Abstract
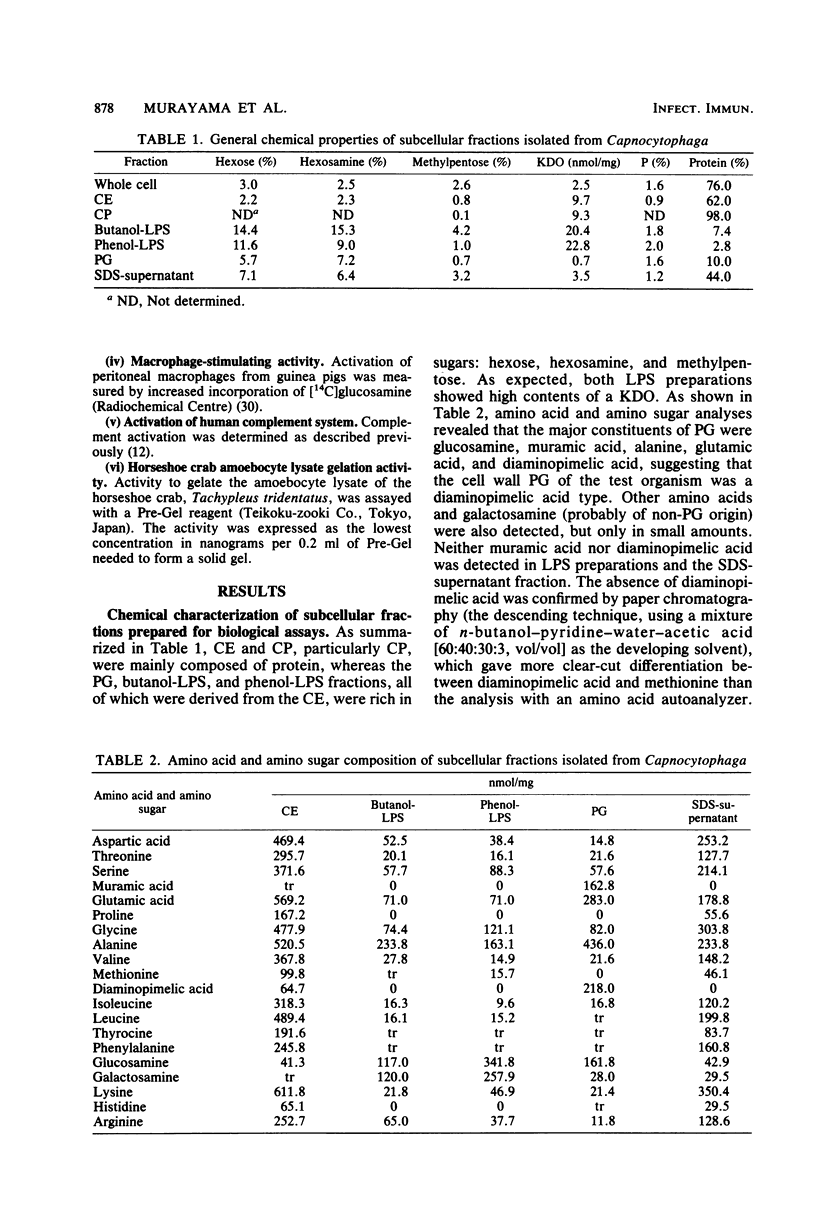
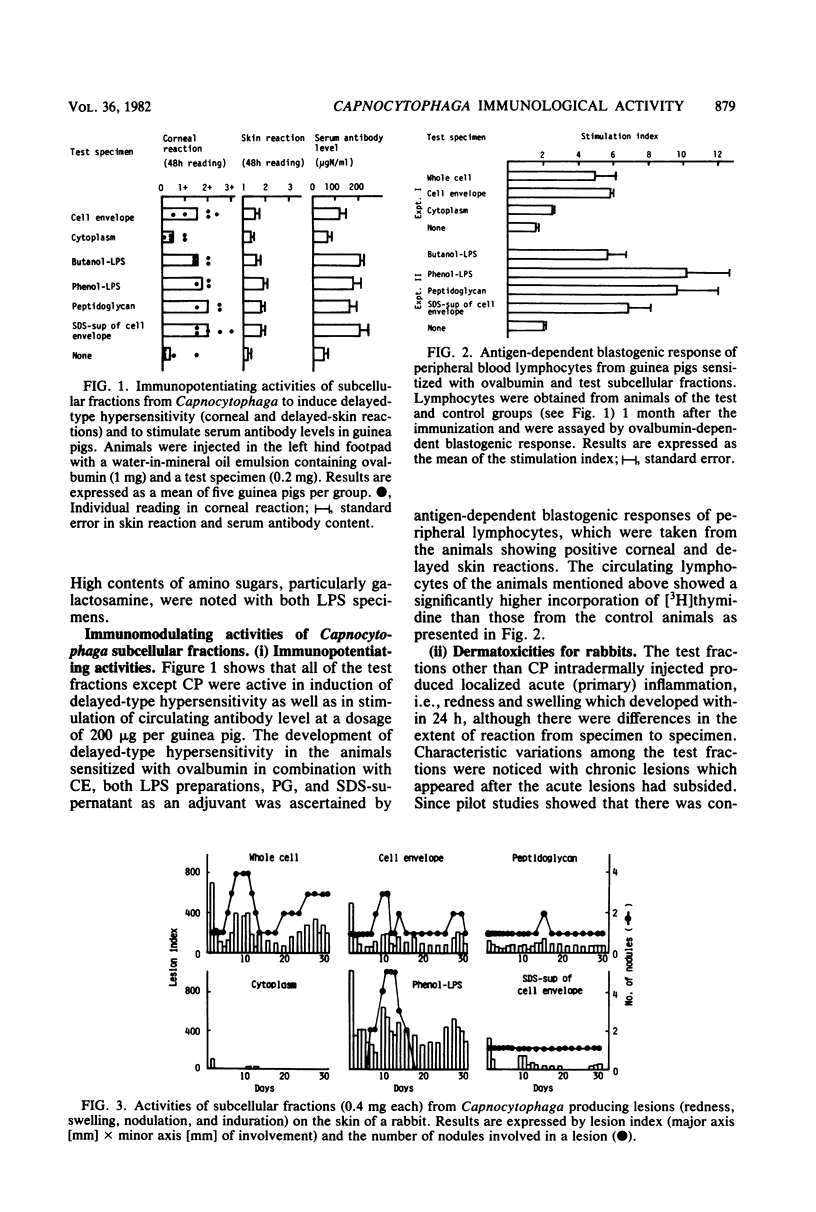
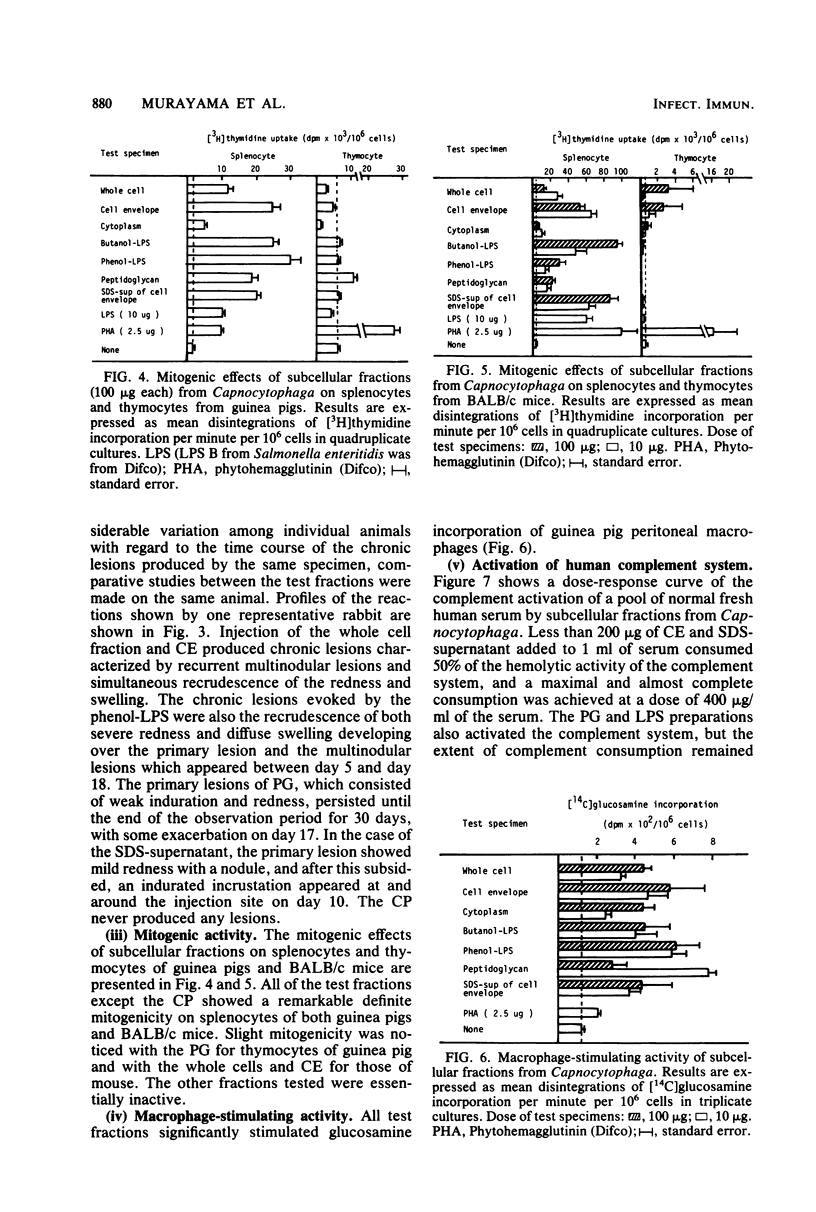
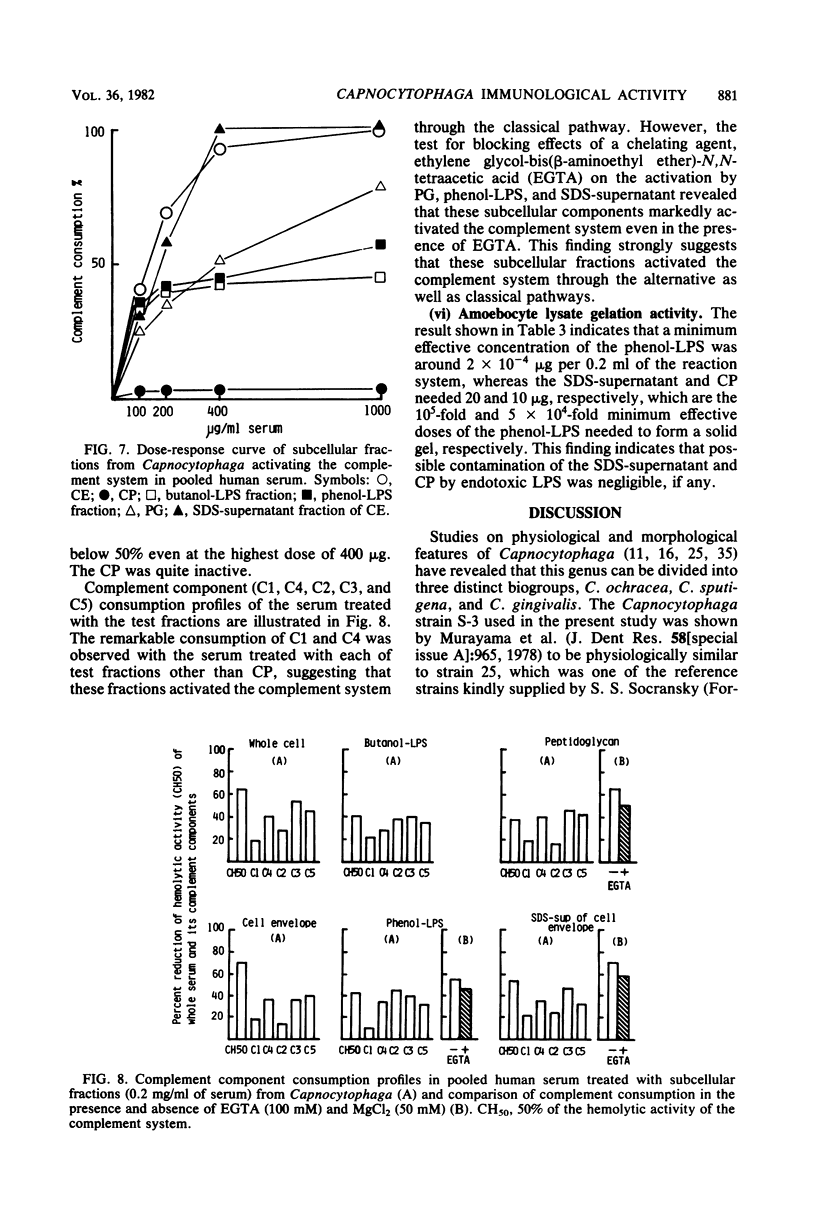
Whole cells of a clinical isolate (strain S-3) of the genus Capnocytophaga were divided into cell envelope (CE) and cytoplasm (CP) fractions by mechanical disintegration followed by differential centrifugation, and a part of the CE fraction was further fractionated by sodium dodecyl sulfate (SDS) treatment into the peptidoglycan and SDS-supernatant fractions. The other part of the CE was extracted with butanol-water or hot phenol-water to isolate butanol-lipopolysaccharide and phenol-lipopolysaccharide, respectively. All of the test fractions except CP exhibited multifold immunomodulating activities, namely, the adjuvant activities to cellular as well as humoral immune responses against ovalbumin in guinea pigs, the mitogenicity on splenocytes of guinea pigs and BALB/c mice (but not on their thymocytes), the stimulation of guinea pig peritoneal macrophages (in terms of increased glucosamine uptake), and the activation of the human complement system through alternative as well as classical pathways. In addition, the test fractions other than the CP evoked dermatoxic reactions on rabbit skin with characteristic variations among them. The immunomodulating activities of SDS-supernatant were noteworthy in view of the fact that this fraction was essentially free of muramic acid and diaminopimelic acid and did not cause the gelation of horseshoe crab amoebocyte lysate except when it was used at the very high dose, suggesting that there was practically no contamination by peptidoglycans and lipopolysaccharides in the SDS-supernatant.