Abstract
Purpose
The purpose of this study was to compare leukemia-free survival (LFS) and other clinical outcomes in patients with acute myelogenous leukemia who underwent a myeloablative allogeneic stem cell transplant with and without total body irradiation (TBI).
Methods and materials
Adult patients with acute myelogenous leukemia undergoing myeloablative allogeneic stem cell transplant at Duke University Medical Center between 1995 and 2012 were included. The primary endpoint was LFS. Secondary outcomes included overall survival (OS), nonrelapse mortality, and the risk of pulmonary toxicity. Kaplan-Meier survival estimates and Cox proportional hazards multivariate analyses were performed.
Results
A total of 206 patients were evaluated: 90 received TBI-based conditioning regimens and 116 received chemotherapy alone. Median follow-up was 36 months. For all patients, 2-year LFS and OS were 36% (95% confidence interval [CI], 29-43) and 39% (95% CI, 32-46), respectively. After adjusting for known prognostic factors using a multivariate analysis, TBI was associated with improved LFS (hazard ratio: 0.63; 95% CI: 0.44-0.91) and OS (hazard ratio: 0.63; 95% CI, 0.43-0.91). There was no difference in nonrelapse mortality between cohorts, but pulmonary toxicity was significantly more common with TBI (2-year incidence 42% vs 12%, P < .001). High-grade pulmonary toxicity predominated with both conditioning strategies (70% and 93% of cases were grade 3-5 with TBI and chemotherapy alone, respectively).
Conclusions
TBI-based regimens were associated with superior LFS and OS but at the cost of increased pulmonary toxicity.
Introduction
Nearly 4 decades ago, 2 groups independently reported improved overall survival for patients with acute myelogenous leukemia (AML) who underwent allogeneic stem cell transplantation (allo-SCT) in first complete remission after being conditioned with cyclophosphamide and total body irradiation (TBI).1, 2 Myeloablative allo-SCT continues to play a fundamental role in the management of AML. Allo-SCT is generally recommended for patients with relapsed disease and in appropriate patients in first complete remission with intermediate- and high-risk disease based on cytogenetics and/or molecular abnormalities.3
The optimal conditioning regimen preparatory to allo-SCT is a subject of continued controversy. Whether TBI is a critical component of the conditioning regimen, or if chemotherapy-alone regimens are sufficient, is a matter of ongoing debate. This stems from conflicting results between older randomized studies4, 5, 6, 7, 8, 9, 10, 11 and more recent retrospective analyses.12, 13, 14, 15, 16 Improvements in chemotherapy delivery, specifically the introduction of intravenous busulfan,12 and technical challenges incorporating TBI into a conditioning regimen, have generally led to decreased utilization of TBI.
Further studies comparing these 2 approaches are needed; therefore, we sought to review our institutional experience in which a consistent TBI-based approach has been used for many years. We also sought to examine known prognostic factors to appropriately compare these 2 conditioning strategies.
Methods
Patients
This Institutional Review Board–approved, retrospective analysis evaluated all adult patients (≥18 years of age) with AML undergoing allo-SCT at Duke University Medical Center between 1995 and 2012. Only patients undergoing a myeloablative conditioning regimen were included. Patient- and treatment-related characteristics, including the specific conditioning regimen, were recorded. The choice of conditioning regimen was made at the discretion of the treating physicians. Refractory disease was defined as >5% blasts by morphology immediately before transplant.
Potential prognostic factors were identified through a literature search. These included age at diagnosis,9, 14, 17, 18 sex, pretransplant performance status, acute graft versus host disease (GVHD),8, 9, 14, 17 year of transplant,14 disease status before transplant,8, 14 and National Comprehensive Cancer Network (NCCN) disease risk category.18, 19 These factors were collected for each patient where available. Two factors of potential prognostic significance, including donor age9 and donor sex,14 were not available for a sufficient number of patients for formal analyses.
A uniform TBI technique was used with patients treated to 13.5 Gy in 1.5 Gy twice-daily fractions using a dose-rate of 15 to 20 cGy/minute. Patients were positioned supine and treated with lateral fields using 4- to 6-MV photons. The lungs were attenuated in all patients to a dose of 8 to 10 Gy using the arms and brass compensators. The degree of lung attenuation was determined individually for each patient based primarily on pretransplant pulmonary function tests and the presence of prior pulmonary disease.
