Abstract
For four decades, an influential hypothesis has posited that competition for food resources between microbes and vertebrates selects for microbes to alter these resources in ways that make them unpalatable to vertebrates. We chose an understudied cross kingdom interaction to experimentally evaluate the effect of fruit infection by fungi on both vertebrate (mammals and birds) fruit preferences and on ecologically relevant fruit traits (volatile compounds, toughness, etc). Our well-replicated field experiments revealed that, in contrast to previous studies, frugivorous mammals and birds consistently preferred infested over intact fruits. This was concordant with the higher level of attractive volatiles (esters, ethanol) in infested fruits. This investigation suggests that vertebrate frugivores, fleshy-fruited plants, and microbes form a tripartite interaction in which each part could interact positively with the other two (e.g. both orange seeds and fungal spores are likely dispersed by mammals). Such a mutualistic view of these complex interactions is opposed to the generalized idea of competition between frugivorous vertebrates and microorganisms. Thus, this research provides a new perspective on the widely accepted plant evolutionary dilemma to make fruits attractive to mutualistic frugivores while unattractive to presumed antagonistic microbes that constrain seed dispersal.
Introduction
Human activity creates numerous opportunities for the appearance of so-called ‘novel interactions’1 between species that otherwise would not coexist. Novel interactions arising from biological invasions have been deeply investigated2 and pair-wise interactions between native and crop species have also received some attention3–5. Surprisingly, novel cross-kingdom (e.g. plant-vertebrate-microbe) interactions remain largely understudied despite their pervasiveness and potential ecological, evolutionary, and economical relevance in natural and humanized ecosystems2, 6. In particular, novel cross-kingdom interactions taking place in agro-ecosystems provide excellent logistical settings to investigate, through easily replicated field experiments, intriguing ecological and evolutionary questions.
Interactions between fruiting groves and frugivorous vertebrates are widespread worldwide7–9. Seeds of domestic species are often ingested by vertebrates and eventually released away from the mother plant (i.e. endozoochory), potentially leading to naturalization of such cultivated plants10. Interestingly, these bipartite interactions can be joined by microorganisms that feed on the pulp and seeds, thus potentially interfering with the domestic plant-seed disperser interaction. Janzen11 suggested that fruits infested by microbes are rarely eaten by vertebrates because microbes produce toxic compounds and reduce the nutritional value of infested fruit. Most empirical investigations of these cross-kingdom interactions12–14 as well as recent theoretical evidence have supported Janzen’s famous prediction15, but see16. Nonetheless, there exist a handful of studies showing that frugivores prefer infested fruits9, 17–19. Also, recent paleogenetic data suggest that during the middle Miocene (~16 MA ago), apes evolved the ability of ingesting fallen microbe-infested fruit20. Such disparate findings are potentially related to frugivore response to fruit infestation changing with vertebrate, plant, and/or pathogen species, as well as with the ecological context14, 21. Surprisingly, however, most available empirical evidence is restricted to interactions involving small fruits and small frugivorous birds (see Supplementary Table S1), and extensively replicated field experiments assessing the spatial and temporal consistency of frugivore responses to microbe infestation are lacking. Furthermore, whether different frugivore functional groups with contrasting foraging modes (e.g. seed disperses, pulp feeders, seed predators22, 23) respond similarly to microbe infestation is unknown. Though species involved in novel interactions do not share a common evolutionary history, these tripartite interactions could shed light on how plants solve the evolutionary dilemma of making their fruits attractive to seed dispersers while unattractive to antagonistic microbes13, 15.
We also know very little concerning the mechanisms underlying vertebrate responses to microbe fruit infestation. In particular, plant volatile organic compounds (VOCs) are secondary metabolites that mediate the attraction of seed dispersers and the avoidance of seed predators24, 25. VOCs emitted by fruits and altered by microorganism infestation are known in some domestic species26, 27. Whether these microbe-induced VOCs changes together with other potential changes in fruit physical and chemical proprieties (e.g. toughness, sweetness, pH) have a significant effect on subsequent fruit interactions with native vertebrate frugivores remains a puzzle19.
Frugivorous vertebrates, domestic Citrus trees, and microorganisms form widespread though understudied tripartite interactions9, 27, 28. In particular, Penicillium digitatum Sacc. infects large fractions of harvested orange fruits worldwide27. In this study, we chose the interaction among the sweet orange tree (Citrus sinensis L. Osb.), several mammalian and avian frugivores, and P. digitatum in tropical and Mediterranean groves to experimentally address the following four questions: i) Does infection by Penicillium alter orange fruit preferences by different vertebrate frugivores (mammals, birds)? If so, and given their contrasting frugivore faunas, ii) is such effect consistent between tropical and Mediterranean orange groves? Also, since different frugivore guilds (i.e. seed dispersers, pulp feeders, granivore rodents) often show contrasting foraging behaviors23, 29, iii) is the effect of fungal infestation consistent among frugivore guilds? Finally, iv) does Penicillium infestation alter physical and/or chemical orange fruit parameters (e.g. toughness, VOC profiles, acid/sugar and ethanol concentrations) and, if so, are such changes consistent with frugivore fruit preferences?
Results
Effect of Penicillium infestation on fruit harvesting
Frugivore tracks and/or other signs such as feces were found by the fruit in all experimental orange trees both in the Mediterranean and the tropical experimental groves. Whereas in the Mediterranean groves, we recorded visits by pulp feeders (mostly rabbits) and rodents, in the tropical groves, in addition to those two frugivore guilds, we also recorded frequent visits by seed dispersers (mostly wild boars; see Supplementary Methods and Results).
