Abstract
Prostacyclin (PGI2) modulates platelet activation to regulate haemostasis. Evidence has emerged to suggest that thrombi are dynamic structures with distinct areas of differing platelet activation. It was hypothesised that PGI2 could reverse platelet spreading by actin cytoskeletal modulation, leading to reduced capability of platelet aggregates to withstand a high shear environment. Our data demonstrates that post-flow of PGI2 over activated and spread platelets on fibrinogen, identified a significant reduction in platelet surface area under high shear. Exploration of the molecular mechanisms underpinning this effect revealed that PGI2 reversed stress fibre formation in adherent platelets, reduced platelet spreading, whilst simultaneously promoting actin nodule formation. The effects of PGI2 on stress fibres were mimicked by the adenylyl cyclase activator forskolin and prevented by inhibitors of protein kinase A (PKA). Stress fibre formation is a RhoA dependent process and we found that treatment of adherent platelets with PGI2 caused inhibitory phosphorylation of RhoA, reduced RhoA GTP-loading and reversal of myosin light chain phosphorylation. Phospho-RhoA was localised in actin nodules with PKA type II and a number of other phosphorylated PKA substrates. This study demonstrates that PGI2 can reverse key platelet functions after their initial activation and identifies a novel mechanism for controlling thrombosis.
Introduction
In healthy blood vessels platelets are exposed to endothelial derived nitric oxide (NO) and prostacyclin (PGI2) which act to inhibit platelet activation1. However, upon vascular damage platelets overcome this inhibition allowing the formation of a thrombus. Within the thrombus, the activation status of the platelet is determined by the relative activatory and inhibitory signalling in the microenvironment of the vascular lesion and its location within the thrombus. Here, fully activated platelets are found within a core region, while those at the periphery are only weakly activated2. However, the diffusion of platelet agonists within the thrombus is not uniform3, and furthermore there is little known on the diffusion of the endogenous platelets inhibitors, NO and PGI2, within the thrombus. Furthermore as endothelial release of PGI2 is induced by the activity of thrombin4, this brings forward the idea that this induction of PGI2 by thrombin is not only limited to the inhibition of non-stimulated platelets, but also to reverse the activation of activated platelets within the thrombus in order to effectively control the extent of the thrombus formation induced by the injury.
In order for platelets to be able to form thrombi effectively within the high shear environment of the vasculature, platelet adhesion at sites of vascular damage leads to significant remodeling of the actin cytoskeleton. The reorganization of the actin cytoskeleton is a complex process requiring co-ordinated modulation of actin polymerization, in order to drive the sequential formation of filopodia, actin nodules, lamellipodia and stress fibres (reviewed in ref. 5). Individual members of the RhoGTPase family have been implicated in this progessive remodeling of the cell architechture. Cdc42 drives filopodia formation6, Rac activation of WASP and the Arp2/3 complex generates lamellipodia7, and RhoA activity drives stress fibre formation8. At present it is unclear which RhoGTPase is responsible for actin nodule formation, although Rac has been identified to be present within the nodule9. This reorganization is dynamic, requiring constant signaling in order to prevent lamellipodial collapse10. Defective cytoskeleton remodelling leads to platelet spreading defects, reduction in thrombus size and an increase in thrombus instability8, 11–13.
Understanding the role of PGI2 is therefore critical to understand platelet function. PGI2 causes platelet inhibition via binding of PGI2 to the IP receptor, leading to the activation of adenylyl cyclase, and the synthesis of cyclic adenosine 3′, 5′ monophosphate (cAMP). Elevated cAMP activates PKA, the primary effector of cAMP signalling in platelets, leading to the inhibition of platelet functions in vitro and diminished platelet accrual at sites of vascular injury in vivo 1. Once activated PKA isoforms blunt multiple aspects of platelet function including Ca2+ mobilisation, integrin activation, secretion, although knowledge of how these specific platelet functions are targeted by cAMP signalling remains unclear14–16. Proteomic studies have demonstrated that cAMP/PKA signalling targets a cluster of potential proteins associated with cytoskeletal reorganisation suggesting that the cytoskeleton is a major target in order to effectively mediate platelet regulation17. In this context, we have recently described a mechanism by which cAMP signalling prevented platelet shape change through inhibition of RhoA signalling and phosphorylation of myosin light chains (MLC)18. However, the mechanisms by which cyclic nucleotide signaling can return platelets to the resting state after activation, thereby preventing permanent attachment to the thrombus or at points distant to the injury, are unknown.
In the present study we examined the role of cAMP in modulating the function of activated platelets. We found that cAMP signaling can reverse stress fibre formation, significantly reduce the surface area coverage of the platelet, and induce actin nodule formation in a cAMP and PKA dependent manner. This reversal of stress fibre formation, and surface area coverage was associated with inhibitory phosphorylation and inactivation of both RhoA and the localisation of pRhoA to actin nodules. Furthermore these changes in the actin cytoskeleton were associated with a reduction in surface area coverage in platelets flowed over fibrinogen in high shear environment. This data identified that PGI2 can reverse platelet activation leading to alteration of the platelets function in a high shear environment.
Results
Effect of PGI2 on Platelet aggregate formation on fibrinogen
Previously we have shown that blood preincubated with PGI2 (100 nM) can inhibit thrombus formation on fibrinogen19. However the effects of PGI2 on activated platelets has not been shown. Therefore, whole blood was flowed over fibrinogen (300 ug/ml) at arterial rates of shear (1000 s−1) resulting in the formation of small thrombi and a surface area coverage of 38.2 ± 6.8% (Fig. 1 and Supplementary Video. 1). Perfusion of these thrombi with PGI2 (100 nM) led to a significant reduction in surface area coverage to 24.4 ± 2.9% (p < 0.05). In contrast, perfusion of the thrombi with buffer alone had no significant effect on surface area coverage (Fig. 1a,b and Supplementary Videos 2 and 3). Further analysis of the platelets identified that there had been a noticable reduction in surface area in the spread platelets, indicating that PGI2 may reverse platelet spreading on fibrinogen.
Figure 1.
