Abstract
Carbasugar sodium-glucose cotransporter 2 (SGLT2) inhibitors are highly promising drug candidates for the treatment of Type 2 diabetes mellitus (T2DM). However, the clinical usage of carbasugar SGLT2 inhibitors has been underexplored, due to the lengthy synthetic routes and the lack of structure-activity relationship (SAR) studies of these compounds. Herein, we report a concise and stereodivergent synthetic route towards some novel carbasugar SGLT2 inhibitors, featuring an underexploited, regioselective, and stereospecific palladium-catalyzed allyl-aryl coupling reaction. This synthetic strategy, together with computational modeling, revealed the unexpected SAR of these carbasugar SGLT2 inhibitors, and enabled the discovery of a highly selective and potent SGLT2 inhibitor.
Introduction
Carbasugars are carbocyclic analogues of carbohydrates, in which the endocyclic oxygen atom of the sugar core was substituted with a methylene unit1. Carbasugars have attracted extensive synthetic, conformational, and biological studies since the 1960s1. Their inhibitory activities towards carbohydrate-processing enzymes, such as α-amylase2, trehalase3, 4, α-glucosidase5, 6, and glycosyltransferase7, 8, have been well explored.
Recently, inhibition of sodium-glucose cotransporter 2 (SGLT2), a transporter protein expressed in the kidneys, has emerged as a promising treatment of diabetes via an insulin-independent mechanism9–12. Most current SGLT2 inhibitors are carbohydrate-based small molecules such as O-, C-, and mixed-glycosides (Compounds 1–4, Fig. 1)13–24 and many recent studies have focused intensely on modifications of the aglycone unit of C-glycoside analogues9–11. Departing from this trend, we have pioneered the design and syntheses of carbasugar SGLT2 inhibitors, in which we modify the sugar core rather than the aglycone25, 26. We found that some of the carbasugar analogues of sergliflozin (1), such as pseudo-O-glycoside 5 and pseudo-N-glycoside 6, exert a high inhibitory activity/selectivity towards SGLT225, 26, and also exhibit a prolonged blood glucose lowering effect25.
Figure 1.
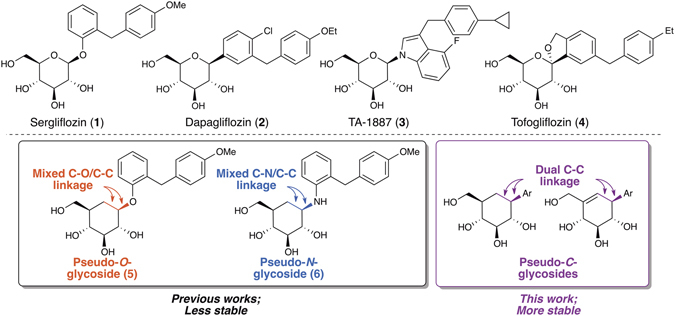
Current SGLT2 inhibitors & carbasugar SGLT2 inhibitors.
After the initial success of 5 & 6, we set out to further expand the synthetic toolkit towards carbasugars, and also to enhance the drug-like properties of carbasugar SGLT2 inhibitors. We postulated that aryl pseudo-C-glycosides, in which a C(sp2)–C(sp3) bond replace a C–O or C–N bond as in 5 & 6, are superior carbasugar SGLT2 inhibitors as they possess higher stability due to the presence of a “dual C–C linkage” (Fig. 1, lower)27. We report herein a concise and stereodivergent synthetic route towards these challenging synthetic targets, and also the unexpected SAR of carbasugar SGLT2 inhibition.
Results and Discussions
Allyl-aryl cross-coupling route towards carbasugars
Retrosynthetic analysis of our synthetic target 7 shows that it is readily accessible from simple starting materials such as D-gluconolactone (11) and arene 12 (Fig. 2). However, a major synthetic challenge is the stereoselective construction of the key C(sp2)–C(sp3) bond between the carbasugar core and aglycone. We therefore utilized an underexploited, stereospecific palladium-catalyzed allyl-aryl coupling reaction to address this challenge.
Figure 2.
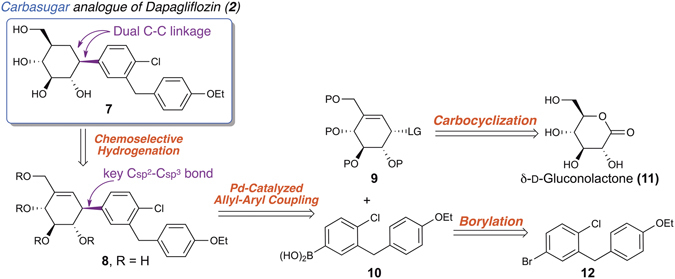
Retrosynthetic analysis of aryl pseudo-C-glycoside 7.