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz S. J., Morrison D. C. Chemical and biologic properties of a protein-rich fraction of bacterial lipopolysaccharides. I. The in vitro murine lymphocyte response. J Immunol. 1977 Oct;119(4):1475–1481. [PubMed] [Google Scholar]
- Bona C., Damais C., Chedid L. Blastic transformtion of mouse spleen lymphocytes by a water-soluble mitogen extracted from Nocardia. Proc Natl Acad Sci U S A. 1974 May;71(5):1602–1606. doi: 10.1073/pnas.71.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Damais C., Dimitriu A., Chedid L., Ciorbaru R., Adam A., Petit J. F., Lederer E., Rosselet J. P. Mitogenic effect of a water-soluble extract of Nocardia opaca: a comparative study with some bacterial adjuvants on spleen and peripheral lymphocytes of four mammalian species. J Immunol. 1974 Jun;112(6):2028–2035. [PubMed] [Google Scholar]
- Ciorbaru R., Adam A., Petit J. F., Lederer E., Bona C., Chedid L. Isolation of mitogenic and adjuvant active fractions from various species of Nocardiae. Infect Immun. 1975 Feb;11(2):257–264. doi: 10.1128/iai.11.2.257-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman G. W., Sultzer B. M. Endotoxin protein is a mitogen and polyclonal activator of human B lymphocytes. J Exp Med. 1979 Mar 1;149(3):713–723. doi: 10.1084/jem.149.