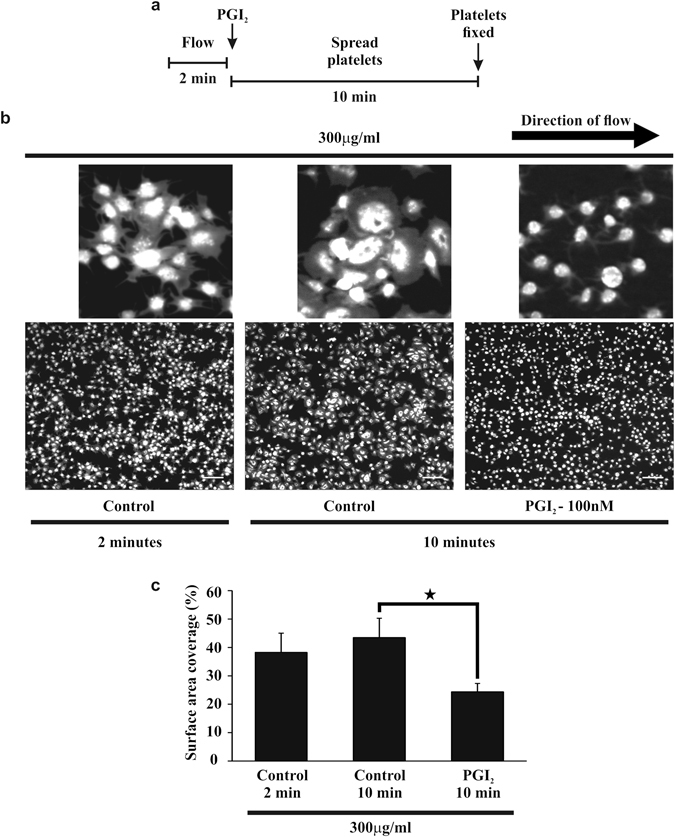
Post perfusion of 100 nM PGI2 induces embolisation of performed thrombi on fibrinogen. Whole blood, stained with 10 μM DiOC6 was flowed over fibrinogen (300 μg/ml) coated slides for 2 minutes at a shear rate of 1000 s−1, to enable the formation of thrombi. After 2 minutes either tyrodes alone or tyrodes containing 100 nM PGI2 were perfused over the preformed thrombi for 10 minutes at 1000 s−1. (a) Schematic of the experimental condition of the flow. (b) Representative images of the thrombi observed under these different experimental conditions. Scale bar is 20 μm. The insert shows an enlarged image of their respective images with a scale bar of 5 μm. (c) The surface area of the thrombi at 2 minutes of flow and after 10 minutes of perfusion with 100 nM PGI2. Thrombi were fixed with 4% paraformaldehyde, before restaining with 10 μM DiOC6 overnight. Thrombi were then imaged using flourescent microscopy, and analysed using ImageJ to obtain the surface area. Analysis completed from n = 3 experiments. p < 0.05.
PGI2 reverses stress fibre formation in spread platelets on fibrinogen
Analysis of platelet spreading in the absence of PGI2 demonstrated within 5 minutes of adhesion, the majority of platelets had formed actin nodules, but by 25 minutes that these actin nodules had been replaced by platelets containing stress fibres (Supplementary Figure S1). These stress fibres were maintained for up to 45 minutes post adhesion (longest time tested) which is consistent with previously published data9. The actin cytoskeleton has been implicated in thrombus stability and is known to be modulated by PKA signaling in multiple cell types20–22. Therefore, in the next series of experiments PGI2 (10 nM) was added to the spread platelets after 25 minutes, and spreading evaluated for a further 60 minutes (Fig. 2a). In the absence of PGI2 the number of platelets with stress fibres did not alter appreciably over this timecourse. In contrast the presence of PGI2 caused a rapid loss of actin stress fibres in spread platelets. This effect was maximal at 10 minutes post PGI2 treatment before returning to control levels after 60 minutes (Fig. 2b,c). Interestingly the loss of stress fibres was associated with the reciprocal appearance of circular dot like F-actin rich structures, which was again maximal at 10 minutes before returning to control values by 60 minutes (Fig. 2b,d). The F-actin circular structures induced by PGI2 were confirmed to be actin nodules, via identification with immunochemistry of the presence of actin nodule markers; the Arp2/3 complex, pan-pTyrosine substrate and WASP (Supplementary Figure S2a,b and data not shown)9. The alterations in actin structure was associated with a dynamic change in platelet surface area. It was observed that as the actin structures rearranged the platelet surface area significantly contracted, but expanded again as the actin stress fibres reformed (Fig. 2b,e). Interestingly, the change in actin structures had no effect upon the number of adherent platelets (Supplementary Figure S3). In order to identify if the reversal of stress fibre formation is due to a reduction in actin polymerization induced by PGI2, platelets were allowed to spread on fibrinogen before stimulation with PGI2 (10 nM) for 10 minutes. The platelets were then lysed and analysed for the content of filamentous actin. Control platelets underwent a 2.0 ± 0.2 fold increase in F-actin polymerisation, which was unaffected by stimulation with PGI2 (2.2 ± 0.3 fold increase), suggesting a targeted modulation of stress fibre formation (Fig. 2f).
Figure 2.
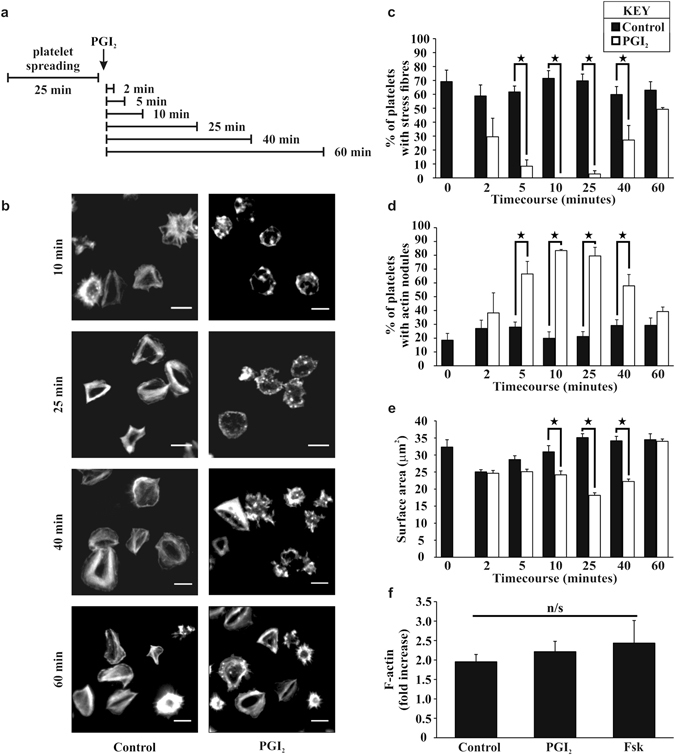
Post treatment of PGI2 induces stress fibre reversal and actin nodule formation in platelets spread on fibrinogen in a time dependent manner. Platelets (2 × 107/ml) were spread on 100 μg/ml fibrinogen for 25 minutes, washed with PBS, and then 10 nM of PGI2 was added, for a further 2–60 minutes as per the representative experimental design in part (a) The platelets were then fixed and stained with FITC-phalloidin before being imaged via fluorescent microscopy. (b) Representative images of each condition of the experiment. (c) The number of spread platelets containing stress fibres was identified in control and PGI2 treated samples. (d) The number of spread platelets containing actin nodules was identified in control and PGI2 treated samples. (e) The average surface area of the spread platelets was analysed for each timepoint in control and PGI2 treated samples. Analysis was performed using Image J. The experiments are an average of n = 5. p < 0.05. Scale-bar 5 μm. (f) Platelets were spread as above for 25 minutes and then treated for 10 minutes with either 10 nM PGI2 or 1 μM forskolin. Assessment of the filamentous actin was performed using the F-actin assay. The experiments are an average of n = 3.