We first set out to synthesize aryl boronic acid 10 as a coupling precursor (Fig. 3, upper). The commercially available bromochlorobenzene 12 was converted into aryl boronic acid 10 by a simple one-pot, three-step borylation sequence. A lithium-bromide exchange yielded the lithiated intermediate 13. Electrophilic trapping of 13 by triisopropyl borate afforded the boronic ester 14, which was hydrolyzed immediately upon acidic quenching to give aryl boronic acid 10.
Figure 3.
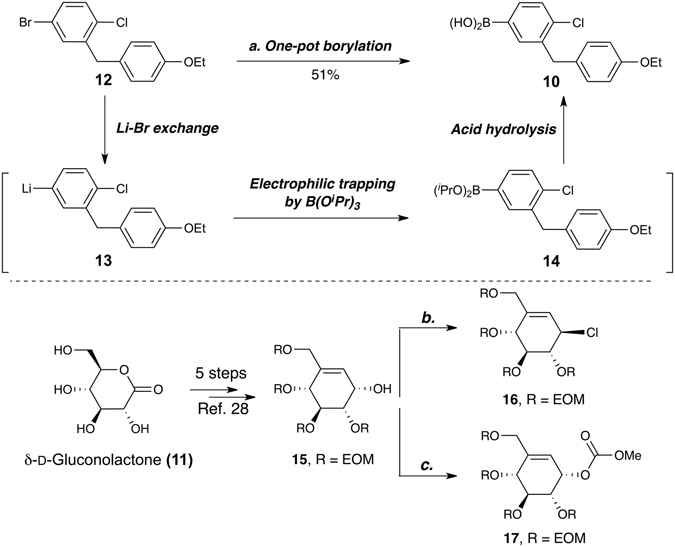
Syntheses of aryl boronic acid 10 and allylic electrophiles 16 & 17 as precursors for the subsequent coupling reactions. (a) 1) nBuLi, THF:Toluene = 1:4, −78 °C; 2) B(OiPr)3, −78 °C to −20 °C; 3) HCl(aq), −20 °C to rt; (b) MsCl, Et3N, 3 A MSn, Bu4NCl, CH2Cl2, 81%; (c) Methyl chloroformate, pyridine, 0 °C to rt, 74% (92% BORSM).
Next, we prepared the allylic electrophiles 16 and 17 for the subsequent allyl-aryl coupling reaction (Fig. 3, lower). Both of them were obtained from a common intermediate, allylic alcohol 15, which is readily accessible from the commercially available, inexpensive sugar 11 28.
With the coupling precursors in hand, we attempted the key Pd-catalyzed allyl-aryl coupling reaction, which has been underexploited in the literature (Fig. 4 and Table 1)29. This seemingly simple transformation, however, was not trivial, and required extensive optimization. The allylic electrophiles 16 and 17 were prone to β-elimination which led to the formation of diene side products. A literature search revealed that allyl-aryl coupling reactions in cyclic systems are rare and challenging30–32, while those in acyclic systems often necessitate elevated reaction temperatures33–35. Thus, a mild reaction condition for cyclic substrates is highly sought-after. Upon extensive reaction optimization, we successfully identified that the combination of Pd(dba)2/K2CO3/1,4-dioxane gave the best reaction outcome for both allylic electrophiles 16 & 17 (Table 1, entries 5 & 10). Notably, the proper choice of solvent, the absence of phosphine ligand, and the mild reaction temperature are critical to a high coupling yield. Remarkably, this phosphine-free Pd-catalyzed allyl-aryl coupling reaction is stereospecific and regioselective, with arylated C-1-α-cyclohexene 18 and C-1-β-cyclohexene 20 obtained as single products.
Figure 4.
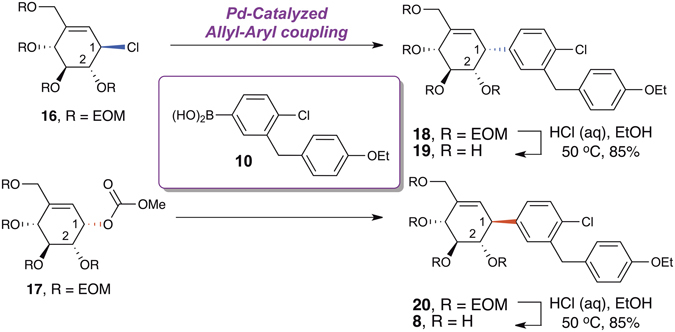
Syntheses of cyclohexene analogues of dapagliflozin via allyl-aryl coupling.