All patients received care in a high-efficiency particulate air-filtered room. Tunneled central venous catheters were placed before beginning the conditioning regimen. Antibiotic prophylaxis was used per standard prophylaxis guidelines and included ciprofloxacin 750 mg by mouth twice daily and metronidazole 500 mg by mouth three times daily. Antiviral therapy included acyclovir 400 mg by mouth twice daily. From 1995 through February 2009, our prescribed fungal prophylaxis was fluconazole 400 mg by mouth daily. After February 2009, prophylaxis was changed to voriconazole 200 mg by mouth twice daily. With the first neutropenic fever, all patients were started on intravenous antibiotic coverage with vancomycin and ceftazidime. Sinusoid obstructive syndrome prophylaxis was prescribed for all patients. From 1995 through June 2009, this was accomplished with low-dose continuous heparin infusion until time of engraftment. After June 2009, patients received ursodeoxycholic acid orally until day 90 following transplant.
T-cell depletion in select patients was accomplished with antithymocyte globulin, monoclonal anti-CD52 antibody-mediated depletion, or CD34 selection. Successful engraftment was defined as platelets >100 × 109/L, neutrophils >1 × 109/L, and transfusion independence.
Statistical methods
Patient characteristics were summarized with median (interquartile range) values for continuous variables and N (%) for categorical variables, for all patients and also by treatment group. The predefined primary outcome of interest was leukemia-free survival, defined as date of transplant to date of relapse or death from any cause, with patients censored at date of last follow-up. Secondary endpoints included overall survival, nonrelapse mortality, and the risk of pulmonary toxicity.20, 21 Overall survival was defined as date of transplant to death from any cause, again with patients censored at last follow-up. Nonrelapse mortality was defined as date of transplant to death not from relapse, with relapse as the competing risk. The cumulative risk of pulmonary toxicity was defined as time from date of transplant to date of any grade pulmonary toxicity, with death as the competing risk. Pulmonary toxicity was scored using the Common Terminology Criteria for Adverse Events, version 4.0.
The Kaplan-Meier method was used to estimate leukemia-free survival and overall survival, for all patients and by treatment group. Six-, 12-, 18-, and 24-month rates were estimated with 95% confidence intervals (CIs) for both endpoints. The log-rank test was used to compare outcomes between treatment groups.
A Cox proportional hazards model was used to estimate hazard ratios (HR) by treatment group after adjustment for known and available covariates. Only covariates that were significant with a P value of ≤.2 on univariate analysis were included in the multivariate model. Factors not significant (P > .2) on univariate analysis, and not included in the multivariate model, were age, sex, pretransplant FEV1/DLCO, history of tobacco abuse, T-cell depletion, preexisting myelodysplastic or myeloproliferative conditions, cytomegalovirus reactivation, stem cell source (cord blood vs peripheral blood), and pretransplant pulmonary disease. There were 24 patients with missing data for NCCN risk group. NCCN risk group was associated with overall survival and leukemia-free survival on univariate modeling (P < .2) but not multivariate modeling, with the analysis restricted to 182 patients with complete information; therefore, this factor was not included in the final analysis. One further patient was excluded from the covariate analysis because of missing values.
HRs with 95% CIs were estimated for nonrelapse mortality and the cumulative risk of pulmonary toxicity with a proportional hazards model that accounted for competing risks using the methods defined by Fine and Gray.22 Cumulative incidence functions were compared between treatment groups, both univariately and after adjustment for known covariates. All statistical analyses were conducting using SAS, version 9.4 (SAS Institute, Cary, NC).
Results
Between 1995 and 2012, 206 patients underwent a myeloablative allo-SCT for AML at our institution, 90 conditioned with a TBI-based regimen and 116 with chemotherapy alone. Median follow-up was 36 months (44 months for the TBI cohort; 26 months for the chemotherapy alone cohort). Patient characteristics are summarized in Table 1. The most notable difference between the 2 groups was disease status before transplant. Patients in first complete remission were more likely to have received chemotherapy alone regimens and patients in second or third complete remission were more likely to receive TBI-based regimens. Patients with refractory disease who had not achieved complete remission before initiating the conditioning regimen were more likely to receive chemotherapy alone. Disease status at the time of allo-SCT was first complete remission in 34%, second complete remission in 39%, with 27% having refractory disease.
Table 1.