To confirm the role for cAMP signalling we used forskolin (Fsk) as a direct activator of adenylyl cyclase. The addition of Fsk (1 μM) to adherent platelets led to a significant and sustained reversal of stress fibre formation (Supplementary Figure S4a,b), increased actin nodule formation (Supplementary Figure S4a,c), a reduction in platelet surface area (Supplementary Figure S4a,e), but did not influence actin polymerisation (Fig. 2f) or platelet adhesion (Supplementary Figure S4d). The effects of Fsk were observed at 10 minutes but in contrast to PGI2 were maintined for up to 60 minutes (longest time tested) (Supplementary Figure S4).
To determine if the reversal of stress fibres driven by PGI2 was independent of effects on secretion, platelets were spread on fibrinogen in the presence of apyrase (2 U/ml) and indomethacin (10 μM) prior to treatment with PGI2 (10 nM). The presence of apyrase and indomethacin reduced the number of adherent platelets, but did not influence surface area or actin structures of the adherent platelets. Importantly treatment of adherent platelets with PGI2 caused a reversal of stress fibre formation, production of actin nodules, and a reduction in surface area. This demonstrates that the effect of PGI2 on the actin cytoskeletal rearrangement is independent of ADP and TXA2 bioavailability (Fig. 3). Therefore PGI2 reversed both stress fibre and lamellipodia formation, whilst inducing actin nodule formation, independent of platelet secretion.
Figure 3.
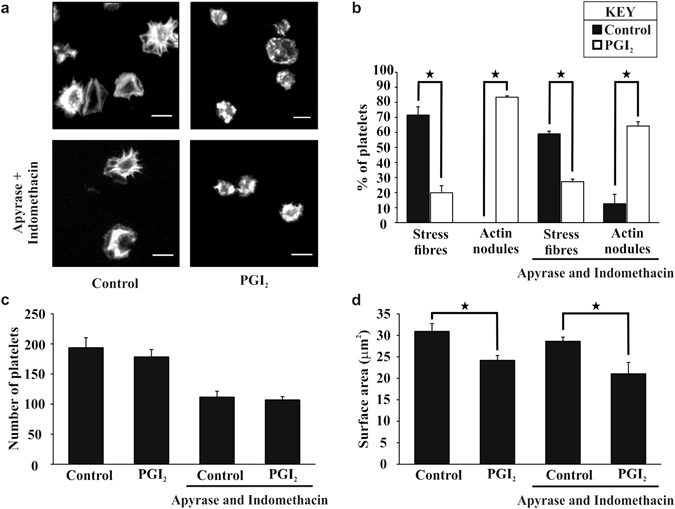
PGI2 reverses stress fibre formation independent of ADP and TXA2. Platelets (2 × 107/ml) were spread on 100 μg/ml fibrinogen for 25 minutes with or without apyrase (2 U/ml) and indomethacin (10 μM). The platelets were washed, and 10 nM PGI2 added for 10 minutes, in the presence or absence of apyrase (2 U/ml) and indomethacin (10 μM). The platelets were then fixed and stained with FITC-phalloidin before being imaged. (a) Images are representative of the experimental conditions. (b) The number of platelets containing stress fibres or actin nodules in the presence or absence of apyrase (2 U/ml) and indomethacin (10 μM) was identified in control and PGI2 treated samples. (c) The total number of platelets adhered, was identified in control and PGI2 treated samples. (d) The average surface area of the spread platelets was identified in control and PGI2 treated samples. The experiments are an average of n = 4. Scale bar is 5 μm. p < 0.05.
PGI2 induces cAMP production and activation of the cAMP/PKA pathway in spread platelets
PGI2 induces generation of cAMP and activation of PKA in platelets, although PKA-independent signalling is shown in other cell types23, 24. Initially it was confirmed that adherent platelets could synthesise cAMP. Treatment of adherent platelets with PGI2 (10 nM) for 2 minutes led to a significant increase in cAMP from 152 ± 52 fmol/μg to 1576 ± 293 fmol/μg (p < 0.05) and was consistent with platelets in suspension stimulated with PGI2 for 1 minute25 (Fig. 4a). To confirm that the actin cytoskeletal rearrangements induced by PGI2 were mediated by PKA the established PKA inhibitors Rp-8cpt-cAMPs (500 μM) and KT5720 (10 μM) were employed26, 27. The presence of Rp-8cpt-cAMPs (500 μM) and KT5720 (10 μM) blocked the ability of PGI2 to reverse stress fibres, induce actin nodule formation, and reduce platelet spreading (Fig. 4b–e). This data suggests PGI2 modulates the actin cytoskeleton in activated spread platelets via a cAMP/PKA dependent mechanism.
Figure 4.
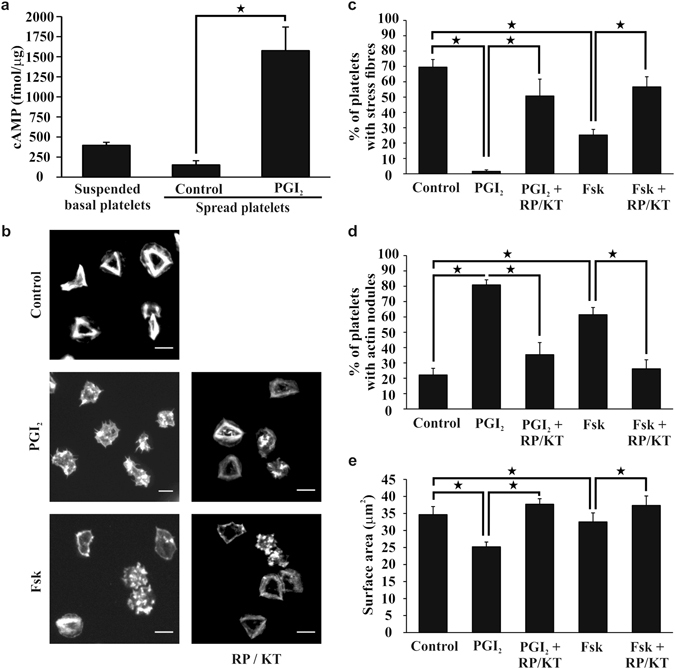
Reversal of platelet stress fibre formation and induction of actin nodule formation is dependent on PKA activation. Platelets (2 × 107/ml) were spread on 100 μg/ml fibrinogen for 25 minutes in the presence or absence of PKA inhibitors; 500 μM RP-8-CPT-cAMP (RP) and 10 μM KT5720 (KT), before being washed with PBS. The platelets were then treated with tyrodes containing 10 nM PGI2 or 1 μM forskolin with or without PKA inhibitors 500 μM RP-8-CPT-cAMP and 10 μM KT5720, for a further 10 minutes. The platelets were then fixed, stained with FITC-phalloidin and imaged. (a) The levels of cAMP were assessed in basal suspended platelets, control and 10 nM PGI2 treated spread platelets. (b) Representative images of spread platelets under different experimental conditions. (c) The number of spread platelets containing stress fibres were identified for each condition in control and treated samples. (d) The number of spread platelets containing actin nodules were identified for each condition in control and treated samples. (e) The average surface area of the spread platelets was analysed for each condition in control and treated samples using Image J. The experiments are an average of n = 3. p < 0.05. Scale bar is 5 μm.