Table 1.
Selected reaction conditions for Pd-catalyzed allyl-aryl couplinga.
| Entry | Reaction conditions | Results |
|---|---|---|
| 1 | 16, Pd(dba)2, K2CO3, CHCl3 | 18: 38% |
| 2 | 16, Pd(dba)2, K3PO4, CHCl3 | 18: 69% |
| 3 | 16, Pd(dba)2, KF, CHCl3 | 18: 62% |
| 4 | 16, Pd(dba)2, K3PO4, 1,4-dioxane | 18: 74% |
| 5 | 16, Pd(dba) 2 , K 2 CO 3 , 1,4-dioxane | 18: 88% |
| 6 | 17, Pd(dba)2, K2CO3, CHCl3 | 20: 38% |
| 7 | 17, Pd(dba) 2, Na2CO3, 1,4-dioxane | 20: 70% |
| 8 | 17, Pd(dba) 2, K2CO3, CH3CN | 20: 22% |
| 9 | 17, Pd(dba) 2, K2CO3, 1,4-dioxane (50 °C) | 20: 35% |
| 10 | 17, Pd(dba) 2 , K 2 CO 3 , 1,4-dioxane | 20: 85% |
| 11 | 17, Pd(dba)2, dppf, K2CO3, 1,4-dioxane | 20: 22% |
aThe reactions were conducted at room temperature unless specified. All of the reported yields are isolated yields. EOM = ethoxymethyl; dppf = 1,1′-bis(diphenylphosphino) ferrocene.
A plausible reaction mechanism is proposed to explain the excellent stereo- and regio-selectivities of the allyl-aryl coupling reaction (Fig. 5). A π-allyl Pd complex is first formed via a back-side attack from the less-hindered face (Fig. 5, pathway I). After the transmetalation step, the aryl group on the π-allyl Pd complex is delivered internally to the less-hindered C-1 position of the carbocycle, resulting a net-inversion of configuration and an exclusive regioselection (pathway III). On the other hand, syn-β-H elimination (pathway IV) of the η1-Pd complex is a viable degradation pathway, which is suppressed at a lower reaction temperture.
Figure 5.
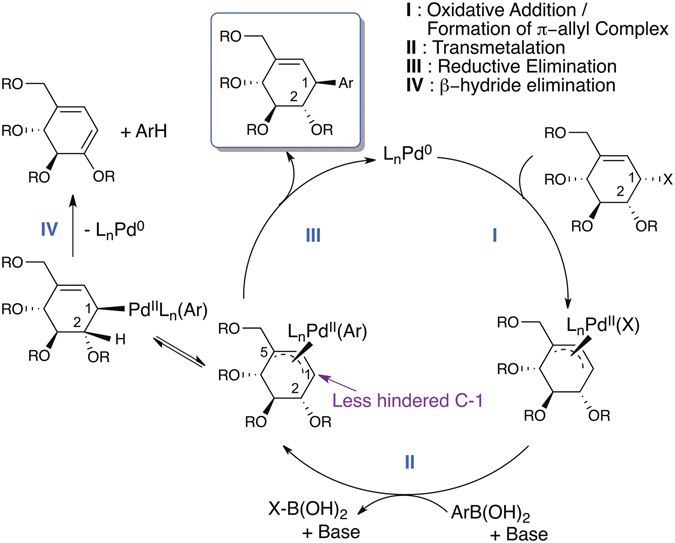
Proposed reaction mechanism of the allyl-aryl coupling reaction.
Next, the arylated cyclohexenes 18 and 20 were subjected to alkene hydrogenation and global deprotection (Fig. 6). Our initial attempts of Pd-, Pt- or Ni-catalyzed hydrogenation led to complete dechlorination of the aglycone moiety. However, the diimide reduction of 18 and 20 was highly chemoselective: the alkene moiety was hydrogenated while the chloride substituent on the proximal benzene ring was preserved. This diimide reduction strategy also provided a stereodivergent entry to both C-1 and C-5 epimers of the cyclohexane analogues of dapagliflozin (Fig. 6). Thus, starting from the common precursor 15, all four possible diastereoisomers (aryl pseudo-C-glycosides 22, 24, 7, and 27) were prepared for the subsequent SAR study. We also synthesized the aryl pseudo-O-glycosides 28–30 for a more comprehensive SAR study.(See Supporting Information)
Figure 6.