Our mixed model indicated that, once the effects of random factors were controlled for, overall fruit harvesting was 8.2-fold higher in the tropical than in the Mediterranean groves (F1, 2897 = 118.90, P < 0.0001; Fig. 1). Frugivores from both regions strongly preferred Penicillium-infested as compared with intact fruits (F1, 2897 = 551.31, P < 0.0001; Fig. 1). Nevertheless, there was a significant interaction between Penicillium infestation and region (F1, 2896 = 93.65, P < 0.0001) indicating that the effect of infestation on fruit harvesting was stronger in the Mediterranean orange groves as compared with the tropical ones (Fig. 1). Specifically, in the Mediterranean groves, harvesting percentage for Penicillium-infested fruit was 21.1-fold higher than for intact fruit (tests of slices, F1, 2896 = 369.01, P < 0.0001), whereas in Brazil fruit harvesting percentage for Penicillium-infested fruit was 2.6-fold higher than for intact fruit (F1, 2897 = 187.42, P < 0.0001). We undertook a second set of analyses to identify guild-specific effects of Penicillium-infestation on fruit harvesting in each region (Table 1). In the Mediterranean groves, there were strong overall significant differences between guilds in the percentages of fruit harvesting (P < 0.0001; Table 1), with pulp feeders harvesting 8.9-times more fruits than rodents (Fig. 2A). Also, we found a strong significant Penicillium infestation effect on fruit harvesting, being 17.23-times higher for infested as compared to intact orange fruits (Table 1; Fig. 2A). The non-significant interaction between infestation and guild (Table 1) indicated that the marked preference for infested fruits was consistent on sign and magnitude for both frugivore guilds (Fig. 2A).
Figure 1.
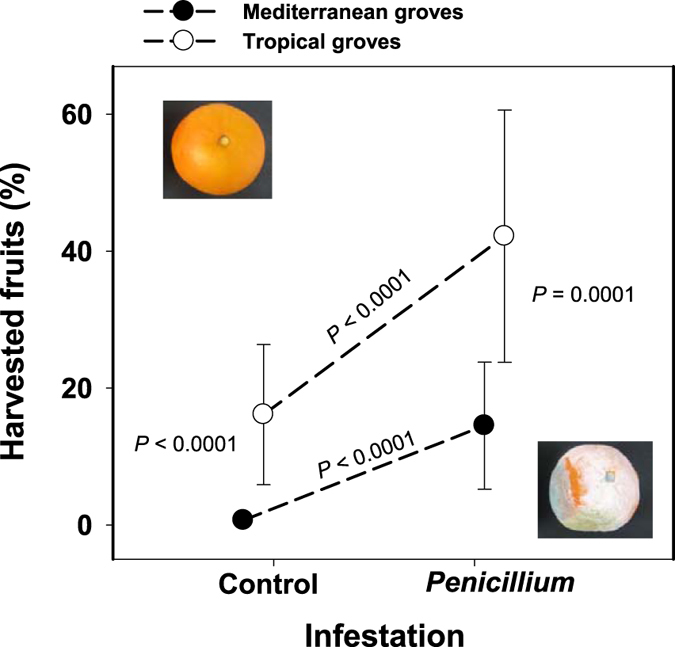
Harvesting by vertebrate frugivores (mammals and birds) of intact and Penicillium-infested oranges. Graphical representation of statistically significant interaction between Penicillium infestation (intact vs. Penicillium-infested) and region (tropical vs. Mediterranean) found for overall fruit harvesting by vertebrate frugivores of sweet orange (Citrus sinensis) fruit in our experimental Mediterranean and tropical groves. The P-values of the tests for the four simple main effects involved in the interaction are shown.
Table 1.
Results of main effect tests using generalized linear mixed models on the effects of Penicillium digitatum infestation (P) and consumer guild (G), as well as their second-order interaction, on percentages of fruit harvesting in Mediterranean and tropical sweet orange (Citrus sinensis) groves.
| Mediterranean groves | Tropical groves | |||||
|---|---|---|---|---|---|---|
| F | d.f. | P | F | d.f. | P | |
| Penicillium (P) | 138.25 | 1, 3062 | <0.0001 | 63.64 | 1, 4215 | <0.0001 |
| Guild (G) | 80.30 | 1, 3062 | <0.0001 | 49.20 | 2, 4215 | <0.0001 |
| P *G | 0.29 | 1, 3062 | 0.561 | 32.51 | 2, 4215 | <0.0001 |
Figure 2.
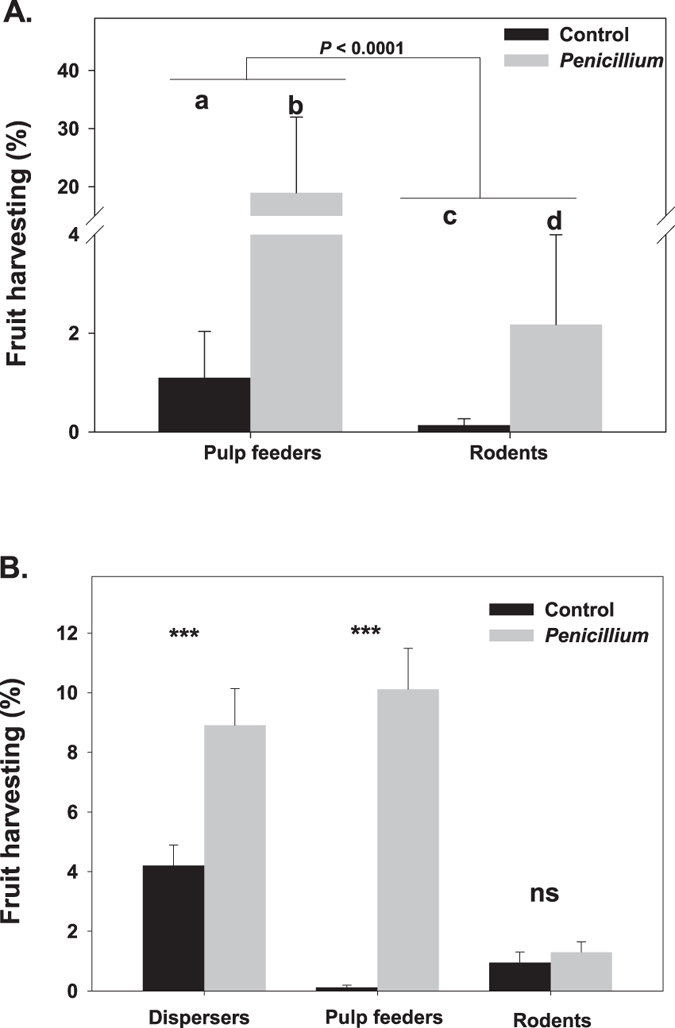
Fruit harvest by different frugivore guilds (i.e. seed dispersers, pulp feeders, granivore rodents). Model corrected mean percentages (±1SE) of sweet orange (Citrus sinensis) fruit harvest by different frugivore guilds as a function of Penicillium infestation in the Mediterranean (A) and tropical groves (B). Different lowercase letters among Penicillium infestation levels denote significant (P < 0.05) differences. ***P < 0.0001; ns, not significant (P > 0.05).
