% compared to 2.93% in unaffected relatives4. This may be partially explained by younger age in the Dutch HeFH cohort, more than one decade, compared to our population and the different criteria used in the diagnosis of T2DM between studies, only auto-reported in the Dutch cohort4. Nevertheless, our study confirms the Dutch results in a completely different population, showing that HeFH subjects present an approximately 50% lower T2DM risk than the general population. This T2DM protection has been questioned in favor of a role for cellular cholesterol metabolism in the pathogenesis of type 2 diabetes4, 5.
Besides this confirmatory information, several important conclusions can be drawn from our study. First, the LDL cholesterol concentration before the start of lipid-lowering treatment did not play a major role in T2DM protection in HeFH in our study. The lower risk of T2DM appeared in a large LDL cholesterol range, and the LDL cholesterol concentration did not significantly affect T2DM risk. Although there is in-vivo and ex-vivo evidence showing that LDL concentration modulates insulin secretion and pancreatic β-cell survival13, large prospective studies showed no significant effect of LDL cholesterol on T2DM risk, in contrast to the association with high triglycerides or low HDL cholesterol that has been repeatedly demonstrated14, 15. In human β cell islets, LDL overload lowered insulin secretion16 and diet-induced hypercholesterolemia also reduced insulin secretion in mice17; however, this diabetogenic effect of hypercholesterolemic diets has also been associated with saturated fatty acid accumulation rather than with cholesterol16, 18. Second, in the present study, no differences were observed in T2DM prevalence according to the presence or absence of causative gene mutations responsible for HeFH. Our study is the first to include clinically-diagnosed patients and analyzed T2DM prevalence according to LDLR, APOB or PCSK9 mutations. Besseling et al. included HeFH only with a confirmed pathogenic mutation, and found a dose-dependent association in HeFH subjects with LDLR negative mutations who had a lower T2DM prevalence than HeFH carriers of defective LDLR or APOB mutations, suggesting that the severity of the genetic defect plays a role in T2DM protection4. The implication of the LDL receptor in glucose homeostasis is controversial, since the deleterious effect of cholesterol overload on insulin secretion by pancreatic β cells was similar in LDLR-/- knockout or wild-type mice19, and oxidized LDL, which does not use the LDL receptor, was found to be a major contributor to β cell dysfunction20. Furthermore, some FH-causing mutations seem to be associated with a higher T2DM risk21. In our study, the presence of any LDLR/APOB/PCSK9 mutation was less common in HeFH with than without T2DM; however, these differences disappeared after adjustment for confounders unrelated to LDL receptor function.
Third, the factors associated with the presence of T2DM in HeFH are the same as those present in the general population, including statin use. The cluster of abnormalities closely associated with insulin resistance: high blood pressure, high triglycerides, low HDL cholesterol, increased BMI and older age22, are also strongly related to the presence of T2DM in HeFH, as previously described23. In this observational, cross-sectional study, we could not calculate whether the impact of T2DM risk factors is different in HeFH than in non-FH; however, we did have a close approximation for statin use as this information was available. Statin use preceded the diagnosis of T2DM in most cases and the risk associated with statin use was identical to that observed in statin trials after adjustment for insulin-resistance markers6, 7, 24.
Thus, if LDL cholesterol or LDL receptor function itself are not responsible for the T2DM protection in HeFH, which mechanisms are involved? Potential explanations have been previously put forward: the observational cross-sectional design without power to establish causality; the possibility of unexplored potential confounders; a healthier lifestyle after receiving the diagnosis of HeFH; or a shorter life-expectancy in patients with HeFH and T2DM4 could be responsible for this observation. The HDL particle is also an unexplored potential protective factor of T2DM in HeFH25. In HeFH, HDL cholesterol is usually within normal range10, 26 and, in contrast with other dyslipidemias, HDL particle size and number are in antiatherogenic range27. Indeed, the Dutch HeFH patients were leaner, smoked less and had lower triglycerides than their unaffected relatives without a clear explanation, except for a healthier lifestyle associated with the diagnosis4. In our cohort of HeFH patients, the prevalence of hypertension, overweight, obesity and tobacco use was much lower than that observed in the Spanish general population28, 29. Altogether, these data suggest that environmental factors could be involved in this T2DM protection in these patients cared for in highly specialized lipid units. Actually, the reduction in BMI compared with the reference population could explain one third of the lower risk of T2DM30. A large case-control study is underway to try to answer these questions.