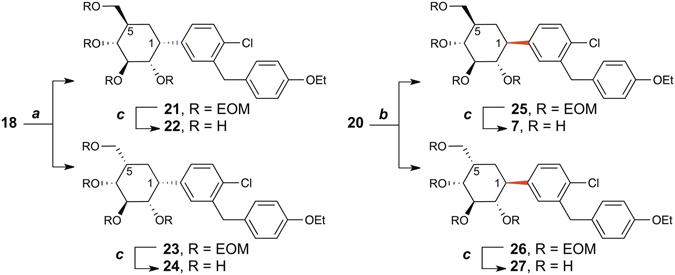
Stereodivergent syntheses of cyclohexane analogues of dapagliflozin. (a) p-TsNHNH2, NaOAc, THF(aq), reflux; (b) p-TsNHNH2, NaOAc, THF(aq), 70 °C; (c) HCl(aq), EtOH, 50 °C, 22: 51% from 18; 24: 7% from 18; 7: 65% from 20; 27: 25% from 20.
Structure-activity relationship (SAR) study
A cellular 14C-α-methyl-D-glucopyranoside (14C-AMG) uptake assay was used to evaluate the SGLT2/SGLT1 inhibitory activities of our carbasugar analogues of dapagliflozin (Fig. 7). In general, the β-C series were more active than the α-C series, hinting that the β-configuration at C-1 is critical for the inhibitory activity. However, within the β-C series, the cyclohexane analogue 7 showed only a moderate SGLT2 inhibitory activity and only 20-fold SGLT2/SGLT1 selectively; whereas the cyclohexene analogue 8 showed a nanomolar SGLT2 inhibitory activity and more than 400 fold SGLT2/SGLT1 selectively. The excellent potency and selectivity of 8 towards SGLT2, together with its enhanced stability (as a result of its dual C-C linkage), suggested that it is a highly promising lead compound for further optimization as a clinically useful SGLT2 inhibitor.
Figure 7.
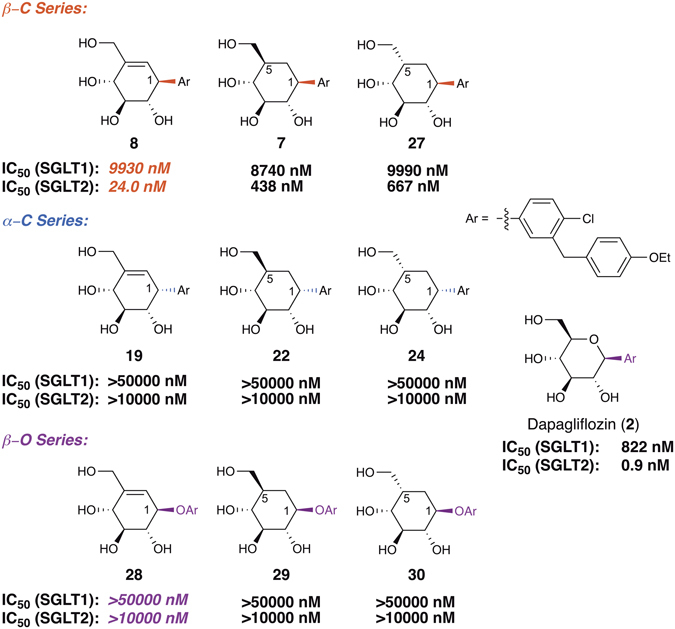
IC50 values for the novel analogues of dapagliflozin towards SGLT1 and SGLT2.
Another piece of unexpected SAR information came from the activity profiles of aryl pseudo-O-glycosides 28, 29, and 30 (β-O series), in which an exocyclic oxygen atom connects the carbasugar and aglycone moiety. We found that this subtle change in spatial connectivity is detrimental to the activity of carbocyclic analogues of dapagliflozin, as suggested by the dramatic loss of inhibitory activity in the β-O series.
Computational modeling
The superior performance of cyclohexene 8, together with the unexpected activity profile of the other carbasugars, prompted us to investigate the binding mode of carbasugar SGLT2 inhibitors by computational modeling. We performed in silico docking analysis by fitting different ligands into a homology model of SGLT2 (Fig. 8). As shown in our homology model, the ligand binding pocket of SGLT2 is shaped by the hydrophobic residues Phe98, Tyr290, and Trp298, together with hydrogen bond donors/acceptors such as His80, Glu99, and Asn10136, 37. (Fig. 8a) We found that the diarylmethane moiety in both 7 & 8 stacked perfectly with the aromatic ring of Tyr290 (Fig. 8b and c), resulting in strong π-π stabilizations. There are also extensive hydrogen bonding interactions between the carbasugar hydroxyl groups and His80, Glu99, and Asn101. Although the hydrogen bond network of 7 and 8 closely resembles each other, the distorted chair conformation of 8 allowed an additional hydrogen bonding interaction between the primary hydroxyl group and Ser287, thus leading to an enhanced binding affinity of 8. (Fig. 8c) Our computational modeling also revealed that pseudo-O-glycoside 28 is a poor ligand of SGLT2 (Fig. 8d). The exocyclic oxygen atom in 28 effectively disrupts the π-π interactions and also withholds the carbasugar core from forming an extensive hydrogen bond network, resulting in the observed lack of SGLT2 inhibitory activity of 28.