Patient characteristics
| Characteristic | All patients (n = 206) | Treatment group: n (%) |
P valuea | |
|---|---|---|---|---|
| TBI + chemotherapy (n = 90) | Chemotherapy alone (n = 116) | |||
| Age: median (IQR) | 43 (33-50) | 41 (31-49) | 44 (36-50) | .06 |
| Sex | ||||
| Female | 94 (46) | 38 (42) | 56 (48) | .39 |
| Male | 112 (54) | 52 (58) | 60 (52) | |
| Pretransplant disease status | .007 | |||
| CR1 | 70 (34) | 24 (27) | 46 (40) | |
| CR2-3 | 80 (39) | 46 (51) | 34 (29) | |
| Refractory | 55 (27) | 20 (22) | 35 (30) | |
| Unavailable | 1 (1) | 0 (0) | 1 (1) | |
| NCCN risk group | .40 | |||
| Low | 18 (9) | 8 (9) | 10 (9) | |
| Intermediate | 125 (61) | 59 (66) | 66 (57) | |
| High | 40 (19) | 14 (16) | 26 (22) | |
| Unavailable | 23 (11) | 9 (10) | 14 (12) | |
| Preexisting MDS/MPD | .62 | |||
| Yes | 31 (15) | 12 (13) | 19 (16) | |
| No | 175 (85) | 78 (87) | 97 (84) | |
| Baseline pulmonary disease | .54 | |||
| Yes | 46 (22) | 22 (24) | 24 (21) | |
| No | 160 (78) | 68 (76) | 92 (79) | |
| KPS: median (IQR) | 90 (80-90) | 90 (80-90) | 90 (80-90) | .53 |
| CMV baseline (patient) | .80 | |||
| Positive | 116 (56) | 50 (56) | 66 (57) | |
| Negative | 68 (33) | 28 (31) | 40 (34) | |
| Unavailable | 22 (11) | 12 (13) | 10 (9) | |
CMV, cytomegalovirus; CR, complete response; IQR, interquartile range; KPS, Karnofsky performance status; MDS, myelodysplastic syndrome; MPD, myeloproliferative disorder; NCCN, National Comprehensive Cancer Network; TBI, total body irradiation.
Wilcoxon rank sum test for continuous variables and χ2 test for categorical variables.
Preexisting pulmonary disease was present in 49 patients, including cryptogenic organizing pneumonia (n = 1), bacterial pneumonia (n = 23), aspergilloma (n = 5), asthma (n = 12), acute respiratory distress syndrome (n = 1), drug-induced pulmonary toxicity (n = 1), diffuse alveolar hemorrhage (n = 1), chronic obstructive pulmonary disease (n = 1), and unknown/other (n = 4).
Treatment programs
Many patients underwent induction chemotherapy at outside institutions before being referred to Duke University Medical Center for consideration of transplant. Several different induction regimens were used, most commonly “7+3” with cytarabine plus idarubicin or another anthracycline. The median number of induction and consolidation cycles was both 2 and similar between the 2 groups.
All patients conditioned with chemotherapy alone received busulfan as part of the preparative regimen, the majority with a busulfan/cyclophosphamide regimen (70%). The proportion receiving intravenous versus oral busulfan was 56% and 44%, respectively. There was more variation in chemotherapy agents combined with TBI but the most common combination was TBI/cyclophosphamide (36%). Other agents used with TBI included melphalan, etoposide, and fludarabine. There was a greater propensity of cord blood (53% vs 13%, P < .001) and matched unrelated donor transplants (66% vs 47%, P = .004) in the TBI cohort. The overall engraftment rate was 87%. The time to engraftment was the same between treatment groups. Treatment characteristics can be found in Table 2.