In the tropical groves, seed dispersers (6.70 ± 0.88%) harvested significantly more fruits than both seed-eating rodents (1.09 ± 0.27%) and pulp feeders (4.87 ± 0.68%; Table 1). As in the Mediterranean groves, overall fruit harvesting was significantly (Table 1) higher for Penicillium-infested as compared with intact fruit (Fig. 2B). Interestingly, however, there was a strongly significant interaction between guild and Penicillium-infestation (Table 1) indicating that the magnitude and/or direction of Penicillium infestation effect on fruit harvesting varied among frugivore guilds (Fig. 2B). In particular, test of slices showed no significant Penicillium infestation effect on rodent fruit harvesting (F1, 4215 = 1.10, P = 0.294), whereas seed dispersers (F1, 4215 = 38.32, P < 0.0001) and, particularly, pulp feeders (F1, 4215 = 60.54, P < 0.0001) markedly selected infested fruit (Fig. 2B). Overall, our results indicated a strong vertebrate frugivore preference for Penicillium-infested fruits. Furthermore, such fruit preference took place in frugivores as contrasting as wild boars, ring-tailed coatis, curl-crested jays and ruddy ground doves in the tropical groves, or European rabbits, field mice, blackbirds, and magpies in the Mediterranean groves.
Effect of Penicillium infestation on VOC emission and on fruit physical and chemical traits
Our VOC analyses of intact and infested oranges identified 55 and 89 different compounds in intact and Penicillium-infested fruits, respectively (the entire list can be found as Supplementary Table S4). Both types of fruits differed significantly in mean percentages of volatile compounds, both for all compound groups considered individually (i.e. GLM univariate tests) and when all volatiles were treated simultaneously in a multivariate analysis of variance (MANOVA, F5, 8 = 63.23, P < 0.0001). For instance, percentage of esters in infested fruits was 5.7-fold higher as compared with intact fruits (F1 = 169.88, P < 0.0001), whereas percentages of hydrocarbons was 1.3-fold higher in intact as compared to infested fruits (F1 = 249.6, P < 0.0001; Fig. 1). The differences between fruit types in percentages of alcohols was small but significant (F1 = 6.67, P < 0.05). In particular, the percentage of ethanol was 5.26-fold higher in infested as compared to intact fruits. The amount of unidentified volatile compounds in Penicillium-infested (7.02%) was similar to that in intact fruits (7.64%). However, when we repeated these analyses considering unidentified volatiles as a sixth compound group, both multivariate and univariate analyses yielded results very similar to those outlined above and, thus, are not detailed here.
We also found overall differences in toughness, pH, and °Brix between intact and infested fruits (F1, 36 = 131.55, P < 0.0001). Specifically, toughness of intact fruits (3.70 ± 0.12 kg) was 4.16 times higher than that of infested fruits (0.89 ± 0.06 kg; F1 = 412.90, P < 0.0001). The pH of intact fruits (2.47 ± 0.03) was slightly, but significantly, lower than for infested fruits (2.67 ± 0.04; F1 = 17.14, P < 0.001). Finally, sweetness of intact fruits (15.48 ± 0.25 °Brix) was 12% higher than that of Penicillium-infested fruits (13.81 ± 0.16 °Brix; F1 = 31.70, P < 0.0001).
Discussion
We chose a novel interaction to experimentally evaluate the effect of fruit infestation by fungi on both fruit appeal to frugivores and vertebrate fruit preferences. Interestingly, and in contrast with most previous work, frugivores consistently preferred infested over intact fruits. Furthermore, the preference for infested fruits was correlated with the higher esters and ethanol levels, but lower sugar levels, in infested as compared to intact fruits24, 30. This investigation thus challenges the widely accepted idea of vertebrate avoidance of microbe-infested fruits and reveals a probable mechanism by which microbes facilitate the naturalization of non-native plant species.
Our experimental results show clearly that all three functional groups of frugivores (seed dispersers, pulp feeders, and rodents) preferred fungi-infested over uninfested orange fruits. Such fruit preference took place in very diverse frugivores differing in many traits such as body sizes (e.g. wild boar vs. ruddy ground dove in the tropical groves) and feeding habits (e.g. rabbit vs. magpie in the Mediterranean groves). Preference for infested fruits was not significant for rodents in the tropical groves. However, our camera traps and field observations indicated that lowland pacas, a frequent rodent visitor, consumed in situ infested fruits whereas they usually carried to the jungle uninfested ones. This observation opens the possibility that some of these uninfested fruits were stored31 till they become spoiled and their physical and chemical traits altered (see below). The pattern of preference for fungi-infested fruits also held true during the three studied fruiting seasons, supporting the robustness of our findings and suggesting that similar results could be expected for comparable systems.
After Janzen11, it has been often reported that frugivores prefer ripe, uninfested fruits over spoiled ones14 (see Supplementary Table S1). Nevertheless, changes in fruit preference by frugivores can be expected to vary with vertebrate, plant, and microbe species14. Regrettably, most studies reporting preference for intact fruits have relied on feeding trials with captive small frugivorous birds and plant species with small-sized fruits (usually ≤ 1 cm in diameter; Supplementary Table S1). Conversely, apart from the current study, very little is known about the effect of microbe infection on large or medium sized fruits. Fruit size might be consequential for frugivore preferences in relation to infestation due to several causes. Large fruit size allows the possibility of fungal infection altering just the outermost fruit parts (i.e. exocarp) and not the pulp, which may be infected in small-sized fruits. In orange fruits, and probably also in other relatively large fruits, Penicillium and other microbes thrive in the exocarp, leaving most of the inner pulp much less infested (Authors personal observation). As we revealed, fungal infection softens fruit exocarps, thereby facilitating foraging by some frugivores. We propose that research bias towards small frugivores birds and towards small-sized fruit with thin exocarps have often led to the wrong perception that microbe infection generally lessens vertebrate fruit consumption19. Further research on plant-vertebrate-microbe systems is undoubtedly needed. Also, though the infested fruit we offered in our field experiments, exhibited well-developed fungal growth, the question remains as to whether, as the fruit continues to rot, it will become less attractive to frugivores.