Figure 8.
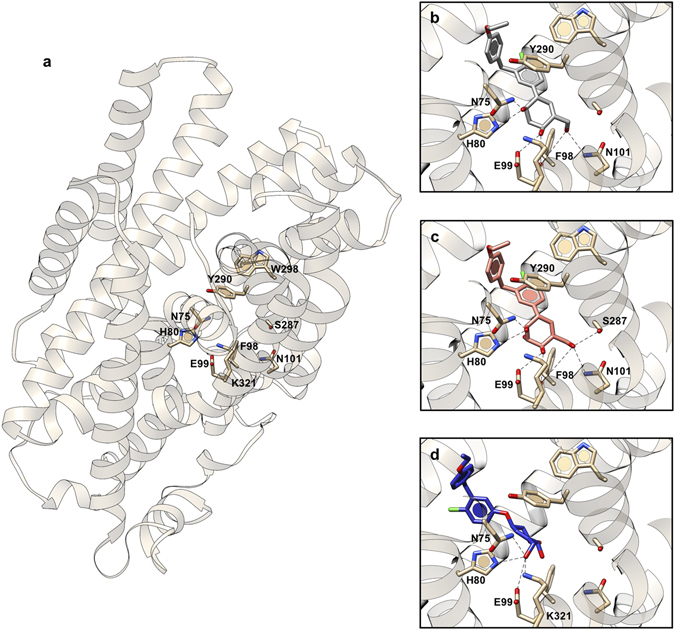
In silico docking analysis of hSGLT2-ligand interactions. (a) Ribbon representation of our hSGLT2 homology model. Molecular docking of (b) compound 7, (c) compound 8 and (d) compound 28 into hSGLT2. Hydrogen bonds are shown as dotted lines, and key interacting residues are labelled.
With the assistance of computational modeling, this SAR study has provided important insights into the ligand space of carbasugar SGLT2 inhibitors, thereby guiding the future exploration of these inhibitors as novel anti-diabetic agents. In addition, recent studies have suggested additional beneficial effects of SGLT2 inhibition, such as reducing body weight, blood pressure, cardiovascular and renal events12, which helped to uncover the hidden therapeutic potentials of our novel carbasugar SGLT2 inhibitors.
Conclusions
In conclusion, we have developed a concise and stereodivergent synthetic route towards some novel carbasugar analogues of dapagliflozin. The key step involves a mild, regioselective, and stereospecific palladium-catalyzed allyl-aryl coupling reaction which has been underexploited in the synthetic community. A chemoselective diimide reduction was also critical to our synthetic route. Notably, this synthetic study revealed unexpected structure-activity relationship (SAR) of carbasugar SGLT2 inhibitors, and also enabled the discovery of the highly potent and selective inhibitor, cyclohexene 8. Our study will therefore guide the future design and syntheses of carbasugar SGLT2 inhibitors, thereby unlocking their potential as novel diabetes therapeutics and beyond.
Methods
Preparation of compound 8
To a stirred solution of cyclohexene 20 (66.6 mg, 0.105 mmol) in EtOH (1.5 mL) was added 1 M HCl (aq) (1.5 mL) at rt. The reaction mixture was stirred at 50 °C for 3 h. Concentration of the reaction mixture under reduced pressure followed by flash chromatography (CHCl3:MeOH, 9:1) yield tetraol 8 (36.9 mg, 87%) as a white solid. For detailed characterization of 8, please refer to supporting information.
Preparation of compound 20
To a mixture of allylic carbonate 17 (71.7 mg, 0.154 mmol) and boronic acid 10 (141.1 mg, 0.486 mmol) in degassed 1,4-dioxane (0.19 mL) was added Pd(dba)2 (5.5 mg, 0.0096 mmol) and K2CO3 (68.1 mg, 0.493 mmol) sequentially. The reaction mixture was degassed for 3 times and stirred for 72 h at rt under nitrogen. Concentration of the reaction mixture under reduced pressured followed by flash chromatography (hexane: EtOAc, 7:2) yielded cyclohexene 20 (82.8 mg, 85%) as a colourless oil. For detailed characterization of 20, please refer to supporting information.