Actin nodules represent a pre-stress fibre actin structure9 and our observation that cAMP signalling was associated with the reversal of stress fibres led us to examine these nodules in more detail. Firstly, adherent platelets were stained for PKARI and RII subunits. Consistent with our previous observations, differential localisation of these isoforms was observed with PKARI found at the periphery of the platelet and PKARII distibuted throughout the platelet (Fig. 5a,b)28. Moreover, PKARII was localized with the actin nodule, while PKARI staining was mutually exclusive to the actin cytoskeleton. PKA activity is localised to particular cell compartments in order to focus its catalytic activity on to specific substrates. Further analysis using immunofluorescence demonstrated the presence of phosphorylated PKA substrates including pRhoAser188 at actin nodules present in spread platelets after being treated with PGI2 (Fig. 5c). This suggested that cAMP maybe focussed on the actin nodule associated RhoA.
Figure 5.
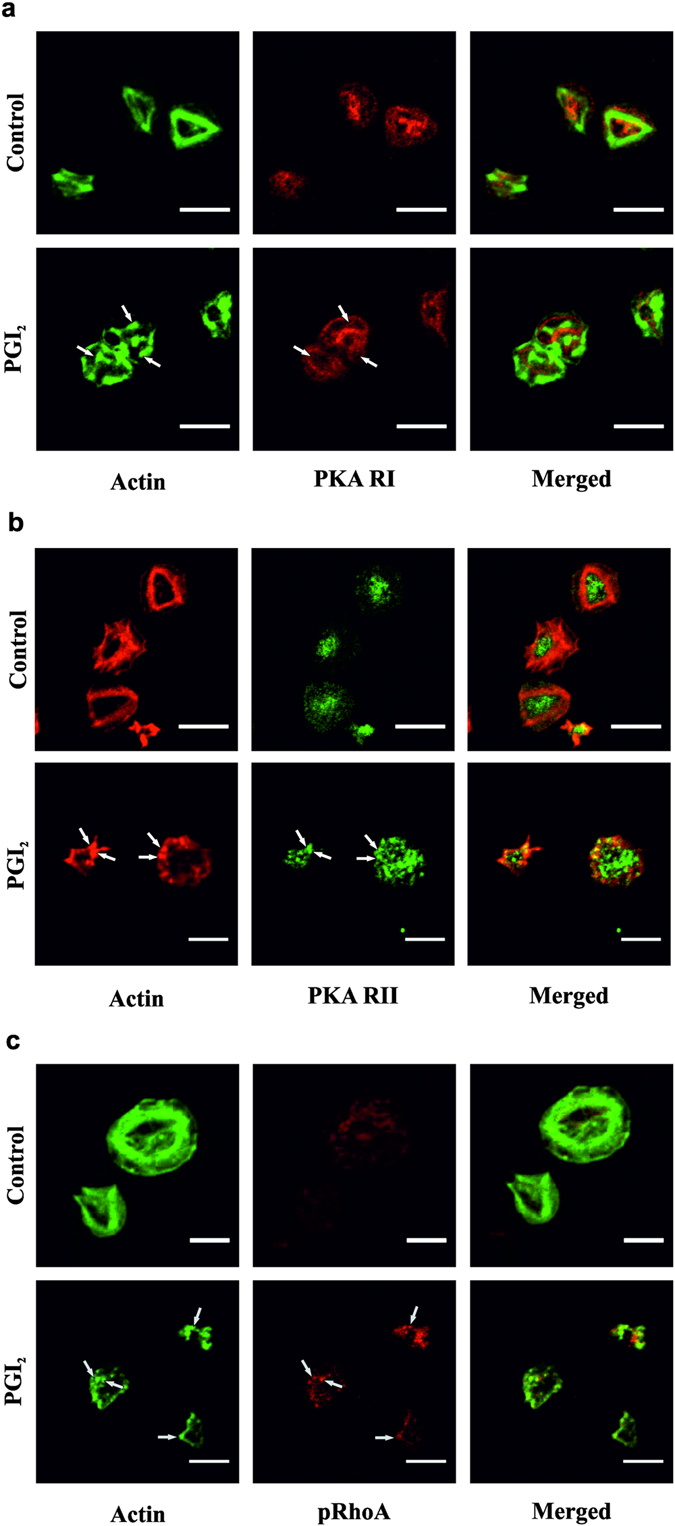
Actin nodules contain PKA signalling proteins. Platelets (2 × 107/ml) were spread on 100 μg/ml fibrinogen for 25 minutes before being washed with PBS. The platelets were then treated with tyrodes with or without 10 nM PGI2 for a further 10 minutes. The platelets were then fixed, lysed and stained for either (a) PKA RI (1:100), (b) PKA RII (1:100) or (c) pRhoA (1:1000); and co-stained with actin (FITC-Phalloidin) for 60 minutes, before mounting and imaging the slides. Images are representative of at least 3 experiments. Scale bar is 5 μm.
PGI2 reverses activation of RhoA in spread platelets
Platelet stress fibre formation requires the activation of RhoA and its downstream effector ROCK9. Platelets incubated with the ROCK inhibitor Y27632 or the RhoA inhibitor Rhosin prior to spreading fail to form stress fibres (Supplementary Figure S5)8. As Fig. 2 had identified a significant reversal of stress fibre formation in the presence of PGI2, this indicated that PGI2 could reverse RhoA activation. To examine this possibility, platelets were spread for 25 minutes, treated with PGI2 (10 nM) or Fsk (1 μM) and the phosphorylation of key proteins evaluated. Confirmation of cAMP signalling in adherent platelets was illustrated by the sustained VASP-ser157 phosphorylation in response to PGI2 or Fsk (Fig. 6a,b, Supplementary Figure S6 and data not shown). In untreated adherent platelets RhoA was non-phosphorylated. In contrast phosphoRhoA-ser188 became apparent after PGI2 (10 nM) and Fsk (1 μM) stimulation, in a PKA dependent manner (Fig. 6a,c, Supplemetary Figure S6 and data not shown). Phosphorylation of RhoA is associated with RhoA inactivation, as it binds the GDI subunit, and therefore can no longer cause the activation of ROCK29. Analysis of RhoA showed it was activated (GTP-bound) in adherent platelets, but that addition of PGI2 significantly reduced the active RhoA present within the spread platelets (Fig. 6e,f and Supplementary Figure S6). Further to this, and in agreement with a reduction in RhoA activity, PGI2 and Fsk reduced MLC-ser19 phosphorylation in spread platelets (Fig. 6a,d and Supplementary Figure S6). These results indicate that PGI2 can inhibit the activated RhoA via phosphorylation by a PKA-dependent mechanism.