Table 2.
Treatment characteristics
| Characteristic | All patients (n = 206) | Treatment group: n (%) |
P valuea | |
|---|---|---|---|---|
| TBI + chemotherapy (n = 90) | Chemotherapy alone (n = 116) | |||
| TBI-based regimen | ||||
| Cyclophosphamide | 32 (16) | 32 (36) | ||
| Melphalan | 13 (6) | 13 (14) | ||
| Etoposide | 2 (1) | 2 (2) | ||
| Fludarabine | 29 (14) | 29 (32) | ||
| Other/unavailable | 14 (7) | 14 (16) | ||
| Chemotherapy-alone regimen | ||||
| Busulfan/cyclophosphamide | 81 (39) | 81 (70) | ||
| Busulfan/fludarabine | 26 (13) | 26 (22) | ||
| Busulfan/melphalan | 9 (4) | 9 (8) | ||
| Transplant type | .004 | |||
| Matched related donor | 87 (42) | 27 (30) | 60 (52) | |
| Matched unrelated donor | 113 (55) | 59 (66) | 54 (47) | |
| Other/unavailable | 6 (3) | 4 (4) | 2 (2) | |
| Donor source | <.001 | |||
| Cord blood | 63 (31) | 48 (53) | 15 (13) | |
| Bone marrow | 1 (1) | 1 (1) | 0 (0) | |
| Peripheral blood | 137 (66) | 41 (46) | 96 (83) | |
| Unavailable | 5 (2) | 0 (0) | 5 (4) | |
| T-cell depletion | <.001 | |||
| Yes | 75 (36) | 18 (20) | 57 (49) | |
| Antithymocyte globulin | 25 (12) | 7 (8) | 18 (16) | |
| CD34 selection | 45 (22) | 9 (10) | 36 (31) | |
| Anti-CD52 antibody | 3 (1) | 0 (0) | 3 (3) | |
| Method unavailable | 2 (1) | 2 (2) | 0 (0) | |
| No | 125 (61) | 67 (74) | 58 (50) | |
| Unavailable | 6 (3) | 5 (6) | 1 (1) | |
| CMV baseline (donor) | .60 | |||
| Positive | 61 (30) | 26 (29) | 35 (30) | |
| Negative | 96 (47) | 45 (50) | 51 (44) | |
| Unknown | 49 (23) | 11 (21) | 30 (26) | |
| Successful engraftment | .18 | |||
| Yes | 179 (87) | 75 (83) | 104 (90) | |
| Nob | 27 (13) | 15 (17) | 12 (10) | |
| Days to engraftment | .26 | |||
| Median (IQR) | 30 (21-39) | 30 (23-42) | 30 (20-36) | |
| CMV reactivation | .09 | |||
| Yes | 76 (37) | 39 (43) | 37 (32) | |
| No | 130 (63) | 51 (57) | 84 (68) | |
| Acute GVHD | .09 | |||
| Grade 0-1 | 128 (62) | 50 (56) | 78 (67) | |
| Grade 2-4 | 78 (38) | 40 (44) | 38 (33) | |
GVHD, graft versus host disease. Other abbreviations as in Table 1.
Wilcoxon rank sum test for continuous variables and χ2 test for categorical variables.
Includes patients who died before engraftment.
Clinical outcomes
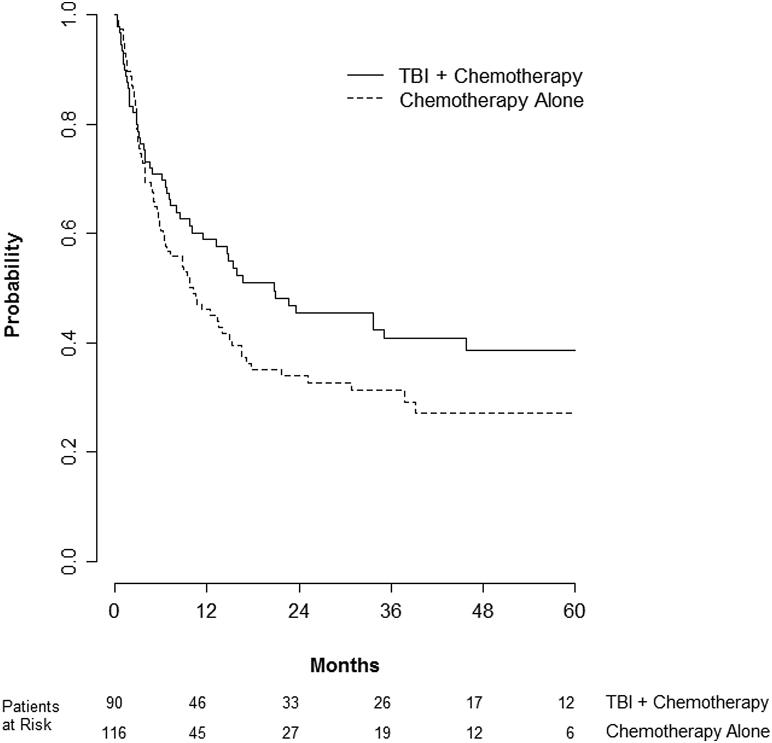
The predefined primary endpoint of the study was leukemia-free survival. For all patients, the median leukemia-free survival was 8 months with a 2-year leukemia-free survival of 36% (95% confidence interval [CI], 29-43). Two-year leukemia-free survival was 43% for TBI and 30% for chemotherapy alone (log-rank P = .12) (Fig 1). Factors associated with improved leukemia-free survival on multivariate analysis included complete remission status before transplant, successful engraftment, more recent year of transplant, and receiving a TBI-based regimen. Compared with chemotherapy-alone regimens, the addition of TBI was associated with a HR of 0.63 (95% CI, 0.44-0.91; P = .01) (Table 3).
Figure 1.
Kaplan-Meier of leukemia-free survival between patients receiving total body irradiation (TBI) plus chemotherapy compared with chemotherapy alone.
Table 3.
