Abstract
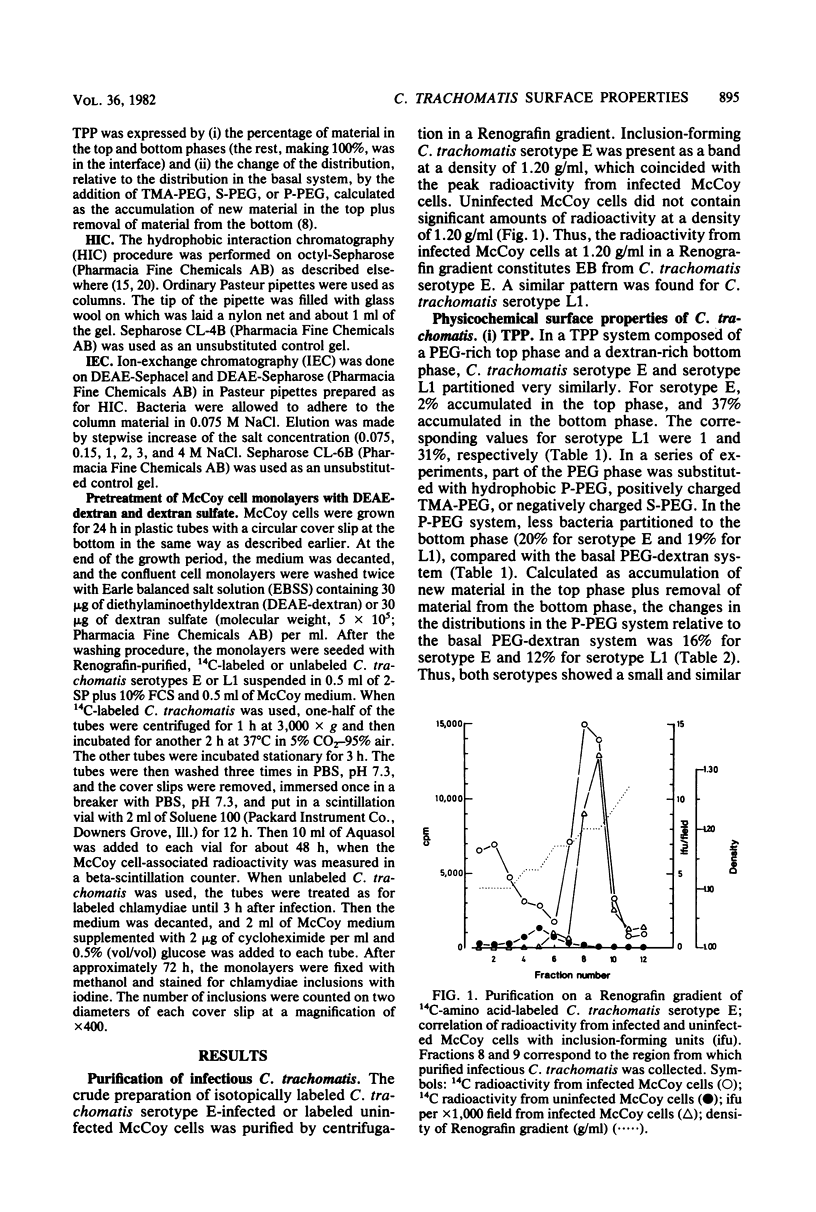
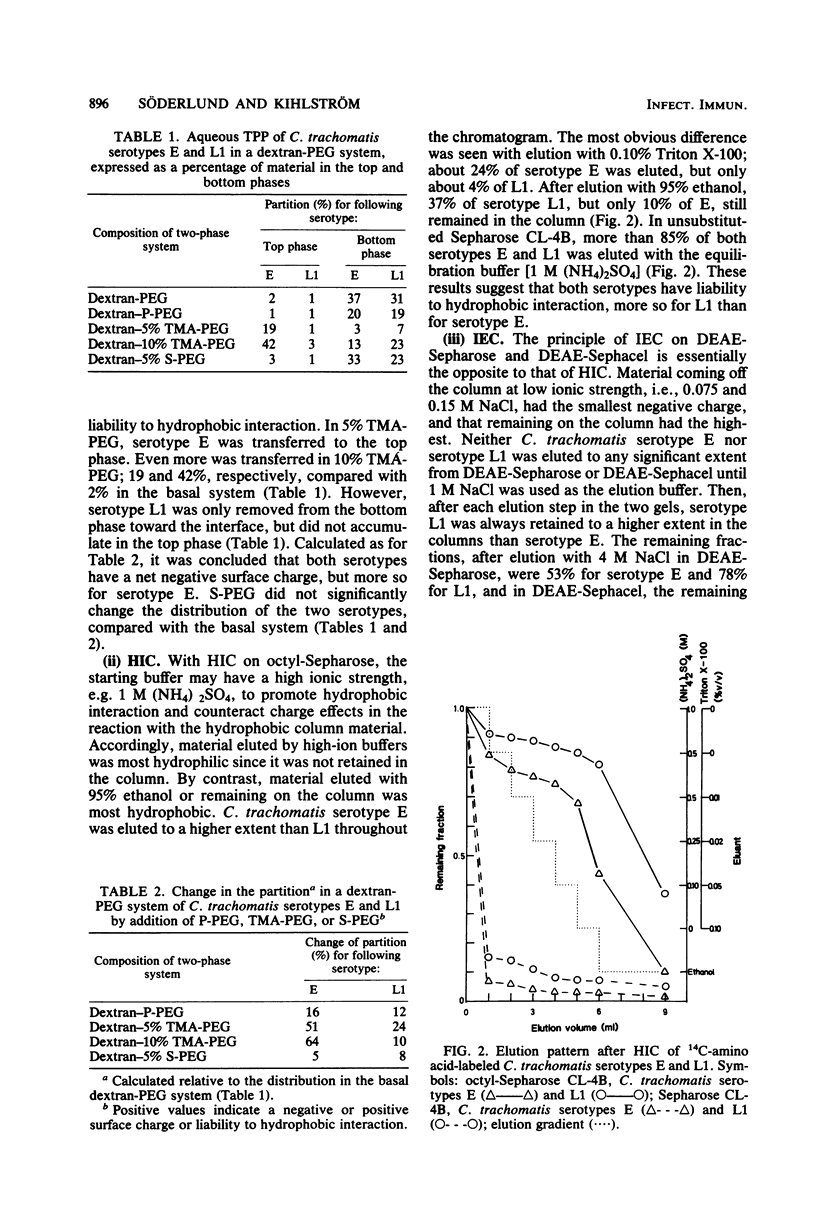
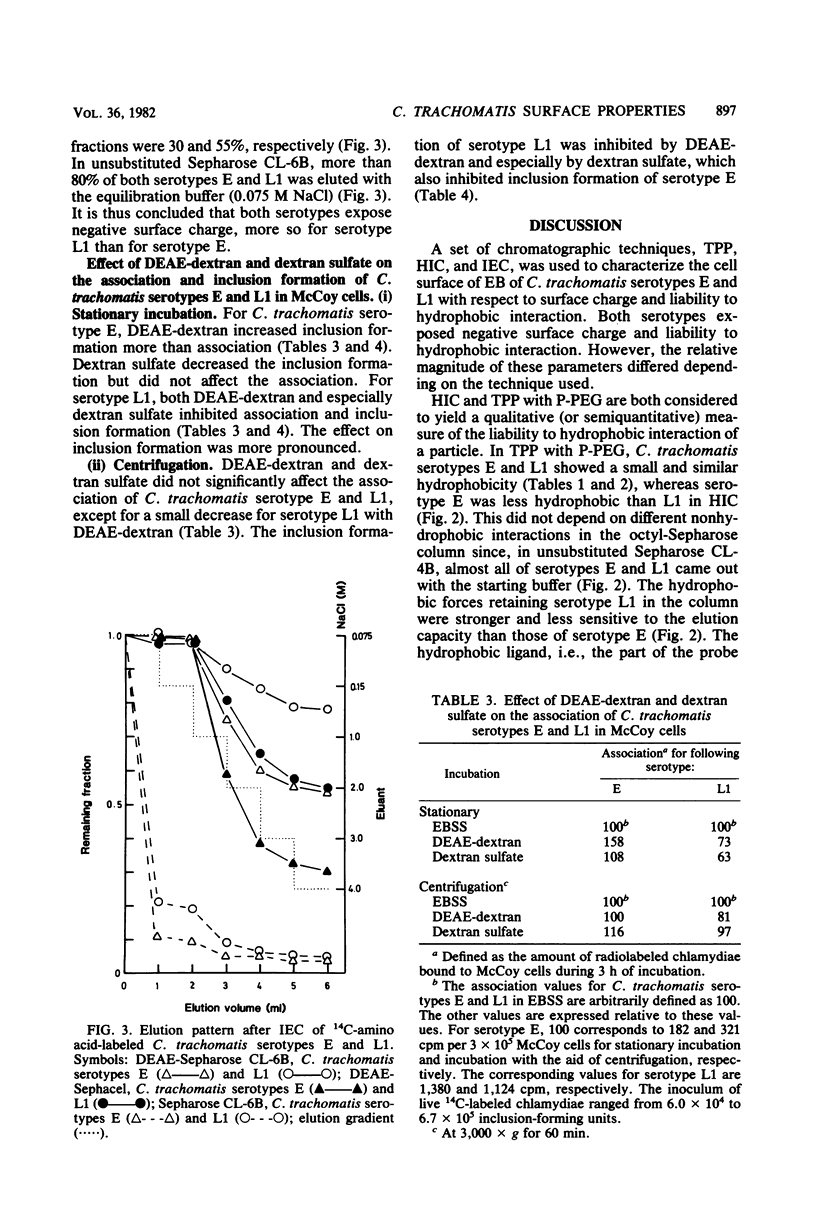
Aqueous biphasic partitioning, hydrophobic interaction chromatography, and ion-exchange chromatography were used to characterize the surface properties of Renografin-purified elementary bodies of Chlamydia trachomatis serotypes E and L1. The two serotypes differed with respect to liability to hydrophobic interaction and negative surface charge. Furthermore, the mutual relative magnitude of these parameters differed between the two serotypes, depending on the chromatographic technique used. This indicates that these chromatographic techniques register different aspects of charge and hydrophobicity on the chlamydial surface. DEAE-dextran and dextran sulfate affected association of, penetration, and intracellular development of C. trachomatis in mouse fibroblasts (McCoy cells). DEAE-dextran affected the association of C. trachomatis serotype E with McCoy cells mainly by charge-dependent forces, whereas both DEAE-dextran and dextran sulfate influenced the association of C. trachomatis serotype L1 mainly by charge-independent forces. These results indicate that the numerous biological differences between lymphogranuloma venereum and non-lymphogranuloma venereum strains of C. trachomatis may be assigned to differences in surface properties between the two strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker Y. The chlamydia: molecular biology of procaryotic obligate parasites of eucaryocytes. Microbiol Rev. 1978 Jun;42(2):274–306. doi: 10.1128/mr.42.2.274-306.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Gregory W. W., Byrne G. I., Gardner M., Moulder J. W. Cytochalasin B does not inhibit ingestion of Chlamydia psittaci by mouse fibroblasts (L cells) and mouse peritoneal macrophages. Infect Immun. 