Frugivore preference for infested orange fruits opens the non-trivial question of what traits (nutritive, chemical, and physical), caused such pattern of fruit selection24, 31–33, despite the lower sugar content of infested compared to control fruits. Many vertebrate frugivores are able to perceive and respond to some odours in the environment through odorant receptors, which are activated by sets of VOCs24, 25, 34. In particular, ethanol accumulation in fruits has been long identified as potential signal of high reward35 and, in our study, infested orange fruit showed a five-fold increase of accumulated ethanol in the endocarp. Thus, high ethanol concentration of infested orange fruits may explain, in part, frugivore preferences in our tropical and Mediterranean groves18, 36, 37.
Other candidate traits are monoterpene hydrocarbons, which were emitted predominantly by intact as compared to infested fruit (69% vs. 54%); however, these compounds were partly transformed during Penicillium infestation to alcohols and esters. Interestingly, these two chemical classes were further transformed into ethyl esters, which went from representing only 2% of total VOCs in intact fruits to ~20% in infested ones (i.e. a 10-fold increase). Animals so diverse as humans, monkeys, rats and flies are known to perceive and respond positively to esters at very low odour thresholds30, 38, 39. Our results thus suggest that esters (within a mixed VOC blend) were perceived as attractive cues by vertebrate frugivores in both tropical and Mediterranean experimental groves. Also the fungus secretes about 50 enzymes involved in plant cell wall degradation40, thus softening plant tissues, as indicated by toughness evaluations. This softening probably facilitated frugivore access to fruit pulp, chiefly in the case of small pulp-feeding birds and some rodents unable to penetrate the thick pericarp of intact orange fruits23, 41, 42. Finally, we cannot rule out the possibility of frugivore self-medication with Penicillium-produced antibiotics, as it has been documented in other similar systems43.
Our study supports that vertebrate frugivores, fleshy-fruited plants, and microbes may form a mutualistic ecological triad44. In orange fruits, monoterpene hydrocarbons are accumulated at very high levels in oil glands of the exocarp, and these compounds are known to favour fruit colonization by Penicillium 27, 45. Spoiled fruit and fungus emit VOCs (e.g. esters) that attract frugivore vertebrates which prefer feeding on soft, alcohol- and sugar-rich fruits. Vertebrates frequently disperse viable orange seeds (e.g. wild boars; Authors unpublished data) facilitating tree naturalization in tropical habitats28 (Authors personal observation). Furthermore, recent progress in fungal dispersal has revealed that passage through vertebrate guts can provide a mechanism of dispersal for fungi as well as seeds46, 47. Therefore, Penicillium and other microbes infecting oranges and other large fruits are likely to benefit from spore dispersal by frugivores. New investigations disentangling the direct and indirect effects likely taking place in these complex multitrophic systems are crucial23.
On the other hand, because fungal infection hastens fruit abscission, it has been generally assumed that it has a negative effect on seed dispersal (i.e. fallen fruits are equated to undispersed ones11, 14). This is probably the case for most bird-dispersed plants, since birds tend to forage on the canopy and not underneath fruiting trees. Conversely, tree species dispersed to some significant extent by non-arboreal mammals, such as lagomorphs, pigs, other ungulates, and carnivores require fruit dropping to archive seed dispersal, and thus fungal infection also enhances in this way tree dispersal success. Such a cooperative relationships in these cross kingdom interactions is opposed to the more generalized view of the competitive nature of interactions between frugivorous vertebrates and microorganisms14, 15, 48 which certainly invites to study in detail other similar systems involving large-fruited plants and contrasting frugivores.
To conclude, we show that frugivores consistently preferred infested over intact orange fruits and that such pattern concords with a high level of attractive volatile compounds in infested fruits. We predict that plant species with large fruits and seeds and dispersed mostly by mammals with acute sense of smell are the most likely to experience enhanced consumption of infested fruits, aided by microbe-induced conspicuous aromas and softened peels. Though some of our target species lack a common evolutionary history, this investigation illustrates a way by which microbes can maintain mutualistic interactions with fleshy-fruited plants and, thus, questioning whether there really is a plant evolutionary dilemma of making their fruits attractive to some frugivores while unattractive to microbes13, 15, 33.
Material and Methods
Study species
The genus Citrus (Rutaceae) comprises several species whose origin is Asian. The orange tree (Citrus sinensis L. Osb.) is an evergreen, flowering tree, with an average height of 4 to 8 m. The fruit is a special type of berry named hesperidium, consisting of fleshy parts divided by segments, the whole being surrounded by a separable skin. This is composed of two major regions: the pericarp, commonly known as the peel, and the endocarp, often called the pulp. The pericarp is composed of external coloured peel known as flavedo (exocarp; rich in oil sacs containing volatile compounds), and the internal usually white and spongy layer known as albedo (mesocarp). The inner flesh or pulp consists of segments surrounding the central axis of the fruit enclosed in a locular membrane in which seeds and juice sacs (vesicles formed by highly vacuolated cells containing juice) grow. The acidity of the juice of citrus fruits is largely due to high contents of citric acid, malic acid and fumaric acid, in order of abundance. Penicillium digitatum Sacc. causes the most damaging postharvest disease of sweet orange fruits27. Dormant Penicillium spores present on the fruit’s surface germinate rapidly and colonize injured peel tissue before and during harvesting and processing.