Procedure for 14C-α-methyl-D-glucopyranoside (14C-AMG) uptake assay
SGLT1- or SGLT2- expressing cells (S1 or S2) were developed by transfecting, respectively, SLC5A1 (NM_000343) or SLC5A2 (NM_003041) human cDNA clone (Origene Technologies Inc., Maryland, USA) into COS-7 cells (monkey fibroblast-like kidney cells, CRL-1651, ATCC, Manassas, USA). The cells were cultured in DMEM supplemented with 10% FBS, 1% PS and G418 (1 mg/ml), and kept in 37 °C humidified incubator supplied with 5% CO2. For the uptake assay, the cells (2 × 105 cells/well) were seeded into 24-well plate and incubated overnight. Then, each well was added with the testing samples at different concentrations and 100 µCi/ml 14C-AMG. The cells were incubated in sodium buffer (140 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM Hepes/Tris, pH 7.5) at 37 °C for 2 hours. After incubation, the plates were washed three times with cold choline stop buffer (140 mM choline chloride, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM Hepes/Tris, pH 7.5) containing 100 µM phlorizin dihydrate. After removing all buffer, the cells were then solubilized with 150 µL of NaOH (0.5 M) and followed by 150 µL of HCl (0.5 M). Then, 200 µL solution was taken out for the measurement of radioactivity and 30 µL solution was used to measure the protein content by BCA protein assay, which was used to normalize the cell number in each well. The amount of glucose uptake was expressed as cpm/mg protein.
Homology Modeling of Human SGLT2
A homology model was generated from the structural template of sodium-galactose symport from Vibrio parahaemolyticus (PDB ID: 3DH4) (Faham et al., 2008). The homology modeling was performed with MODELLER (Sali and Blundell, 1993) in UCSF Chimera (Yang et al., 2012). UCSF Chimera alpha version 1.12 was used for the modeling. For validation of this homology model, please refer to supporting information.
In silico Docking Analyses
The receptor macromolecule and ligands were prepared by using the Dock Prep tool in UCSF Chimera. During preparation, solvent and non-complexed ions were deleted, truncated sidechains were repaired, hydrogen atoms were added, partial charges were assigned, files were saved as Mol2 format. Docking was subsequently performed in UCSF Chimera (alpha version 1.12) using the AutoDock Vina tool version 1.1.2. The ViewDock tool in UCSF Chimera was used to analyze the receptor-ligand docking results. For details of the docking results, please refer to supporting information.
Acknowledgements
We thank Dr. Sonal Jhaveri (DFCI) and Wing-Hin Ng (CUHK) for useful comments on this manuscript. W.L.N. thanks HKRGC for a Hong Kong PhD Fellowship. This work has been supported by a Hong Kong RGC GRF grant (ref. no. 2130348).
Author Contributions
W.L.N. and T.K.M.S. designed the project. W.L.N. and H.C.L. conducted chemical syntheses. K.M.L. performed 14C-AMG uptake assay. W.L.N. and A.K.N.C. performed computational analysis. W.L.N. and T.K.M.S. wrote the manuscript. T.K.M.S. and C.B.S.L. guided the project. All authors read and commented on the manuscript.
Competing Interests
We would also like to declare the following competing financial interest(s): CUHK has filed a patent on the compounds disclosed in this manuscript. If licensed, the authors will receive royalty payments in line with standard university practice.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arjona O, Gómez AM, López JC, Plumet J. Synthesis and conformational and biological aspects of carbasugars. Chem Rev. 2007;107:1919–2036. doi: 10.1021/cr0203701. [DOI] [PubMed] [Google Scholar]
- 2.Lahiri R, Ansari AA, Vankar YD. Recent developments in design and synthesis of bicyclic azasugars, carbasugars and related molecules as glycosidase inhibitors. Chem Soc Rev. 2013;42:5102–5118. doi: 10.1039/c3cs35525j. [DOI] [PubMed] [Google Scholar]
- 3.Kameda Y, Asano N, Yamaguchi T, Matsui K. Validoxylamines as trehalase inhibitors. J Antibiot (Tokyo). 1987;40:563–565. doi: 10.7164/antibiotics.40.563. [DOI] [PubMed] [Google Scholar]