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman M. G., Parks D. E., Weigle W. O. Immunologic responsiveness of the C3H/HeJ mouse: differential ability of butanol-extracted lipopolysaccharide (LPS) to evoke LPS-mediated effects. J Exp Med. 1978 Mar 1;147(3):800–813. doi: 10.1084/jem.147.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R., Socransky S. S. Capnocytophaga: new genus of gram-negative gliding bacteria. II. Morphology and ultrastructure. Arch Microbiol. 1979 Jul;122(1):17–27. doi: 10.1007/BF00408041. [DOI] [PubMed] [Google Scholar]
- Inal S., Nagaki K., Ebisu S., Kato K., Kotani S. Activation of the alternative complement pathway by water-insoluble glucans of streptococcus mutans: the relation between their chemical structures and activating potencies. J Immunol. 1976 Oct;117(4):1256–1260. [PubMed] [Google Scholar]
- Ivanyi L., Lehner T. Stimulation of lymphocyte transformation by bacterial antigens in patients with periodontal disease. Arch Oral Biol. 1970 Nov;15(11):1089–1096. doi: 10.1016/0003-9969(70)90121-4. [DOI] [PubMed] [Google Scholar]
- Kato K., Iwata S., Suginaka H., Namba K., Kotani S. Chemical structure of the peptidoglycan of Vibrio parahaemolyticus A55 with special reference to the extent of interpeptide cross-linking. Biken J. 1976 Dec;19(4):139–150. [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Leadbetter E. R., Holt S. C., Socransky S. S. Capnocytophaga: new genus of gram-negative gliding bacteria. I. General characteristics, taxonomic considerations and significance. Arch Microbiol. 