Proportional hazards multivariate model (N = 205)
| Variable | Leukemia-free survival |
Overall survival |
Pulmonary toxicity |
|---|---|---|---|
| Hazard ratio (95% confidence interval) | |||
| Karnofsky performance status | 0.98 (0.96-1.00) | 0.98 (0.96-1.01) | 0.96 (0.93-0.99) |
| Pretransplant disease status | |||
| CR1 | 0.63 (0.40-0.98) | 0.72 (0.46-1.14) | 0.75 (0.38-1.48) |
| CR2-3 | 0.51 (0.33-0.79) | 0.55 (0.35-0.87) | 0.57 (0.27-1.22) |
| Refractory | Reference | Reference | Reference |
| Acute GVHD | 1.16 (0.80-1.69) | 1.27 (0.86-1.85) | 0.89 (0.51-1.54) |
| Successful engraftment | 0.17 (0.10-0.27) | 0.16 (0.10-0.27) | 0.38 (0.20-0.75) |
| Year of transplant | 0.91 (0.87-0.95) | 0.91 (0.87-0.95) | 0.97 (0.92-1.03) |
| Treatment group | |||
| Chemotherapy alone | Reference | Reference | Reference |
| TBI + chemotherapy | 0.63 (0.44-0.91) | 0.63 (0.43-0.91) | 5.19 (2.65-10.15) |
The median overall survival for all patients was 13 months with a 2-year overall survival of 39% (95% CI, 32-46). Two-year overall survival was 45% for TBI-based regimens compared with 34% for chemotherapy alone (log-rank P = .08) (Fig 2). Factors associated with improved overall survival on multivariate analysis included successful engraftment, more recent year of transplant, and receiving TBI. Compared with chemotherapy alone, the HR for overall survival for TBI-based regimens was 0.63 (95% CI, 0.43-0.91; P = .02) (Table 3).
Figure 2.
Kaplan-Meier of overall survival between patients receiving total body irradiation (TBI) plus chemotherapy compared with chemotherapy alone.
Complications
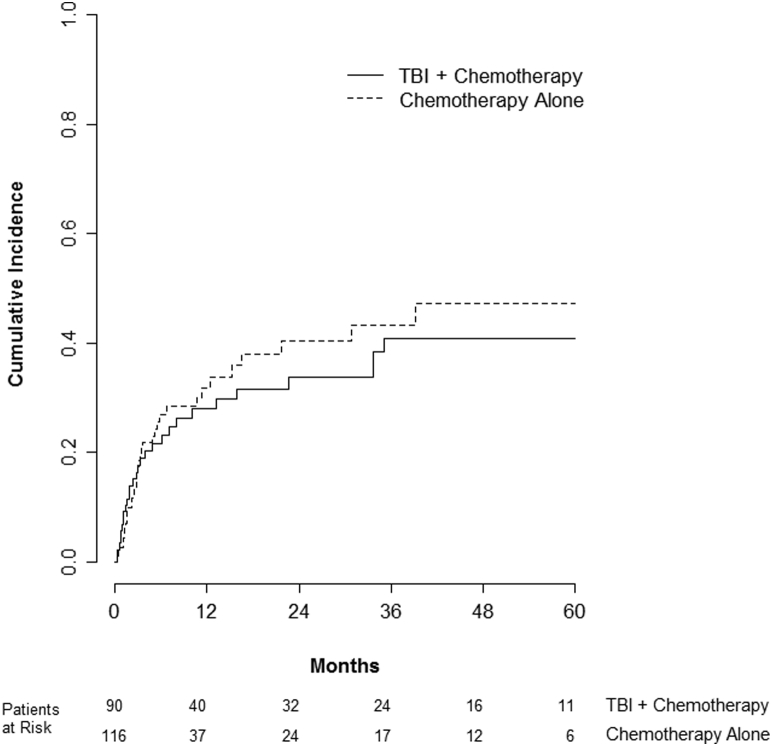
Nonrelapse mortality did not differ between cohorts with 2-year rates of 29% with TBI and 31% with chemotherapy alone (P = .83) (Fig 3). However, pulmonary toxicity was significantly more common in patients receiving TBI. The cumulative incidence of pulmonary toxicity at 2 years was 42% with TBI compared with 12% with chemotherapy alone (P < .001) (Fig 4). High-grade pulmonary toxicity predominated with both conditioning strategies (70% and 93% of cases were grade 3-5 with TBI and chemotherapy alone, respectively) (Table 4). On multivariate analysis, improved performance status and successful engraftment were associated with a decreased risk of pulmonary toxicity. Alternatively, receiving TBI was associated with an increased risk of posttransplant pulmonary toxicity. Compared with chemotherapy alone, TBI-based regimens had an HR of 5.19 (95% CI, 2.65-10.15; P < .001).
Figure 3.
Cumulative incidence of nonrelapse mortality between patients receiving total body irradiation (TBI) plus chemotherapy compared with chemotherapy alone.
Figure 4.