- 4.Asano N, Takeuchi M, Kameda Y, Matsui K, Kono Y. Trehalase inhibitors, validoxylamine A and related compounds as insecticides. J Antibiot (Tokyo). 1990;43:722–726. doi: 10.7164/antibiotics.43.722. [DOI] [PubMed] [Google Scholar]
- 5.Shing TKM, et al. Enantiospecific synthesis of pseudoacarviosin as a potential antidiabetic agent. Org Lett. 2008;10:3145–3148. doi: 10.1021/ol8010503. [DOI] [PubMed] [Google Scholar]
- 6.Shing TKM, Cheng HM. Intramolecular direct aldol reactions of sugar diketones: syntheses of valiolamine and validoxylamine G. Org Lett. 2008;10:4137–4139. doi: 10.1021/ol801889n. [DOI] [PubMed] [Google Scholar]
- 7.Yuasa H, Palcic MM, Hindsgaul O. Synthesis of the carbocyclic analog of uridine 5′-(α-D-galactopyranosyl diphosphate) (UDP-Gal) as an inhibitor of β (1 → 4)-galactosyltransferase . Can. J. Chem. 1995;73:2190–2195. doi: 10.1139/v95-272. [DOI] [Google Scholar]
- 8.Cai S, Stroud MR, Hakomori S, Toyokuni T. Synthesis of carbocyclic analogs of guanosine 5′-(α-L-fucopyranosyl diphosphate) (GDP-fucose) as potential inhibitors of fucosyltransferases. J. Org. Chem. 1992;57:6693–6696. doi: 10.1021/jo00051a004. [DOI] [Google Scholar]
- 9.Chao EC, Henry RR. SGLT2 inhibition - a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010;9:551–559. doi: 10.1038/nrd3180. [DOI] [PubMed] [Google Scholar]
- 10.Jones D. Diabetes field cautiously upbeat despite possible setback for leading SGLT2 inhibitor. Nat Rev Drug Discov. 2011;10:645–646. doi: 10.1038/nrd3546. [DOI] [PubMed] [Google Scholar]
- 11.Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010;95:34–42. doi: 10.1210/jc.2009-0473. [DOI] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Norton L, Abdul-Ghani M. Renal, metabolic and cardiovascular considerations of SGLT2 inhibition. Nat Rev Nephrol. 2017;13:11–26. doi: 10.1038/nrneph.2016.170. [DOI] [PubMed] [Google Scholar]
- 13.Nunoi K, et al. Beneficial effect of T-1095, a selective inhibitor of renal Na+-glucose cotransporters, on metabolic index and insulin secretion in spontaneously diabetic GK rats. Clin Exp Pharmacol Physiol. 2002;29:386–390. doi: 10.1046/j.1440-1681.2002.03671.x. [DOI] [PubMed] [Google Scholar]
- 14.Ueta K, et al. Long-term treatment with the Na+-glucose cotransporter inhibitor T-1095 causes sustained improvement in hyperglycemia and prevents diabetic neuropathy in Goto-Kakizaki rats. Life Sci. 2005;76:2655–2668. doi: 10.1016/j.lfs.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 15.Kees KL, et al. New potent antihyperglycemic agents in db/db mice: synthesis and structure-activity relationship studies of (4-substituted benzyl) (trifluoromethyl)pyrazoles and -pyrazolones. J Med Chem. 1996;39:3920–3928. doi: 10.1021/jm960444z. [DOI] [PubMed] [Google Scholar]
- 16.Grempler R, et al. Empagliflozin, a novel selective sodium glucose cotransporter-2 (SGLT-2) inhibitor: characterisation and comparison with other SGLT-2 inhibitors. Diabetes Obes Metab. 2012;14:83–90. doi: 10.1111/j.1463-1326.2011.01517.x. [DOI] [PubMed] [Google Scholar]
- 17.Nomura S, et al. Discovery of canagliflozin, a novel C-glucoside with thiophene ring, as sodium-dependent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem. 2010;53:6355–6360. doi: 10.1021/jm100332n. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, et al. EGT1442, a potent and selective SGLT2 inhibitor, attenuates blood glucose and HbA(1c) levels in db/db mice and prolongs the survival of stroke-prone rats. Pharmacol Res. 2011;63:284–293. doi: 10.1016/j.phrs.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto Y, et al. N-Glucosides as human sodium-dependent glucose cotransporter 2 (hSGLT2) inhibitors. Bioorg Med Chem Lett. 2013;23:5641–5645. doi: 10.1016/j.bmcl.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Yao CH, et al. Discovery of novel N-β-D-xylosylindole derivatives as sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes. J Med Chem. 2011;54:166–178. doi: 10.1021/jm101072y. [DOI] [PubMed] [Google Scholar]