Figure 6.
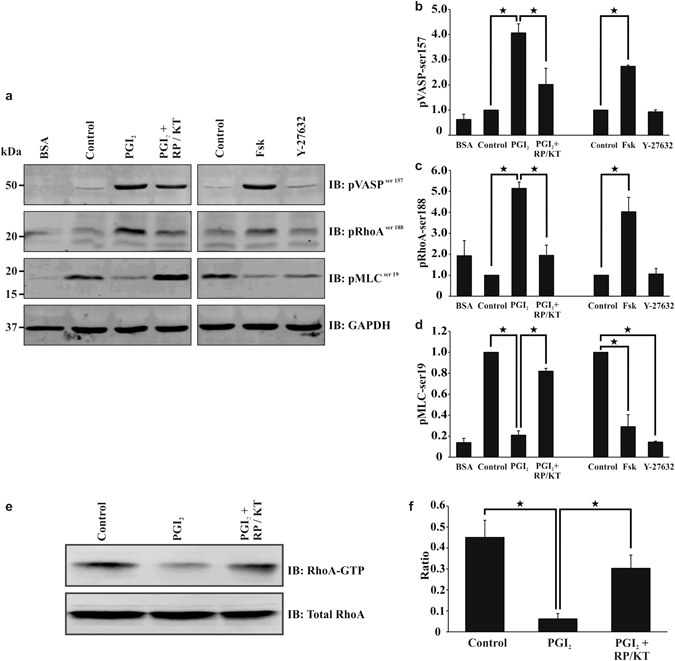
PGI2 induces a PKA signalling response in spread platelets. Platelets (2 × 108/ml) were spread on 100 μg/ml fibrinogen for 25 minutes in the presence or absence of PKA inhibitors 100 μM RP-8CPT-cAMP (RP) and 2 μM KT5720 (KT), before being washed with PBS. (a) The platelets were then treated with tyrodes containing 10 nM PGI2 with or without PKA inhibitors (100 μM RP-8CPT-cAMP and 2 μM KT5720), or 1 μM forskolin, or Y27632 (10 μM), for a further 10 minutes. The samples were then lysed with laemelli buffer before being western blotted for pVASPser159, pMLCser19, pRhoAser188, and GAPDH. Cropped gel Images are representative of at least three experiments (full length gels are illustrated in Supplementary Figure S6). (b–d) Densitometry for the western blots; pVASPser159, pMLCser19 and pRhoAser188 using GAPDH as the loading control. The ratios were standardised to the control. (e) Spreading the platelets as above, they were treated with tyrodes containing 10 nM PGI2 with or without PKA inhibitors 100 μM RP-8CPT-cAMP and 2 μM KT5720 for a further 10 minutes. The samples were then lysed, before the addition of RhoA GTP beads. Samples were then western blotted for active RhoA and total RhoA. Cropped gel Images are representative of at least three experiments (full length gels are illustrated in Supplementary Figure S6). (f) Images of RhoA pull down were analysed for densitometry (full length gels are illustrated in Supplementary Figure S6). Analysis is an average of at least n = 3 experiments. p < 0.05.
Discussion
Haemostasis relies on stable platelet adhesion for subsquent thrombus formation. The ability of platelets to undergo a rapid remodelling of the actin cytoskeleton is critical in order to withstand shear stress. Indeed an inability to form lamellipodia or stress fibres upon platelet activation and spreading is linked to thrombus instability and embolisation8, 12, 13. However, under physiological conditions these key changes occur in situations where platelets are continually exposed to PGI2. Given the key role of RhoGTPases in actin remodelling we examined the influence of cAMP signalling on activated platelets, using the platelet spreading and in vitro flow assays. We show here that (i) PGI2 can cause the reversal of surface area of platelet aggregates under high shear (ii) PGI2 can reverse platelet spreading via dissolution of stress fibres, and the formation of actin nodules in a cAMP/PKA dependent mechanism, (iii) PKA localises to the actin nodule, using it as a platform to mediate PKA mediated inhibitory phosporylation of RhoA. These data suggest that in addition to its already known roles in causing platelet inhibition prior to thrombus formation, cAMP signalling can control the size and stability of existing platelet aggregates via modulating the actin cytoskeleton, causing the aggregates to become vulnerable to high shear stress.
PGI2 and the PKA signaling pathway have been implicated in actin cytoskeleton modulation in multiple cell types15, 20, 21. Although prior incubation of platelets with PGI2 and Fsk leads to inhibition of platelet adhesion, spreading and thrombus formation30–33, it is unclear if PGI2 can actively modulate a fully activated platelet. This is a critical idea as it demonstrates that PGI2 plays a dual role in the control of thrombus formation, both inhibition of platelets prior to activation, and reversal of platelets once they are already active. Both effects could then lead to reduction in thrombus formation. The reversal of platelet activation links into recent modelling of thrombus formation that suggests a dynamic structure with a core area, within which the platelets are fully activated and surrounded by a pheripheral area in which the activatory signals are much weaker2. The balance between the activatory and inhibitory signals acting on the platelets likely determines their activatory status. Therefore we set out to explore how PGI2 affected preexisting platelet aggregates. Using an in vitro flow assay we show for the first time that post perfusion of thrombi on fibrinogen with PGI2 caused a significant reduction in surface area. The ability of the platelet to withstand high shear is driven, at least in part, by the formation of remodelled actin structures within the platelet and we focussed our investigation on these processes. Platelet spreading was associated with progressive changes in actin structures including filopodia, actin nodules, lamellipodia and ultimately stress fibres. Addition of PGI2 to spread platelets induced cytoskeletal remodelling associated with stress fibre dissolution consistent with previous observations in other cell types22, 34. The effects of PGI2 were mimicked by forskolin, but blocked by PKA inhibitors, indicating a central role for the cAMP-PKA signalling cascade.
To examine the mechanism that underpinned stress fibre disassembly and actin nodule formation we used a pharmacolgical approach. Incubation of platelets with RhoA and ROCK inhibitors, led to adherent platelets rich in actin nodules and an absence of stress fibres as reported previously8. Since the addition of PGI2 led to a loss of stress fibres, we speculated that PGI2 may target RhoA signalling in adherent platelets. Consistent with our previous studies in suspended platelets we found that PGI2 increased the inhibitory phosphorylation of RhoA at ser188 and reduced the GTP-loading of RhoA. Here we have extended our original findings to show that cAMP signalling can switch off activated RhoA in addition to blocking its activation. The inhibition of RhoA/ROCK signalling led to dephosphorylation of MLC, which likely occurs through the ability of cAMP signalling to induce disinhibition of MLCP. Moreover we show that the inhibitory phosphorylation of RhoA identified by immunoblotting is primarily located at the actin nodule. The actin nodule was identified as an F-actin rich structure that was formed in spreading platelets prior to lamellipodia formation9 that required both the Arp2/3 complex and WASp, and are sites of tyrosine kinase signalling activity35. Given that RhoA phosphorylation is mediated by PKA we also examined the presence of the kinase. cAMP signalling events are highly regulated and involve the selective coupling of cAMP signalling complexes to specific substrates or regions within the cell (Reviewed in ref. 36). Our data suggest that the actin nodule may act as such a signalling platform allowing localisation of PKAII, but not PKAI, and several phosphorylated PKA substrates. These data suggest that PKAII specifically targets RhoA and that this inhibitory phosphorylation prevents sustained stress fibre formation allowing reversion to actin nodules. This is in addition to the previous identification of Rac puts forward the interesting hypothesis that the nodule is under the control of both Rac and RhoA, and that the interplay between the level of activation of both is critical to actin nodule formation and dissolution.