Cumulative incidence of pulmonary toxicity (grades 1-5) between patients receiving total body irradiation (TBI) plus chemotherapy compared with chemotherapy alone.
Table 4.
Pulmonary toxicity
| Type of toxicity | Conditioning regimen |
|
|---|---|---|
| TBI + chemotherapy (n = 40) | Chemotherapy alone (n = 14) | |
| Infectious | 4 | 1 |
| Diffuse alveolar hemorrhage | 7 | 4 |
| Pulmonary edema | 3 | 0 |
| Pneumonitis | 1 | 0 |
| Radiographic changes only | 3 | 0 |
| Multifactorial | 9 | 2 |
| Pulmonary toxicity NOSa | 13 | 7 |
| Total | 40 | 14 |
| Grades 1-2 | 12 | 1 |
| Grades 3-4 | 16 | 9 |
| Grade 5b | 12 | 4 |
| Total | 40 | 14 |
NOS, not otherwise specified.
Underlying etiology unclear.
Grade 5 indicates death from respiratory failure.
Acute grades 0-1 and 2-4 GVHD developed in 62% and 38% of patients, respectively, and were not different between cohorts. Lower grade acute GVHD (0-1) was more common than higher grade GVHD (2-4) for both the chemotherapy (67% and 33%, respectively) and chemotherapy plus TBI groups (56% and 44%, respectively). Limited chronic GVHD developing more than 1 year from transplant and limited to 1 organ system was more common than extensive chronic GVHD (13% and 7%, respectively).
Discussion
In this study, after correcting for known and available prognostic factors, leukemia-free survival and overall survival were improved when TBI was included in the conditioning regimen before myeloablative allo-SCT for AML. There are several reasons why a TBI-based conditioning regimen might be advantageous. It provides a noncross-resistant modality that complements chemotherapy. A combined modality approach has been a successful strategy across numerous oncologic disease sites. Further, there are no issues with blood supply and metabolism/excretion issues are avoided. Finally, although not as important with AML compared with acute lymphoblastic leukemia, TBI treats sanctuary sites within the central nervous system and testicles.
Historically, TBI was a standard component of AML conditioning regimens. Chemotherapy alone strategies, most commonly busulfan plus cyclophosphamide, were introduced in the 1980s in an attempt to reduce transplant-related morbidity, particularly radiation pneumonitis, and also increase the likelihood of long-term remission.11 These regimens also expanded the availability of transplant in centers with limited or no access to TBI. Potential drawbacks to the traditional busulfan plus cyclophosphamide regimen include poor tolerability and unpredictable pharmacodynamics with the oral busulfan formulations.23 The relatively recent development of intravenous busulfan may serve to counteract some of these issues.14
Despite several decades of experience with both approaches, the optimal conditioning regimen for AML is uncertain with conflicting conclusions in the literature. There are only a few randomized studies. Most are small and underpowered and many enrolled patients with other leukemia subtypes such as acute lymphoblastic leukemia and chronic myelogenous leukemia. An analysis of long-term follow-up of 4 randomized studies, only 2 of which enrolled patients with AML,4, 9 suggested that clinical outcomes were improved when TBI was used in the conditioning regimen.11 Ten-year differences in disease-free survival (57% vs 47%, P = .051) and overall survival (63% vs 51%, P = .068) appeared to be clinically relevant, though not statistically significant.
In contrast, numerous comparisons using registry data have shown no consistent differences between the 2 most common conditioning regimens: TBI/cyclophosphamide and busulfan/cylophosphamide.13, 14, 15, 16 A recent analysis by Copelan et al observed that regimens containing oral busulfan appeared to be equivalent to TBI/cyclophosphamide in regards to overall and leukemia free survival12; however, they found regimens containing intravenous busulfan to be superior to TBI/cyclophosphamide. Shi-Xia and colleagues conducted a meta-analysis of 18 controlled trials, some randomized but most not, and found that TBI was associated with improved disease-free survival in AML but with a greater risk of interstitial pneumonia.10
Given these inconsistencies in the literature, we sought to explore our institutional experience. In addition to the observation that TBI was associated with improved leukemia-free and overall survival, one of the most striking differences between the 2 regimens was the risk of pulmonary toxicity. Patients receiving TBI-based regimens were far more likely to develop pulmonary toxicity (2-year rates 42% vs 12%; HR, 5.19; P < .001). Although increased rates of pulmonary toxicity with TBI, specifically interstitial pneumonitis, have been observed in some series,10, 15 others have not noted a significant difference.4, 7, 9, 11 Notably, Ringden et al reported an increased rate of obstructive bronchiolitis in patients receiving chemotherapy regimens compared with TBI.8 In our series, pretransplant Karnofsky performance status (HR, 0.96) and whether patients ultimately engrafted (HR, 0.38) were also risk factors for pulmonary toxicity. We have previously demonstrated that the number of prior chemotherapy regimens20 and poor exercise tolerance24 may also affect the risk of pulmonary toxicity.