Our study has some limitations. In this respect, the number of HeFH with T2DM was relatively low, but the concurrence with previous data supports the T2DM protection. Our study was cross-sectional, and results other than prevalence should thus be taken with caution. The sample was composed of middle-aged men and women from a Mediterranean country, with traditionally lower coronary heart disease rates than other populations, albeit with a high prevalence of T2DM11. Furthermore, patients included in the Spanish Dyslipidemia Registry are treated with a standardized protocol promoting the use of high-intensity lipid-lowering treatment in highly specialized Lipid Clinics; thus, the results cannot be easily extrapolated to other clinical settings. Finally, the observed prevalence of T2DM in HeFH could be underestimated since patients with HeFH are at increased risk for cardiovascular disease and some will have died before their inclusion in the Dyslipidemia Spanish registry. If HeFH was associated with T2DM, the possibility of early death from cardiovascular causes was even higher. On the other hand, T2DM prevalence increases with age and HeFH patients who died prematurely might have been less likely to develop T2DM; thus, an overestimation of T2DM in our cohort is also possible. In any event, the mean age of HeFH patients was around 55 years, and the prevalence of T2DM increased with age in both HeFH patients and in the general population.
Conclusions
In summary, the absolute prevalence of T2DM in patients with HeFH was 40% lower than that observed in the general population matched for age and sex. Risk factors independently associated with the presence of T2DM in subjects with HeFH were age, male sex, BMI, baseline triglycerides, hypertension and years of statin treatment. LDL cholesterol concentrations and the presence of mutations in FH-causing genes were not associated with the presence of T2DM in HeFH subjects.
Electronic supplementary material
Acknowledgements
We thank the Lipid Units for inclusion of cases in the Dyslipidemia Registry of the Spanish Arteriosclerosis Society, and Miss Christine O’Hara for review of the English version of the manuscript. CIBEROBN is an initiative of ISCIII, Spain. S. Perez-Calahorra: grants from Amgen, Sanofi and Pfizer. E.Ros has received lecture honoraria, consultancy fees and/or research funding from Aegerion, Alexion, MSD, Pfizer and Sanofi. F. Civeira: grants, consulting fees, and/or honoraria from Amgen, Merck, Pfizer, and Sanofi-Aventis. J. Pedro-Botet has received lecture honoraria, consultancy fees and/or research funding from Astra-Zeneca, Esteve, Merck, Mylan and Sanofi-Aventis.
Author Contributions
E.C.: acquisition, analysis, and interpretation of data for the work; drafting the work; S.P.-C.: acquisition and statistical analysis of data. V.M.-B.: analysis and interpretation of data for the work. N.P.: interpretation of data for the work, revising it critically. R.S.: acquisition and statistical analysis of data. E.R.: interpretation of data for the work, revising it critically. J.F.A.: interpretation of data for the work, revising it critically. J.P.: data acquisition. F.A.: data acquisition. C.L.: data acquisition. F.C.: conception and design of the work; analysis and interpretation of data for the work. J.P.-B.: conception and design of the work; analysis and interpretation of data for the work; drafting the work. All authors have read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06101-6
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hovingh GK, Davidson MH, Kastelein JJ, O’Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur. Heart. J. 2013;34:962–971. doi: 10.1093/eurheartj/eht015. [DOI] [PubMed] [Google Scholar]
- 2.Cenarro A, et al. The p.Leu167del mutation in APOE gene causes autosomal dominant hypercholesterolemia by down-regulation of LDL receptor expression in hepatocytes. J. Clin. Endocrinol. Metab. 2016;101:2113–2121. doi: 10.1210/jc.2015-3874. [DOI] [PubMed] [Google Scholar]
- 3.Vohl MC, et al. Comparison of the effect of two low-density lipoprotein receptor class mutations on coronary heart disease among French-Canadian patients heterozygous for familial hypercholesterolaemia. Eur. J. Clin. Invest. 1997;27:366–373. doi: 10.1046/j.1365-2362.1997.1250669.x. [DOI] [PubMed] [Google Scholar]
- 4.Besseling J, Kastelein JJ, Defesche JC, Hutten BA, Hovingh GK. Association between familial hypercholesterolemia and prevalence of type 2 diabetes mellitus. JAMA. 2015;313:1029–1036. doi: 10.1001/jama.2015.1206. [DOI] [PubMed] [Google Scholar]
- 5.Preiss D, Sattar N. Does the LDL receptor play a role in the risk of developing type 2 diabetes? JAMA. 2015;313:1016–1017. doi: 10.1001/jama.2015.1275. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 7.Preiss D, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305:2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 8.Katsiki N, Rizzo M, Mikhailidis DP, Mantzoros CS. New-onset diabetes and statins: throw the bath water out, but, please, keep the baby! Metabolism. 2015;64:471–475. doi: 10.1016/j.metabol.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Miller PE, Martin SS. Approach to statin use in 2016: an update. Curr. Atheroscler. Rep. 2016;18:20. doi: 10.1007/s11883-016-0578-1. [DOI] [PubMed] [Google Scholar]