1979 Jul;122(1):9–16. doi: 10.1007/BF00408040. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. C., Kline L. F. Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J Immunol. 1977 Jan;118(1):362–368. [PubMed] [Google Scholar]
- Narita T. [Comparative studies on biological and immunological properties of bacterial and fungal cell walls, and solubilization and modification of active principle(s) by cell wall lytic enzymes]. Osaka Daigaku Shigaku Zasshi. 1974 Jun;19(1):81–99. [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster G. S., Hayashi J. A., Bahn A. N. Toxic properties of the cell wall of gram-positive bacteria. J Bacteriol. 1967 Jan;93(1):47–52. doi: 10.1128/jb.93.1.47-52.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurin S. B., Socransky S. S., Sweeney E., Stossel T. P. A neutrophil disorder induced by capnocytophaga, a dental micro-organism. N Engl J Med. 1979 Oct 18;301(16):849–854. doi: 10.1056/NEJM197910183011601. [DOI] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Socransky S. S., Holt S. C., Leadbetter E. R., Tanner A. C., Savitt E., Hammond B. F. Capnocytophaga: new genus of gram-negative gliding bacteria. III. Physiological characterization. Arch Microbiol. 1979 Jul;122(1):29–33. doi: 10.1007/BF00408042. [DOI] [PubMed] [Google Scholar]