- 21.Nomura S, et al. Novel indole-N-glucoside, TA-1887 as a sodium glucose cotransporter 2 inhibitor for treatment of type 2 diabetes. ACS Med Chem Lett. 2013;5:51–55. doi: 10.1021/ml400339b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki M, et al. Tofogliflozin, a potent and highly specific sodium/glucose cotransporter 2 inhibitor, improves glycemic control in diabetic rats and mice. J Pharmacol Exp Ther. 2012;341:692–701. doi: 10.1124/jpet.112.191593. [DOI] [PubMed] [Google Scholar]
- 23.Ikeda S, et al. A novel and selective sodium-glucose cotransporter-2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:984–993. doi: 10.1111/dom.12538. [DOI] [PubMed] [Google Scholar]
- 24.Mascitti V, et al. On the importance of synthetic organic chemistry in drug discovery: reflections on the discovery of antidiabetic agent ertugliflozin. Med. Chem. Comm. 2013;4:101–111. doi: 10.1039/C2MD20163A. [DOI] [Google Scholar]
- 25.Shing TKM, Ng WL, Chan JY, Lau CB. Design, syntheses, and SAR studies of carbocyclic analogues of sergliflozin as potent sodium-dependent glucose cotransporter 2 inhibitors. Angew Chem Int Ed. 2013;52:8401–8405. doi: 10.1002/anie.201302543. [DOI] [PubMed] [Google Scholar]
- 26.Ng WL, Lau KM, Lau CB, Shing TKM. Palladium-catalyzed arylation of carbasugars enables the discovery of potent and selective SGLT2 inhibitors. Angew Chem Int Ed. 2016;55:13818–13821. doi: 10.1002/anie.201608758. [DOI] [PubMed] [Google Scholar]
- 27.Ohtake Y, et al. C-Aryl 5a-carba-β-D-glucopyranosides as novel sodium glucose cotransporter 2 (SGLT2) inhibitors for the treatment of type 2 diabetes. Bioorg Med Chem. 2012;20:4117–4127. doi: 10.1016/j.bmc.2012.04.053. [DOI] [PubMed] [Google Scholar]
- 28.Shing TKM, Chen Y, Ng WL. Short and efficient syntheses of gabosine I, streptol, 7-O-acetylstreptol, 1-epi-streptol, gabosine K, and carba-α-D-glucose from δ-D-gluconolactone. Synlett. 2011;2011:1318–1320. doi: 10.1055/s-0030-1260547. [DOI] [Google Scholar]
- 29.Sidera M, Fletcher SP. Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids. Nat. Chem. 2015;7:935–939. doi: 10.1038/nchem.2360. [DOI] [PubMed] [Google Scholar]
- 30.Kayaki Y, Koda T, Ikariya T. A highly effective (triphenyl phosphite) palladium catalyst for a cross−coupling reaction of allylic alcohols with organoboronic acids. Eur. J. Org. Chem. 2004;24:4989–4993. doi: 10.1002/ejoc.200400621. [DOI] [Google Scholar]
- 31.Kobayashi Y, Ikeda E. Nickel-catalysed substitution reactions of allylic carbonates with aryl- and alkenyl-borates. J. Chem. Soc., Chem. Commun. 1994;1994:1789–1790. doi: 10.1039/c39940001789. [DOI] [Google Scholar]
- 32.Li Z, Ip FC, Ip NY, Tong R. Highly trans-selective arylation of Achmatowicz rearrangement products by reductive γ-deoxygenation and Heck-Matsuda reaction: asymmetric total synthesis of (−)-musellarins A-C and their analogues. Chem. Eur. J. 2015;21:11152–11157. doi: 10.1002/chem.201501713. [DOI] [PubMed] [Google Scholar]
- 33.Ye J, Zhao J, Xu J, Mao Y, Zhang YJ. Pd-Catalyzed stereospecific allyl-aryl coupling of allylic alcohols with arylboronic acids. Chem. Commun. 2013;49:9761–9763. doi: 10.1039/c3cc45053h. [DOI] [PubMed] [Google Scholar]
- 34.Li C, et al. Pd-catalyzed regioselective and stereospecific Suzuki-Miyaura coupling of allylic carbonates with arylboronic acids. Org. Lett. 2012;14:390–393. doi: 10.1021/ol203154j. [DOI] [PubMed] [Google Scholar]
- 35.Li MB, Wang Y, Tian SK. Regioselective and stereospecific cross-coupling of primary allylic amines with boronic acids and boronates through palladium-catalyzed C-N bond cleavage. Angew. Chem., Int. Ed. 2012;51:2968–2971. doi: 10.1002/anie.201109171. [DOI] [PubMed] [Google Scholar]
- 36.Abramson J, et al. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–615. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- 37.Faham S, et al. The crystal structure of a sodium galactose transporter reveals mechanistic insights into Na+/sugar symport. Science. 2008;321:810–814. doi: 10.1126/science.1160406. [DOI] [PMC free article] [PubMed] [Google Scholar]