Pulmonary toxicity following both TBI-based and chemotherapy-alone preparative regimens can include infectious pneumonia, interstitial pneumonitis, acute respiratory distress syndrome, and diffuse alveolar hemorrhage. The severity of pulmonary toxicity varies widely from asymptomatic radiographic changes to respiratory failure requiring intubation and sometimes death. Additionally, it can be quite difficult to differentiate between these various etiologies in the clinical setting without invasive interventions, which are often not pursued. The primary method to reduce the risk of pulmonary toxicity in patients receiving TBI is lung shielding. We have previously evaluated the use of cardiopulmonary exercise testing to risk stratify patients prior to transplant.24 Given high rates of severe pulmonary toxicity with TBI, further strategies to mitigate this risk are clearly warranted.
Our study has several limitations. It is a retrospective evaluation of a single center's experience; thus, patient selection and choice of conditioning regimen were not controlled. Numerous conditioning regimens were used, even between the 2 cohorts, making it difficult to draw firm conclusions about the efficacy of a specific regimen. Further, the graft source varied between TBI-based and chemotherapy alone conditioning regimens, with cord blood transplants conditioned primarily with TBI. However, most studies have shown comparable clinical outcomes after allo-SCT for AML regardless of graft source.25 Given the era studied, we did not have known prognostic factors for all patients. This limited the breadth of our multivariate analysis. Finally, only a minority of patients received intravenous busulfan, which is now a standard at our institution. The primary strength of our study was the utilization of a uniform TBI protocol within an experienced transplant center.
Conclusion
In our experience, TBI-based regimens were associated with superior leukemia-free and overall survival but at the cost of increased pulmonary toxicity. Efforts and strategies to reduce pulmonary toxicity are warranted.
Footnotes
Sources of support: This work was supported by the Department of Radiation Oncology, Duke University Medical Center, Durham, North Carolina.
Conflict of Interest: None.
References
- 1.Beutler E., Blume K.G., Bross K.J. Bone marrow transplantation as the treatment of choice for “good risk” adult patients with acute leukemia. Trans Assoc Am Physicians. 1979;92:189–195. [PubMed] [Google Scholar]
- 2.Thomas E.D., Buckner C.D., Clift R.A. Marrow transplantation for acute nonlymphoblastic leukemia in first remission. N Engl J Med. 1979;301:597–599. doi: 10.1056/NEJM197909133011109. [DOI] [PubMed] [Google Scholar]
- 3.O'Donnell M.R., Abboud C.N., Altman J. National Comprehensive Cancer Network. Acute myeloid leukemia. J Natl Compr Canc Netw. 2011;9:280–317. doi: 10.6004/jnccn.2011.0027. [DOI] [PubMed] [Google Scholar]
- 4.Blaise D., Maraninchi D., Archimbaud E. Allogeneic bone marrow transplantation for acute myeloid leukemia in first remission: A randomized trial of a busulfan-cytoxan versus cytoxan-total body irradiation as preparative regimen: A report from the group d'etudes de la greffe de moelle osseuse. Blood. 1992;79:2578–2582. [PubMed] [Google Scholar]
- 5.Blaise D., Maraninchi D., Michallet M. Long-term follow-up of a randomized trial comparing the combination of cyclophosphamide with total body irradiation or busulfan as conditioning regimen for patients receiving hla-identical marrow grafts for acute myeloblastic leukemia in first complete remission. Blood. 2001;97:3669–3671. doi: 10.1182/blood.v97.11.3669. [DOI] [PubMed] [Google Scholar]
- 6.Blume K.G., Kopecky K.J., Henslee-Downey J.P. A prospective randomized comparison of total body irradiation-etoposide versus busulfan-cyclophosphamide as preparatory regimens for bone marrow transplantation in patients with leukemia who were not in first remission: A southwest oncology group study. Blood. 1993;81:2187–2193. [PubMed] [Google Scholar]
- 7.Hartman A.R., Williams S.F., Dillon J.J. Survival, disease-free survival and adverse effects of conditioning for allogeneic bone marrow transplantation with busulfan/cyclophosphamide vs total body irradiation: A meta-analysis. Bone Marrow Transplant. 1998;22:439–443. doi: 10.1038/sj.bmt.1701334. [DOI] [PubMed] [Google Scholar]
- 8.Ringden O., Remberger M., Ruutu T. Increased risk of chronic graft-versus-host disease, obstructive bronchiolitis, and alopecia with busulfan versus total body irradiation: Long-term results of a randomized trial in allogeneic marrow recipients with leukemia. Nordic bone marrow transplantation group. Blood. 1999;93:2196–2201. [PubMed] [Google Scholar]