Study sites
The study was conducted from June 2013 to May 2015 in two very distinct geographical regions, a Mediterranean in eastern Spain and a Tropical in southern Brazil. In both regions we used several experimental groves where frugivore activity was known. In Spain, we selected two Mediterranean sites within the Valencia province in the municipalities of Moncada and Sagunto. In Moncada, we used a 0.6 ha experimental field within the Instituto Valenciano de Investigaciones Agrarias (IVIA; latitude 39°35′N, longitude 0°23′W; 50 m a.s.l.). The Sagunto field site is located near the Sierra Calderona Natural Park (latitude 39°42′N, longitude 0°15′W; 30 m a.s.l), within extensive orange monocultures. Within this site, we used two different orange groves (0.2 and 0.4 ha, respectively) 250 meters apart. The most common frugivore species in these two Mediterranean sites were rabbits (Oryctolagus cuniculus L.) and small birds that acted as pulp feeders (i.e. they consume the fruit pulp but without ingesting the seeds23). Granivore rodents (e.g. Mus spretus L.) and rats (Rattus rattus L.) were also relatively frequent visitors. The tropical field site (called Cambuhy; latitude 21°38′S, longitude 48°31′W, 600 m above sea level; 14,083 ha) is located next to a dry tropical forest in Matâo, Sâo Paulo, southern Brazil. Inside the farm there is a large (2,168 ha) semi-deciduous forest of dry Mata Atlantica called Mata da Virgínia. We selected two orange groves (8.1 and 11.5 ha, respectively) about 1.3 km apart of each other. The most frequent frugivore visitors were introduced wild boars (Sus scrofa L.) which together with the scarce ring-tailed coati (Nasua nasua L.) ingested whole fruits and delivered intact viable seeds (Authors unpublished data). Several species of small rodents were also frequent visitors and together with lowland pacas (Cuniculus paca Brisson) comprised the guild seed-eating rodents. Pulp feeding bird species such as curl-crested jay (Cyanocorax cristatellus Temminck), pale-breasted thrush (Turdus leucomelas Vieillot), and ruddy ground dove (Columbina talpacoti Temminck) frequently visited and picked the pulp of our experimental fruits. Further detail on the study sites and their frugivore assemblages are documented in Supplementary methods S3 (Supporting Information). All field experiments were done under permission of IVIA, Fundecitrus, and all orange grove owners both in the tropical and the Mediterranean sites.
Field experimental design
To evaluate vertebrate frugivore preference in the field, intact and Penicillium-infested fruit types were simultaneously offered underneath orange trees simulating natural fruit drop on circular sand beds (1-meter of diameter23). All frugivores (e.g. mammals, birds) had access to fruit since no exclusion system was implemented23. Intact and infested fruits (2 or 3 per type) were alternately arranged, with ~10 cm spacing, beneath each experimental tree. In the experiments of Sagunto and Moncada, 15 fruit depots haphazardly distributed in the field during the months of April to June 2014 and June to December 2013, respectively, coinciding with the ripening seasons. In Brazil, fruits were offered in two parallel rows (10 fruit depots each). Within each row, fruit depots were 60 m apart. Fruit harvesting was recorded during seven and twelve consecutive days in July 2014 and May 2015, respectively. Every one or two days early in the morning the numbers of fruits either consumed in situ or removed were recorded and replaced by new fruits of the corresponding treatment. Frugivore identification was based on frugivore tracks on fine sand23, 49, 50 and on the way fruits were manipulated and eaten by different frugivores. To confirm the origin of some animal traces and signs, some Bushnell Trophy Cameras with motion sensors were used in Brazil fields. The timing of our experiments was intended to coincide with the ripening of offered fruits.
To inoculate fruit with P. digitatum, we used fruits not sprayed with any insecticide and fungicide during at least the previous three months. Fully developed orange fruits were taken to the lab and then their surfaces were disinfested with 1-min immersion in a sodium hypochlorite solution (4 gL−1), rinsed with fresh water and left to air dry at room temperature. P. digitatum isolated NAV-7 was obtained from the culture collection of the Laboratory of Pathology, Postharvest Technology Center (IVIA, Moncada, Spain). Oranges were inoculated by wounding the rind with a stainless steel tip and introducing 2 µL of a known concentration of 1 × 106 P. digitatum NAV-7 spores mL−1 in two opposite incisions in the equator of the fruit45. Inoculated fruit were placed on closed plastic bags and incubated at 20 °C and 80% relative humidity (RH). Infested fruits were use in the field experiment 7–11 days after inoculation, when the halo of the fungus covered the entire fruit surface (see Fig. 1). A second set of fully developed oranges were used as controls and were treated as infected fruits except that were no wounded and inoculated with P. digitatum. We evaluated the possibility that the tiny wounds induce changes on VOC profiles and thus were partly responsible of possible differences between control and Penicillium-infested fruits. However, our results unmistakably showed no differences between wounded and unwounded (control) orange fruits for all volatile classes (see Supplementary Figure S2).