- Stevens R. H., Sela M. N., McArthur W. P., Nowotny A., Hammond B. F. Biological and chemical characterization of endotoxin from Capnocytophaga sputigena. Infect Immun. 1980 Jan;27(1):246–254. doi: 10.1128/iai.27.1.246-254.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Sela M. N., Shapira J., Hammond B. F. Detection of a fibroblast proliferation inhibitory factor from Capnocytophaga sputigena. Infect Immun. 1980 Jan;27(1):271–275. doi: 10.1128/iai.27.1.271-275.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Hirachi Y., Hashizume H., Kotani S. Mitogenic activity of cytoplasmic membranes isolated from L-forms of Staphylococcus aureus. Microbiol Immunol. 1980;24(11):1079–1090. doi: 10.1111/j.1348-0421.1980.tb02913.x. [DOI] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kato K., Kotani S., Kusumoto S., Inage M., Shiba T., Yano I., Kawata S., Yokogawa K. Macrophage activation by bacterial cell walls and related synthetic compounds. Infect Immun. 1979 Jul;25(1):48–53. doi: 10.1128/iai.25.1.48-53.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Hammond B. F., Baehni P., McArthur W. P., Taichman N. S. Interaction of inflammatory cells and oral microorganisms. VI. Exocytosis of PMN lysosomes in response to gram-negative plaque bacteria. J Periodontal Res. 1978 Nov;13(6):504–512. doi: 10.1111/j.1600-0765.1978.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Williams B. L., Hollis D., Holdeman L. V. Synonymy of strains of Center for Disease Control group DF-1 with species of Capnocytophaga. J Clin Microbiol. 1979 Oct;10(4):550–556. doi: 10.1128/jcm.10.4.550-556.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