- 10.Nordestgaard BG, et al. European Atherosclerosis Society Consensus Panel. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: guidance for clinicians to prevent coronary heart disease: consensus statement of the European Atherosclerosis Society. Eur. Heart. J. 2013;34:3478–3490. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soriguer F, et al. Prevalence of diabetes mellitus and impaired glucose regulation in Spain: the Di@bet.es Study. Diabetologia. 2012;55:88–93. doi: 10.1007/s00125-011-2336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stef MA, et al. A DNA microarray for the detection of point mutations and copy number variation causing familial hypercholesterolemia in Europe. J. Mol. Diagn. 2013;15:362–372. doi: 10.1016/j.jmoldx.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Paul R, Choudhury A, Choudhury S, Mazumder MK, Borah A. Cholesterol in pancreatic β-cell death and dysfunction: underlying mechanisms and pathological implications. Pancreas. 2016;45:317–324. doi: 10.1097/MPA.0000000000000486. [DOI] [PubMed] [Google Scholar]
- 14.von Eckardstein A, Schulte H, Assmann G. Risk for diabetes mellitus in middle-aged Caucasian male participants of the PROCAM study: implications for the definition of impaired fasting glucose by the American Diabetes Association. Prospective Cardiovascular Münster. J. Clin. Endocrinol. Metab. 2000;85:3101–3108. doi: 10.1210/jcem.85.9.6773. [DOI] [PubMed] [Google Scholar]
- 15.Imamura F, et al. Risk factors for type 2 diabetes mellitus preceded by β-cell dysfunction, insulin resistance, or both in older adults: the Cardiovascular Health Study. Am. J. Epidemiol. 2013;177:1418–1429. doi: 10.1093/aje/kws440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutti S, et al. Low and high density lipoproteins modulate function, apoptosis and proliferation of primary human and murine pancreatic beta cells. Endocrinology. 2009;150:4521–4530. doi: 10.1210/en.2009-0252. [DOI] [PubMed] [Google Scholar]
- 17.Kruit JK, et al. Cholesterol efflux via ATP-binding cassette transporter A1 (ABCA1) and cholesterol uptake via the LDL receptor influences cholesterol-induced impairment of beta cell function in mice. Diabetologia. 2010;53:1110–1119. doi: 10.1007/s00125-010-1691-2. [DOI] [PubMed] [Google Scholar]
- 18.Boslem E, et al. Alteration of endoplasmic reticulum lipid rafts contributes to lipotoxicity in pancreatic β-cells. J. Biol. Chem. 2013;288:26569–26582. doi: 10.1074/jbc.M113.489310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonfleur ML, et al. Primary hypercholesterolaemia impairs glucose homeostasis and insulin secretion in low-density lipoprotein receptor knockout mice independently of high-fat diet and obesity. Biochim. Biophys. Acta. 2010;1801:183–190. doi: 10.1016/j.bbalip.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Abderrahmani A, et al. Human high-density lipoprotein particles prevent activation of the JNK pathway induced by human oxidised low-density lipoprotein particles in pancreatic beta cells. Diabetologia. 2007;50:1304–1314. doi: 10.1007/s00125-007-0642-z. [DOI] [PubMed] [Google Scholar]
- 21.Saavedra YG, Dufour R, Baass A. Familial hypercholesterolemia: PCSK9 InsLEU genetic variant and prediabetes/diabetes risk. J. Clin. Lipidol. 2015;9:786–793. doi: 10.1016/j.jacl.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 22.Reaven GM. Statins, familial hypercholesterolemia and type 2 diabetes. J. Intern. Med. 2016;280:421–422. doi: 10.1111/joim.12537. [DOI] [PubMed] [Google Scholar]
- 23.Fuentes F, et al. SAFEHEART Investigators. Statins do not increase the risk of developing type 2 diabetes in familial hypercholesterolemia: The SAFEHEART study. Int. J. Cardiol. 2015;201:79–84. doi: 10.1016/j.ijcard.2015.07.107. [DOI] [PubMed] [Google Scholar]
- 24.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Eckardstein A, Sibler RA. Possible contributions of lipoproteins and cholesterol to the pathogenesis of diabetes mellitus type 2. Curr. Opin. Lipidol. 2011;22:26–32. doi: 10.1097/MOL.0b013e3283412279. [DOI] [PubMed] [Google Scholar]
- 26.Civeira F, et al. Comparison of genetic versus clinical diagnosis in familial hypercholesterolemia. Am. J. Cardiol. 2008;102:1187–1193. doi: 10.1016/j.amjcard.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 27.Jarauta E, et al. Carotid atherosclerosis and lipoprotein particle subclasses in familial hypercholesterolaemia and familial combined hyperlipidaemia. Nutr. Metab. Cardiovasc. Dis. 2012;22:591–597. doi: 10.1016/j.numecd.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Grau M, et al. Cardiovascular risk factors in Spain in the first decade of the 21st century, a pooled analysis with individual data from 11 population-based studies: The DARIOS Study. Rev. Esp. Cardiol. 2011;64:295–304. doi: 10.1016/j.recesp.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 29.García-Esquinas E, et al. Obesity, fat distribution, and risk of frailty in two population-based cohorts of older adults in Spain. Obesity (Silver Spring) 2015;23:847–55. doi: 10.1002/oby.21013. [DOI] [PubMed] [Google Scholar]
- 30.Vazquez G, Duval S, Jacobs DR, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol. Rev. 2007;29:115–28. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