Fruit volatile emissions, physical and chemical traits
To assess how VOC profiles relate to frugivore fruit preferences, chemical analysis of volatiles emitted from intact and Penicillium-infested fruits were performed. For Penicillium-infested fruits, volatile analyses were conducted in days 7, 8, 9, 10, and 11 after infection, with two fruits per day. The volatile compounds were extracted by headspace solid-phase microextraction (HS-SPME) and analysed by GC-MS essentially as described in Rodríguez et al.45. Briefly, samples were introduced into glass beakers of 1 L volume (Labbox Labware) closed with foil. 10 μg of 2-octanol (Aldrich, purity ≥99.5%) was added as internal standard. After 1 h of equilibration at room temperature, a 100 μm fiber coated with polydimethylsiloxane (PDMS, Supelco, USA), previously conditioned in the GC injector as indicated by the manufacturer, and was inserted into the glass and exposed for 40 min. The adsorbed volatiles were injected to a gas chromatograph-mass spectrometer (GC-MS) by desorption at 250 °C during 1 min in splitless mode in the injection port of a 6890 N gas chromatograph (Agilent Technologies). Volatile compounds were separated on an Agilent J&W DB-5 ms GC Column (60 m × 0.25 mm × 1.00 μm) coupled to a Termo-DSQ mass spectrometer. The GC interface and MS source temperatures were 260 °C and 230 °C, respectively. Oven programming conditions were 40 °C for 2 min, 5 °C/min ramp until 250 °C, and a final hold at 250 °C for 5 min. Helium was the carrier gas at 1.5 mL/min in the splitless mode. Data was recorded in a 5975B mass spectrometer (Agilent Technologies) in the 35–250 m/z range at 7 scans, with electronic impact ionization at 70 eV. Chromatograms were processed by means of the Enhanced ChemStation E.02.02 software (Agilent Technologies). Compounds in HS-SPME extractions were identified by matching the acquired mass spectra with those stored in the reference library (National Institute of Standards and Technology) and/or by comparison with authentic standard compounds when available. The relative emission rate of every compound in each sample was calculated as its corrected peak area (by fruit weight) divided by the recovery rate of the internal standard. The results are reported as the mean values of peak area percent ± SE. Infested fruits showed comparable VOC profiles independently of the day of sampling after Penicillium infection and thus data were pooled. All identified volatile compounds were grouped into five main types (ethers, alcohols, ketones, hydrocarbons, and esters). The results in Fig. 3 are reported as the mean values of peak area percent. For individual volatiles, the results (Supplementary Table S4) are reported as the mean values of peak area ± standard error and the correspondent peak area percent. GC-MS was performed at the Metabolomics Service in Instituto de Biología Molecular y Celular de Plantas, Consejo Superior de Investigaciones Científicas-Universidad Politécnica de Valencia (Spain).
Figure 3.
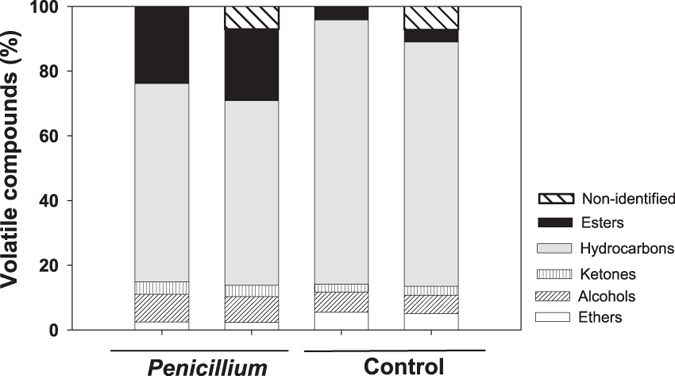
GCMS analysis of the volatile compounds emission in control and Penicillium-infested sweet orange (Citrus sinensis) fruits. Constituents are classified by chemical class: alcohols, esters, hydrocarbons, ketones, aldehydes, ethers and epoxides. For each treatment (control and Penicillium-infested) percentages are shown first without considering non-identified compounds and, then, considering them as an additional class.
In addition, 20 intact and 20 Penicillium-infested orange fruits harvested from 10 trees (2 fruit per tree) were chosen to perform sugar/acid analyses of the juice of each fruit, according to Citrus Handbook (1998). Acidity was measured using a pH-meter (CRISON Basic 207) and sugar content using a refractometer (HANNA HI 96811, expressed in °BRIX ± standard error). Fruit resistance to pressure was measured using a hand-held penetrometer (Penetrometer Fruit Pressure Tester FT011) and expressed in kg as the mean of the peak force at rupture ± standard error following Shmulevich et al. (2003). Two measurements were performed on each fruit with the penetrometer.
Statistical analyses
The results were analysed by fitting generalized linear mixed models using the Proc Glimmix from SAS51, which allows the modelling of non-normal response variables as well as the usage of both fixed and random factors52. We first evaluated overall frugivore preference for infested vs. intact fruits. In this model, the percentage of fruit harvesting was the response variable, whereas fruit infestation and region (tropical, Mediterranean) were specified as fixed factors. Then, we evaluated guild-specific fruit preferences by fitting a second model with frugivore guild (seed dispersers, seed predators, and pulp consumers) and fruit infestation (infested vs. intact fruits) as fixed factors. To evaluate the consistence of the effect of one factor at the different levels of other factors, in both models we also included second-order interactions among main effects. When the interaction between any two factors was significant, tests for the effect of a given factor at the different levels of the other factor (i.e. tests of slices) were performed using the SLICE option in the LSMEANS statement of the MIXED procedure51. Season (2013, 2014, and 2015) and block (nested within parcel) were included as random factors in both models. To compare the effects of different levels of any significant main factor, we calculated the difference between their least-square means. Because of the binomial nature of the response variables (percentage of fruit harvested), binomial error and logit link function were specified.
Identified VOCs emitted by orange fruits were classified into one of five main groups (ethers, alcohols, ketones, monoterpenes hydrocarbons, and esters). Multivariate analysis of variance (MANOVA) in GLM procedure51, 52 were done to test overall differences in the percentages of the five main types of VOCs between Penicillium-infested and intact orange fruits. Once overall significant differences were detected, we applied univariate analyses for each group of VOCs. Differences in toughness, pH, and Brix degrees were also tested with multivariate and univariate analyses.
Electronic supplementary material
Acknowledgements
JMF was funded by a Marie Curie Intraeuropean fellowship (FP7-PEOPLE-2011-IEF-298137) and a Portuguese FCT grant (IF/00728/2013). We sincerely thank Sharon Strauss for wise comments and improvements of clarity and readability on a previous draft. We thank A. Tachibana for allowing us to work at Fazenda Cambuhy Agricola and to his staff for helping to perform our field experiments in Brazil. We thank Fundecitrus for funding and technical support.
Author Contributions
L.P. and J.M.F. conceived the study, J.E.P. performed the field experiments, J.E.P. and A.R. performed biochemical analyses, J.M.F. analysed the data, L.P. J.M.F., J.E.P. wrote the first draft, and all authors contributed substantially to reviewing the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-05643-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Leandro Peña, Email: lpenya@fundecitrus.com.br.
José María Fedriani, Email: fedriani@isa.ulisboa.pt.