- 9.Ringden O., Ruutu T., Remberger M. A randomized trial comparing busulfan with total body irradiation as conditioning in allogeneic marrow transplant recipients with leukemia: A report from the nordic bone marrow transplantation group. Blood. 1994;83:2723–2730. [PubMed] [Google Scholar]
- 10.Shi-Xia X., Xian-Hua T., Hai-Qin X., Bo F., Xiang-Feng T. Total body irradiation plus cyclophosphamide versus busulphan with cyclophosphamide as conditioning regimen for patients with leukemia undergoing allogeneic stem cell transplantation: A meta-analysis. Leuk Lymph. 2010;51:50–60. doi: 10.3109/10428190903419130. [DOI] [PubMed] [Google Scholar]
- 11.Socie G., Clift R.A., Blaise D. Busulfan plus cyclophosphamide compared with total-body irradiation plus cyclophosphamide before marrow transplantation for myeloid leukemia: Long-term follow-up of 4 randomized studies. Blood. 2001;98:3569–3574. doi: 10.1182/blood.v98.13.3569. [DOI] [PubMed] [Google Scholar]
- 12.Copelan E.A., Hamilton B.K., Avalos B. Better leukemia-free and overall survival in aml in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. 2013;122:3863–3870. doi: 10.1182/blood-2013-07-514448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litzow M.R., Perez W.S., Klein J.P. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide-total body irradiation versus busulphan-cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol. 2002;119:1115–1124. doi: 10.1046/j.1365-2141.2002.03973.x. [DOI] [PubMed] [Google Scholar]
- 14.Nagler A., Rocha V., Labopin M. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: Comparison of intravenous busulfan plus cyclophosphamide (cy) versus total-body irradiation plus cy as conditioning regimen—a report from the acute leukemia working party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2013;31:3549–3556. doi: 10.1200/JCO.2013.48.8114. [DOI] [PubMed] [Google Scholar]
- 15.Ringden O., Labopin M., Tura S. A comparison of busulphan versus total body irradiation combined with cyclophosphamide as conditioning for autograft or allograft bone marrow transplantation in patients with acute leukaemia. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT) Br J Haematol. 1996;93:637–645. doi: 10.1046/j.1365-2141.1996.d01-1681.x. [DOI] [PubMed] [Google Scholar]
- 16.Uberti J.P., Agovi M.A., Tarima S. Comparative analysis of bu and cy versus cy and tbi in full intensity unrelated marrow donor transplantation for aml, cml and myelodysplasia. Bone Marrow Transplant. 2011;46:34–43. doi: 10.1038/bmt.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grigg A.P., Szer J., Beresford J. Factors affecting the outcome of allogeneic bone marrow transplantation for adult patients with refractory or relapsed acute leukaemia. Br J Haemotol. 1999;107:409–418. doi: 10.1046/j.1365-2141.1999.01713.x. [DOI] [PubMed] [Google Scholar]
- 18.Wingard J.R., Majhail N.S., Brazauskas R. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29:2230–2239. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koreth J., Schlenk R., Kopecky K.J. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: Systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–2361. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelsey C.R., Horwitz M.E., Chino J.P. Severe pulmonary toxicity after myeloablative conditioning using total body irradiation: an assessment of risk factors. Int J Radiat Oncol Biol Phys. 2011;81(3):812–818. doi: 10.1016/j.ijrobp.2010.06.058. [DOI] [PubMed] [Google Scholar]
- 21.Soule B.P., Simone N.L., Savani B.N. Pulmonary function following total body irradiation (with or without lung shielding) and allogeneic peripheral blood stem cell transplant. Bone Marrow Transplant. 2007;40:573–578. doi: 10.1038/sj.bmt.1705771. [DOI] [PubMed] [Google Scholar]
- 22.Fine J.P., Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 23.Richard Hoppe M., Theodore Phillips M.D., Mack Roach M.D. Saunders; Philadelphia, PA: 2010. Leibel and Phillips Textbook of Radiation Oncology. [Google Scholar]
- 24.Kelsey C.R., Scott J.M., Lane A. Cardiopulmonary exercise testing prior to myeloablative allo-SCT: a feasibility study. Bone Marrow Transplant. 2014;49(10):1330–1336. doi: 10.1038/bmt.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballen K.K., Lazarus H. Cord blood transplant for acute myeloid leukaemia. BrJ Haematol. 2016;173:25–36. doi: 10.1111/bjh.13926. [DOI] [PubMed] [Google Scholar]