This reversal of stress fibres on fibrinogen links with the thrombus defect as stress fibre formation is critical to withstand high shear8. The physiogical importance of our observations relates to the environment of the growing thrombus. The data indicates that the reversal of platelet aggregate formation by PGI2 is due to its effect on the upper layers of the thrombus, which are linked by the exposure of fibrinogen. Furthermore, thrombin and TXA2 generated at the sites of thrombus formation further activate platelets but also stimulate the biosynthesis of PGI2 by the blood vessel wall4. It is possible that in addition to preventing excessive platelet activation the release of PGI2 in areas of vascular damage could modulate thrombus formation through regulating thrombus stability. Indeed the model proposed by Welsh et al.3 of graded platelet activation through the thrombus could be explained at least in part by our observations. Thrombi have been postulated to be made up of a core area, within which the platelets are fully activated as they have a multitude of activatory signals. This core area is surrounded by a peripheral area where the activatory signals are much weaker, and platelets are known to bind transiently to the growing thrombi. Exposure of these peripheral platelets to PGI2 may facilitate their embolisation and contribute to the control of thrombosis. It would be highly interesting to extend this work through understanding how this effect of platelet reversal interplays with the activation of the coagulation system, and the formation of fibrin, through the action of thrombin. Thrombin activation of platelets has previously been identified to be inhibited by PGI2, so can PGI2 reverse its activation? How would this reversal affect the formation of fibrin? Indeed, is PGI2 reversal of platelet activation required to prevent excessive fibrinogen exposure, and therefore excessive fibrin formation. This potentially ties in with the altered fibrin formation associated with thrombi from patients with pulmonary arterial hypertension. Within this condition there is reduced PGI2 production, and thrombi with altered fibrin formation, that is resistant to lysis37. Therefore is the reduction in PGI2 inducing excessive fibrin formation due to an inability to reverse platelet activation? Furthermore given the importance of PGI2 in both the initial inhibition, and also the reversal of thrombus formation, this data strengthens the case for the development of anti-platelet drug therapies that target cAMP elevation for the control of thrombus formation.
However, importantly this report identifies PGI2 mediated reversal of platelet activation. This reversal of activation, through the control of activity of RhoA, could have a signficant effect upon the capability of the platelet to work effectively in a high shear environment. This report therefore demonstrates a novel mechanism by which PGI2 regulates platelet activation through continous modulation of RhoA and stress fibre formation.
Material and Methods
Materials
PGI2 (Cayman Chemical, Michigan, USA), Forskolin (Sigma-Aldrich, UK), Fibrinogen (Enzyme Research, Swansea, UK), Y-27632 (Abcam, Cambridge, UK), Rp-8CPT-cAMP (Biolog, Bremen, Germany), KT5720 (Abcam, Cambridge, UK), pRhoAser188 (Santa-Cruz Biotechnology, Heidelberg, Germany), pMLCser19 and pVASPser157 (New England Biolabs, Hitchin, UK), GAPDH and Arp2/3 (Millipore, Watford, Hertfordshire, UK), PKA RI (Cell Signalling Technology, Leiden, Netherlands), PKARII (BD Biosciences, Oxford, UK), pPKA substrate (Cell Signalling Technology, Leiden, Netherlands), WASP (Santa-Cruz Biotechnology, Heidelberg, Germany), pTyrosine (Cytoskeleton, Denver, UK), RhoA pulldown kit (Cytoskeleton, Denver, UK), cAMP assay and ProLong Diamond Antifade Mountant (GE healthcare, Little Chalfont, Buckinghamshire, UK), Flourescent secondary anti-mouse 800 and anti-rabbit 680 antibodies (LI-COR Biotechnology, Cambridge, UK) All other chemicals were from Sigma Ltd (Poole, UK) unless otherwise stated.
Platelet preparation
Whole blood was mixed with acid-citrate dextrose (ACD; 5:1) (114 mM glucose, 30 mM Tris-Na Citrate, 72.6 mM NaCl and 3.0 mM citric acid pH 6.4) and platelets were isolated using low pH as previously described18. Platelets were allowed to rest for 30 minutes prior to experimentation. Written informed consent was acquired for the donation of blood. The work was conducted in accordance with the relevant guidelines and regulations and was completed under the ethical permission granted by the Hull York Medical School ethical committee, for “The study of platelet activation, signalling and metabolism”.
Platelet Spreading
Coverslips were incubated with fibrinogen (100 μg/ml) overnight at 4 °C, washed with PBS and blocked with denatured fatty acid free BSA (5 mg/ml) for 1 hour at room temperature. Platelets (2 × 107/ml) were adhered to immobilised proteins for 25 minutes at 37 °C in the presence or absence of Apyrase (2 U/ml), Indomethacin (10 μM), or Rp-8-CPT-cAMP (500 μM) and KT5720 (10 μM). After 25 minutes the coverslips were washed twice with PBS to remove nonadhered platelets and then treated with PGI2 (1-1000 nM) or forskolin (0.1–100 μM) in the presence or absence of Apyrase (2 U/ml), Indomethacin (10 μM), or Rp-8-CPT-cAMP (500 μM) and KT5720 (10 μM). At the required timepoint the platelets were fixed with 4% paraformaldehyde for 10 minutes, lysed with 0.1% Triton X-100, stained with FITC-phalloidin and if required the relevant primary antibody (PKARI, and PKARII (1:100), pRhoAser188, Arp2/3, and 4G10 (1:1000)), followed by the secondary antibody (1:200), mounted and visualized using a Zeiss Axio Observer (Zeiss, Cambridge, UK) with a x63 oil immersion objective (1.4 NA) and Zen Pro software (Carl Zeiss, Cambridge, UK). Images of spread platelets were analysed using ImageJ software (NIH, Bethesda, USA) to identify the surface area, the platelets containing either actin nodules or stress fibres, and platelet adhesion. In some cases platelets (2 × 107/ml) were preincubated with PGI2 (1–1000 nM) or Forskolin (0.1–100 μM), for 2 minutes prior to adhesion for 45 minutes.