References
- 1.Parker IM, Gilbert GS. The evolutionary ecology of novel plant-pathogen interactions. Annu. Rev. Ecol. Syst. 2004;35:675–700. doi: 10.1146/annurev.ecolsys.34.011802.132339. [DOI] [Google Scholar]
- 2.Traveset A, Richardson DM. Mutualistic interactions and biological invasions. Annu. Rev. Ecol. Syst. 2014;45:89–113. doi: 10.1146/annurev-ecolsys-120213-091857. [DOI] [Google Scholar]
- 3.Ellstrand, N. C. Dangerous liaisons? When cultivated plants mate with their wild relatives. JHU Press (2003).
- 4.Schroth, G. et al. (Ed.). Agroforestry and biodiversity conservation in tropical landscapes. Island Press (2004).
- 5.Bhagwat SA, Willis KJ, Birks HJB, Whittaker RJ. Agroforestry: a refuge for tropical biodiversity? Trends Ecol. Evol. 2008;23:261–267. doi: 10.1016/j.tree.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Desprez-Loustau ML, et al. The fungal dimension of biological invasions. Trends Ecol. Evol. 2007;22:472–480. doi: 10.1016/j.tree.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Fedriani JM, Fuller TK, Sauvajot RM. Does availability of anthropogenic food enhance densities of omnivorous mammals? An example with coyotes in southern California. Ecography. 2001;24:325–331. doi: 10.1111/j.1600-0587.2001.tb00205.x. [DOI] [Google Scholar]
- 8.Rey PJ. Preserving frugivorous birds in agro‐ecosystems: lessons from Spanish olive orchards. J. Appl. Ecol. 2011;48:228–237. doi: 10.1111/j.1365-2664.2010.01902.x. [DOI] [Google Scholar]
- 9.Peris JE, Fedriani JM, Peña L. Los mamíferos frugívoros prefieren frutos de cítricos infectados por Penicillium: ¿se equivocaba Janzen? Ecosistemas. 2015;24:5–13. doi: 10.7818/ECOS.2015.24-3.02. [DOI] [Google Scholar]
- 10.Richardson DM, et al. Naturalization and invasion of alien plants: concepts and definitions. Div. and Distrib. 2000;6:93–107. doi: 10.1046/j.1472-4642.2000.00083.x. [DOI] [Google Scholar]
- 11.Janzen DH. Why fruits rot, seeds mold, and meat spoils. Am. Nat. 1977;111:691–713. doi: 10.1086/283200. [DOI] [Google Scholar]
- 12.Borowicz VA. Do vertebrates reject decaying fruit? An experimental test with Cornus amomum fruits. Oikos. 1998;53:74–78. doi: 10.2307/3565665. [DOI] [Google Scholar]
- 13.Buchholz R, Levey DJ. The evolutionary triad of microbes, fruits, and seed dispersers: an experiment in fruit choice by cedar waxwings. Bombycilla cedrorum. Oikos. 1990;59:200–204. doi: 10.2307/3545535. [DOI] [Google Scholar]
- 14.Cipollini ML, Stiles EW. Fungi as biotic defense agents of fleshy fruits: Alternative hypotheses, predictions, and evidence. Am. Nat. 1993;141:663–673. doi: 10.1086/285498. [DOI] [PubMed] [Google Scholar]
- 15.Ruxton GD, Wilkinson DM, Schaefer HM, Sherratt TN. Why fruit rots: theoretical support for Janzen’s theory of microbe –macrobe competition. Proc. R. Soc. B. 2014;281:20133320. doi: 10.1098/rspb.2013.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherratt TN, Wilkinson DM, Bain RS. Why fruits rot, seeds mold and meat spoils: a reappraisal. Ecol. Mod. 2006;192:618–626. doi: 10.1016/j.ecolmodel.2005.07.030. [DOI] [Google Scholar]
- 17.Dominy NJ, Lucas PW. Significance of color, calories, and climate to the visual ecology of catarrhines. Am. J. Prim. 2004;62:189–207. doi: 10.1002/ajp.20015. [DOI] [PubMed] [Google Scholar]
- 18.Milton K. Ferment in the family tree: does a frugivorous dietary heritage influence contemporary patterns of human ethanol use? Integr. Comp. Biol. 2004;44:304–314. doi: 10.1093/icb/44.4.304. [DOI] [PubMed] [Google Scholar]
- 19.Asplund, J., Gauslaa, Y. & Merinero, S. The role of fungal parasites in tri‐trophic interactions involving lichens and lichen‐feeding snails. New Phytol., doi:10.1111/nph.13975 (2016). [DOI] [PubMed]
- 20.Carrigan MA, et al. Hominids adapted to metabolize ethanol long before human-directed fermentation. Proc. Nat. Ac. Sci.USA. 2015;112:458–463. doi: 10.1073/pnas.1404167111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perea R, Delibes M, Polko M, Suárez A, Fedriani JM. Context-dependent fruit-frugivore interactions: partner identities and spatio-temporal variations. Oikos. 2013;122:943–951. doi: 10.1111/j.1600-0706.2012.20940.x. [DOI] [Google Scholar]
- 22.Fedriani JM, Delibes M. Dangerous liaisons disperser the Mediterranean dwarf palm: defensive flesh-pulp role against seed predators. Ecology. 2011;92:304–312. doi: 10.1890/09-2194.1. [DOI] [PubMed] [Google Scholar]
- 23.Fedriani JM, Delibes M. Pulp feeders alter plant interactions with subsequent animal associates. J. Ecol. 2013;101:1581–1588. doi: 10.1111/1365-2745.12146. [DOI] [Google Scholar]
- 24.Lomáscolo SB, Levey DG, Kimball RT, Bolker BM, Alborn HT. Dispersers shape fruit diversity in Ficus (Moraceae) Proc. Nat. Ac. Sci.USA. 2010;107:14668–14672. doi: 10.1073/pnas.1008773107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valenta, K. et al. Colour and odour drive fruit selection and seed dispersal by mouse lemurs. Sci. Rep. 3, doi:10.1038/srep02424 (2013). [DOI] [PMC free article] [PubMed]
- 26.Vikram A, Prithiviraj B, Kushalappa AC. Use of volatile metabolite profiles to discriminate fungal diseases of Cortland and empires apples. J. Plant Path. 2004;86:215–225. [Google Scholar]