1979 Jul;25(1):463–466. doi: 10.1128/iai.25.1.463-466.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson G. Studies on aqueous dextran-poly (ethylene glycol) two-phase systems containing charged poly (ethylene glycol). I. Partition of albumins. Biochim Biophys Acta. 1970 Nov 24;222(2):381–389. doi: 10.1016/0304-4165(70)90127-3. [DOI] [PubMed] [Google Scholar]
- Johansson G. The effect of poly(ethyleneglycol) esters on the partition of proteins and fragmented membranes in aqueous biphasic systems. Biochim Biophys Acta. 1976 Dec 21;451(2):517–529. doi: 10.1016/0304-4165(76)90147-1. [DOI] [PubMed] [Google Scholar]
- Kihlström E., Magnusson K. E. Association with HeLa cells of LPS mutants of Salmonella typhimurium and Salmonella minnesota in relation to their physicochemical surface properties. Cell Biophys. 1980 Sep;2(3):177–189. doi: 10.1007/BF02790448. [DOI] [PubMed] [Google Scholar]
- Kraaipoel R. J., van Duin A. M. Isoelectric focusing of Chlamydia trachomatis. Infect Immun. 1979 Nov;26(2):775–778. doi: 10.1128/iai.26.2.775-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976 Apr;13(4):1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T. Effect of polycations, polyanions and neuraminidase on the infectivity of trachoma-inclusin conjunctivitis and lymphogranuloma venereum organisms HeLa cells: sialic acid residues as possible receptors for trachoma-inclusion conjunction. Infect Immun. 1973 Jul;8(1):74–79. doi: 10.1128/iai.8.1.74-79.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C., Wang S., Grayston J. T. Differentiation of TRIC and LGV organisms based on enhancement of infectivity by DEAE-dextran in cell culture. J Infect Dis. 1972 Mar;125(3):313–317. doi: 10.1093/infdis/125.3.313. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Moulder J. W. Persistent infection of mouse fibroblasts (McCoy cells) with a trachoma strain of Chlamydia trachomatis. Infect Immun. 1981 May;32(2):822–829. doi: 10.1128/iai.32.2.822-829.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy N. J. Wheat germ agglutinin blockage of chlamydial attachment sites: antagonism by N-acetyl-D-glucosamine. Infect Immun. 1979 Sep;25(3):946–953. doi: 10.1128/iai.25.3.946-953.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Edebo L. Phagocytosis of mutants of Salmonella typhimurium by rabbit polymorphonuclear cells. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(4):481–488. doi: 10.1111/j.1699-0463.1972.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Normann B., Edebo L. Influence of O and K antigens on the surface properties of Escherichia coli in relation to phagocytosis. Acta Pathol Microbiol Scand B. 1979 Apr;87B(2):85–91. doi: 10.1111/j.1699-0463.1979.tb02408.x. [DOI] [PubMed] [Google Scholar]
- Stjernström I., Magnusson K. E., Stendahl O., Tagesson C. Liability to hydrophobic and charge interaction of smooth Salmonella typhimurium 395 MS sensitized with anti-MS immunoglobulin G and complement. Infect Immun. 1977 Nov;18(2):261–265. doi: 10.1128/iai.18.2.261-265.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. W., Jr, Hatch T. P. Surface properties of Chlamydia psittaci. Infect Immun. 1980 Jul;29(1):175–180. doi: 10.1128/iai.29.1.175-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L. The cell periphery. Int Rev Cytol. 1969;26:63–105. doi: 10.1016/s0074-7696(08)61634-4. [DOI] [PubMed] [Google Scholar]