In vitro Flow Assay
Flow studies were performed with multichannel biochips (Cellix, Dublin Ireland). Biochips were coated with 300 μg/ml fibrinogen overnight at 4 °C and blocked with denatured BSA (5 mg/ml) for 1 hour. Whole blood was stained with DIOC6 (10 μM) and flowed through the biochips for 2 minutes at a shear rate of 1000 s−1 at 37 °C. Biochips then underwent a post-flow with Tyrode’s buffer supplemented with or without PGI2 (100 nM; 1000 s−1 at 37 °C) for 10 minutes, before fixation with 4% paraformaldehyde, staining overnight with DiOC6 (10 μM) and imaging using a Apotome.2 confocal unit on a Zeiss Axio Observer (Zeiss, Cambridge, UK) with a x63 oil immersion objective (1.4 NA) and Zen Pro software (Carl Zeiss, Cambridge, UK). Analysis of the surface area coverage of each condition was performed using ImageJ software (NIH, Bethesda, USA).
Immunoblotting
Six well plates were coated with fibrinogen as per platelet spreading. Platelets (2 × 108/ml) were adhered for 25 minutes before removal of non-adherent platelets. Adherent platelets were then treated with PGI2 (10 nM), FSK (1 μM), or Y27632 (10 μM) in the presence or absence of Rp-8CPT-cAMP (500 μM) and KT5720 (10 μM). Platelets were lysed with Laemelli buffer and the lysates were separated by SDS-PAGE, transferred and immunoblotted with the required antibodies (pVASPser157 (1:1000), pRhoAser188 (1:1000), pMLCser19 (1:1000), or GAPDH (1:6000)). The blots were imaged by Odyssey CLx Infrared Imaging system (LI-COR Biotechnology, Cambridge, UK), and densitometry was analysed by Image Studio ver.5.2 (LI-COR Biotechnology, Cambridge, UK).
F-actin Analysis
Six well plates were coated with fibrinogen as per platelet spreading. Platelets (2 × 108/ml) platelets were adhered for 25 minutes before being washed with PBS. The platelets were then treated with Tyrode’s buffer, PGI2 (10 nM) or FSK (1 μM) for 10 minutes. The samples were processed as previously described38. Aliquots of unstimulated platelets (1 × 108/ml to 8 × 108/ml) were used to calculate both the protein concentration and F-actin concentration at these platelet numbers as an unstimulated control.
Measurement of cAMP and RhoA Pull-down assay
Six well plates were coated with fibrinogen as per platelet spreading. Platelets (2 × 108/ml) were spread for 25 minutes before washing, and treatment with Tyrodes buffer or PGI2 (10 nM) for 2 minutes. Platelets were lysed and cAMP concentrations measured using a commerical available assay kit18. For the RhoA pull down platelets the samples were prepared as above, but then lysed and lysates incubated for 90 minutes at 4 °C with Rhotekin-RBD-beads. Bead pellets were washed once and Laemmeli buffer was added prior to immunoblotting as previously published39.
Statistical analysis
Results are shown as mean ± standard error of mean (SEM). Data was arcsin transformed as appropriate and then subject to statistical analysis. Statistical analysis was performed by using one-way ANOVA with a P value of < 0.05.
Electronic supplementary material
Acknowledgements
The authors would like to thank Hafsa Khan for her help with image analysis. This study was funded by grants from the British Heart Foundation (FS/15/36/31525, PG/11/37/28884 and PG/12/49/29441).
Author Contributions
M.Y.Z. designed the research, performed experiments, analyzed data, and wrote the manuscript; L.A., A.A., and Z.R., performed experiments; S.G.T. wrote the manuscript; K.M.N. and S.D.J.C. designed the research, analysed the data, and wrote the manuscript. All authors reviewed the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05817-9
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schwarz UR, Walter U, Eigenthaler M. Taming platelets with cyclic nucleotides. Biochemical pharmacology. 2001;62:1153–1161. doi: 10.1016/S0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- 2.Stalker TJ, et al. A systems approach to hemostasis: 3. Thrombus consolidation regulates intrathrombus solute transport and local thrombin activity. Blood. 2014;124:1824–1831. doi: 10.1182/blood-2014-01-550319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh JD, et al. A systems approach to hemostasis: 1. The interdependence of thrombus architecture and agonist movements in the gaps between platelets. Blood. 2014;124:1808–1815. doi: 10.1182/blood-2014-01-550335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marcus AJ, Weksler BB, Jaffe EA, Broekman MJ. Synthesis of prostacyclin from platelet-derived endoperoxides by cultured human endothelial cells. J Clin Invest. 1980;66:979–986. doi: 10.1172/JCI109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aslan JE, McCarty OJ. Rho GTPases in platelet function. Journal of thrombosis and haemostasis: JTH. 2013;11:35–46. doi: 10.1111/jth.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pleines I, et al. Multiple alterations of platelet functions dominated by increased secretion in mice lacking Cdc42 in platelets. Blood. 2010;115:3364–3373. doi: 10.1182/blood-2009-09-242271. [DOI] [PubMed] [Google Scholar]
- 7.McCarty OJ, Calaminus SD, Berndt MC, Machesky LM, Watson SP. von Willebrand factor mediates platelet spreading through glycoprotein Ib and alpha(IIb)beta3 in the presence of botrocetin and ristocetin, respectively. Journal of thrombosis and haemostasis: JTH. 2006;4:1367–1378. doi: 10.1111/j.1538-7836.2006.01966.x. [DOI] [PubMed] [Google Scholar]
- 8.Calaminus SD, et al. MyosinIIa contractility is required for maintenance of platelet structure during spreading on collagen and contributes to thrombus stability. Journal of thrombosis and haemostasis: JTH. 2007;5:2136–2145. doi: 10.1111/j.1538-7836.2007.02696.x. [DOI] [PubMed] [Google Scholar]
- 9.Calaminus SD, Thomas S, McCarty OJ, Machesky LM, Watson SP. Identification of a novel, actin-rich structure, the actin nodule, in the early stages of platelet spreading. Journal of thrombosis and haemostasis: JTH. 2008;6:1944–1952. doi: 10.1111/j.1538-7836.2008.03141.x. [DOI] [PubMed] [Google Scholar]
- 10.Tomlinson MG, et al. Collagen promotes sustained glycoprotein VI signaling in platelets and cell lines. Journal of thrombosis and haemostasis: JTH. 2007;5:2274–2283. doi: 10.1111/j.1538-7836.2007.02746.x. [DOI] [PubMed] [Google Scholar]