- 27.Droby S, et al. Role of citrus volatiles in host recognition, germination and growth of Penicillium digitatum and Penicillium italicum. Postharvest Biol. Tec. 2008;49:386–396. doi: 10.1016/j.postharvbio.2008.01.016. [DOI] [Google Scholar]
- 28.Stampella PC, Delucchi G, Keller HA, Hurrell JA. Etnobotánica de Citrus reticulate (Rutaceae, Aurantioideae) naturalizada en la Argentina. Bonplandia. 2014;23:151–162. [Google Scholar]
- 29.Fedriani JM, Zywiec M, Delibes M. Thieves or mutualists? Pulp feeders enhance local endozoochore recruitment. Ecology. 2012;93:575–587. doi: 10.1890/11-0429.1. [DOI] [PubMed] [Google Scholar]
- 30.Laska M, Seibt A. Olfactory sensitivity for aliphatic esters in squirrel monkeys and pigtail macaques. Behav. Brain Res. 2002;134:165–174. doi: 10.1016/S0166-4328(01)00464-8. [DOI] [PubMed] [Google Scholar]
- 31.Fedriani JM, Boulay R. Foraging by fearful frugivores: combined effect of fruit ripening and predation risk. Func. Ecol. 2006;20:1070–1079. doi: 10.1111/j.1365-2435.2006.01199.x. [DOI] [Google Scholar]
- 32.Rodríguez A, Alquézar B, Peña L. Fruit aromas in mature fleshy fruits as signals of readiness for predation and seed dispersal. New Phytol. 2013;197:36–48. doi: 10.1111/j.1469-8137.2012.04382.x. [DOI] [PubMed] [Google Scholar]
- 33.Cipollini ML, Levey DJ. Secondary metabolites of fleshy vertebrate dispersed fruits: adaptive hypotheses and implications for seed dispersal. Am. Nat. 1997;150:346–372. doi: 10.1086/286069. [DOI] [PubMed] [Google Scholar]
- 34.Borges RM, Ranganathan Y, Krishnan A, Ghara M, Pramanik G. When should fig fruit produce volatiles? Pattern in a ripening process. Acta Oecol. 2011;37:611–618. doi: 10.1016/j.actao.2011.06.003. [DOI] [Google Scholar]
- 35.Schaefer, H. M. & Ruxton, G. D. Plant-animal communication. OUP Oxford (2011).
- 36.Dudley R. Evolutionary origins of human alcoholism in primate frugivory. Quart. Rev. Biol. 2000;75:3–15. doi: 10.1086/393255. [DOI] [PubMed] [Google Scholar]
- 37.Siegel, R. K. Intoxication: The universal drive for mind-altering substances. Inner Traditions/Bear & Co (2005).
- 38.Keller A, Vosshall LB. Influence of odorant receptor repertoire on odor perception in humans and fruit flies. P. Natl. Acad. Sci. USA. 2007;104:5614–5619. doi: 10.1073/pnas.0605321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomez-Marin A, Stephens GJ, Louis M. Active sampling and decision making in Drosophila chemotaxis. Nature comm. 2011;2:441. doi: 10.1038/ncomms1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marcet-Houben M, et al. Genome sequence of the necrotrophic fungus Penicillium digitatum, the main postharvest pathogen of citrus. BMC Genomics. 2012;13:646. doi: 10.1186/1471-2164-13-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balcomb SR, Chapman CA. Bridging the gap: influence of seed deposition on seedling recruitment in a primate-tree interaction. Ecol. Mon. 2003;73:625–642. doi: 10.1890/02-4036. [DOI] [Google Scholar]
- 42.Grant, P. R. & Grant, B. R. 40 years of evolution: Darwin’s finches on Daphne Major island. Princeton University Press (2014).
- 43.Baker M. Fur Rubbing: Use of Medicinal Plants by Capuchin Monkeys (Cebus capucinus) Am. J. Primatol. 1996;38:263–270. doi: 10.1002/(SICI)1098-2345(1996)38:3<263::AID-AJP5>3.0.CO;2-X. [DOI] [Google Scholar]
- 44.Herrera CM. Avian interference of insect frugivory: an exploration into the plant-bird-fruit pest evolutionary triad. Oikos. 1984;42:203–210. doi: 10.2307/3544794. [DOI] [Google Scholar]
- 45.Rodríguez A, et al. Terpene down-regulation in orange reveals the role of fruit aromas in mediating interactions with insect herbivores and pathogens. Plant Physiol. 2011;156:793–802. doi: 10.1104/pp.111.176545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tesson SV, et al. Integrating microorganism and macroorganism dispersal: modes, techniques and challenges with particular focus on co-dispersal. Ecoscience. 2016;22:1–16. [Google Scholar]
- 47.Andras JP, Ebert D. A novel approach to parasite population genetics: experimental infection reveals geographic differentiation, recombination and host-mediated population structure in Pasteuria ramosa, a bacterial parasite of Daphnia. Mol Ecol. 2013;22:972–986. doi: 10.1111/mec.12159. [DOI] [PubMed] [Google Scholar]
- 48.Tewksbury JJ. Fruits, frugivores and the evolutionary arms race. New Phytol. 2002;156:137–139. doi: 10.1046/j.1469-8137.2002.00522.x. [DOI] [PubMed] [Google Scholar]
- 49.Fedriani JM, Delibes M. Seed dispersal in the Iberian pear Pyrus bourgaeana: a role for infrequent mutualists. Ecoscience. 2009;16:311–321. doi: 10.2980/16-3-3253. [DOI] [Google Scholar]
- 50.Balcomb SR, Chapman CA. Bridging the gap: influence of seed deposition on seedling recruitment in a primate–tree interaction. Ecol. Monog. 2003;73:625–642. doi: 10.1890/02-4036. [DOI] [Google Scholar]
- 51.SAS Institute. Base SAS 9.4. Procedures Guide: Statistical Procedures. SAS Institute (2014).
- 52.Bolker BM, et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecol. & Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