- 11.Calaminus SD, et al. A major role for Scar/WAVE-1 downstream of GPVI in platelets. Journal of thrombosis and haemostasis: JTH. 2007;5:535–541. doi: 10.1111/j.1538-7836.2007.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarty OJ, et al. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. The Journal of biological chemistry. 2005;280:39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schoenwaelder SM, et al. RhoA sustains integrin alpha IIbbeta 3 adhesion contacts under high shear. The Journal of biological chemistry. 2002;277:14738–14746. doi: 10.1074/jbc.M200661200. [DOI] [PubMed] [Google Scholar]
- 14.Cavallini L, Coassin M, Borean A, Alexandre A. Prostacyclin and sodium nitroprusside inhibit the activity of the platelet inositol 1,4,5-trisphosphate receptor and promote its phosphorylation. The Journal of biological chemistry. 1996;271:5545–5551. doi: 10.1074/jbc.271.10.5545. [DOI] [PubMed] [Google Scholar]
- 15.Halbrugge M, Walter U. Purification of a vasodilator-regulated phosphoprotein from human platelets. Eur J Biochem. 1989;185:41–50. doi: 10.1111/j.1432-1033.1989.tb15079.x. [DOI] [PubMed] [Google Scholar]
- 16.Manganello JM, Djellas Y, Borg C, Antonakis K, Le Breton GC. Cyclic AMP-dependent phosphorylation of thromboxane A(2) receptor-associated Galpha(13) The Journal of biological chemistry. 1999;274:28003–28010. doi: 10.1074/jbc.274.39.28003. [DOI] [PubMed] [Google Scholar]
- 17.Beck F, et al. Time-resolved characterization of cAMP/PKA-dependent signaling reveals that platelet inhibition is a concerted process involving multiple signaling pathways. Blood. 2014;123:e1–e10. doi: 10.1182/blood-2013-07-512384. [DOI] [PubMed] [Google Scholar]
- 18.Aburima A, et al. cAMP signaling regulates platelet myosin light chain (MLC) phosphorylation and shape change through targeting the RhoA-Rho kinase-MLC phosphatase signaling pathway. Blood. 2013;122:3533–3545. doi: 10.1182/blood-2013-03-487850. [DOI] [PubMed] [Google Scholar]
- 19.Roberts W, Magwenzi S, Aburima A, Naseem KM. Thrombospondin-1 induces platelet activation through CD36-dependent inhibition of the cAMP/protein kinase A signaling cascade. Blood. 2010;116:4297–4306. doi: 10.1182/blood-2010-01-265561. [DOI] [PubMed] [Google Scholar]
- 20.Feoktistov I, Goldstein AE, Biaggioni I. Cyclic AMP and protein kinase A stimulate Cdc42: role of A(2) adenosine receptors in human mast cells. Mol Pharmacol. 2000;58:903–910. doi: 10.1124/mol.58.5.903. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor KL, Mercurio AM. Protein kinase A regulates Rac and is required for the growth factor-stimulated migration of carcinoma cells. The Journal of biological chemistry. 2001;276:47895–47900. doi: 10.1074/jbc.M107235200. [DOI] [PubMed] [Google Scholar]
- 22.Ellerbroek SM, Wennerberg K, Burridge K. Serine phosphorylation negatively regulates RhoA in vivo. The Journal of biological chemistry. 2003;278:19023–19031. doi: 10.1074/jbc.M213066200. [DOI] [PubMed] [Google Scholar]
- 23.Ravni A, et al. A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol Pharmacol. 2008;73:1688–1708. doi: 10.1124/mol.107.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martini CN, Plaza MV, Vila Mdel C. PKA-dependent and independent cAMP signaling in 3T3-L1 fibroblasts differentiation. Mol Cell Endocrinol. 2009;298:42–47. doi: 10.1016/j.mce.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 25.Raslan Z, Naseem KM. Compartmentalisation of cAMP-dependent signalling in blood platelets: The role of lipid rafts and actin polymerisation. Platelets. 2015;26:349–357. doi: 10.3109/09537104.2014.916792. [DOI] [PubMed] [Google Scholar]
- 26.de Wit RJ, et al. Inhibitory action of certain cyclophosphate derivatives of cAMP on cAMP-dependent protein kinases. Eur J Biochem. 1984;142:255–260. doi: 10.1111/j.1432-1033.1984.tb08279.x. [DOI] [PubMed] [Google Scholar]
- 27.Kase H, et al. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291X(87)90293-2. [DOI] [PubMed] [Google Scholar]
- 28.Raslan Z, Magwenzi S, Aburima A, Tasken K, Naseem KM. Targeting of type I protein kinase A to lipid rafts is required for platelet inhibition by the 3′,5′-cyclic adenosine monophosphate-signaling pathway. Journal of thrombosis and haemostasis: JTH. 2015;13:1721–1734. doi: 10.1111/jth.13042. [DOI] [PubMed] [Google Scholar]
- 29.Faure J, Dagher MC. Interactions between Rho GTPases and Rho GDP dissociation inhibitor (Rho-GDI) Biochimie. 2001;83:409–414. doi: 10.1016/S0300-9084(01)01263-9. [DOI] [PubMed] [Google Scholar]
- 30.Weiss HJ, Turitto VT. Prostacyclin (prostaglandin I2, PGI2) inhibits platelet adhesion and thrombus formation on subendothelium. Blood. 1979;53:244–250. [PubMed] [Google Scholar]
- 31.Higgs EA, et al. Effect of prostacyclin (PGI2) on platelet adhesion to rabbit arterial subendothelium. Prostaglandins. 1978;16:17–22. doi: 10.1016/0090-6980(78)90197-1. [DOI] [PubMed] [Google Scholar]
- 32.Higgs EA, Higgs GA, Moncada S, Vane JR. Prostacyclin (PGI2) inhibits the formation of platelet thrombi in arterioles and venules of the hamster cheek pouch. British journal of pharmacology. 1978;63:535–539. doi: 10.1111/j.1476-5381.1978.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sim DS, Merrill-Skoloff G, Furie BC, Furie B, Flaumenhaft R. Initial accumulation of platelets during arterial thrombus formation in vivo is inhibited by elevation of basal cAMP levels. Blood. 2004;103:2127–2134. doi: 10.1182/blood-2003-04-1133. [DOI] [PubMed] [Google Scholar]
- 34.Lang P, et al. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- 35.Poulter NS, et al. Platelet actin nodules are podosome-like structures dependent on Wiskott-Aldrich syndrome protein and ARP2/3 complex. Nat Commun. 2015;6:7254. doi: 10.1038/ncomms8254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pidoux G, Tasken K. Specificity and spatial dynamics of protein kinase A signaling organized by A-kinase-anchoring proteins. J Mol Endocrinol. 2010;44:271–284. doi: 10.1677/JME-10-0010. [DOI] [PubMed] [Google Scholar]
- 37.Miniati M, et al. Fibrin resistance to lysis in patients with pulmonary hypertension other than thromboembolic. Am J Respir Crit Care Med. 2010;181:992–996. doi: 10.1164/rccm.200907-1135OC. [DOI] [PubMed] [Google Scholar]
- 38.Machesky LM, Hall A. Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization. The Journal of cell biology. 1997;138:913–926. doi: 10.1083/jcb.138.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wraith KS, et al. Oxidized low-density lipoproteins induce rapid platelet activation and shape change through tyrosine kinase and Rho kinase-signaling pathways. Blood. 2013;122:580–589. doi: 10.1182/blood-2013-04-491688. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


