Abstract
Purpose
There is growing evidence supporting incorporating multiparametric (mp) magnetic resonance imaging (MRI) scans into risk stratification, active surveillance, and treatment paradigms for prostate cancer. The purpose of our study was to determine whether demographic disparities exist in staging MRI utilization for prostate cancer patients.
Methods and materials
An institutional database of 705 nonmetastatic prostate cancer patients treated with radiation therapy from 2005 through 2013 was used to identify patients undergoing versus not undergoing pretreatment diagnostic prostate mpMRI. Uni- and multivariable logistic regression evaluated the relationship of clinical and demographic characteristics with MRI utilization.
Results
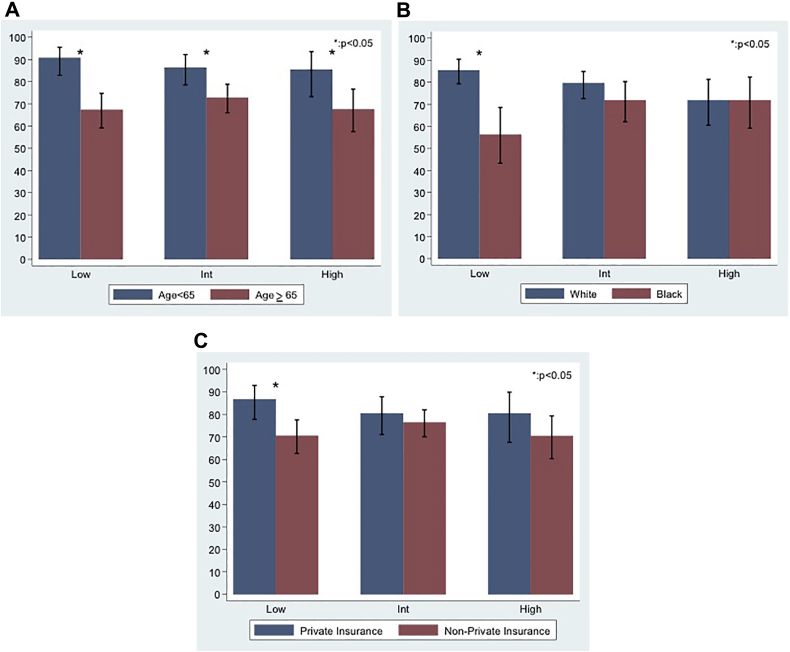
All demographic variables assessed, except the other race category, were significantly associated with MRI utilization (all P < .05), including age (odds ratio [OR], 0.92), black race (OR, 0.51), poverty (OR, 0.53), closer distance to radiation facility (OR, 1.79), and nonprivate primary insurance (OR, 0.57) on univariable analysis, while clinical stage T3 (OR, 3.37) was the only clinical characteristic. On multivariable analysis stratified by D'Amico risk group, age remained significant across all risk groups, whereas the black versus white racial (OR, 0.21; 95% confidence interval, 0.08-0.55) and nonprivate versus private insurance type (OR, 0.37; 95% confidence interval, 0.16-0.86) disparities persisted in the low-risk group. Clinical stage T3 remained associated in the high-risk group. For race specifically, the percentages of whites, blacks, and others undergoing MRI in the overall cohort and by risk group were, respectively: overall, 80% (343/427), 68% (156/231), and 85% (40/47); low risk, 86%, 56%, and 63%; intermediate risk, 79%, 72%, and 95%; and high risk, 72%, 72%, and 100%.
Conclusions
In this urban, academic center cohort, older patients across all risk groups and black or nonprivate insurance patients in the low risk group were less likely to undergo staging prostate MRI scans. Further research should investigate these differences to ensure equitable utilization across all demographic groups considering the burden of prostate cancer disparities.
Introduction
Well-documented disparities exist in prostate cancer incidence, morbidity, and mortality in black versus white males in the United States.1, 2 Identifying appropriate candidates for active surveillance using only traditional risk stratification parameters (prostate specific antigen [PSA], Gleason score, and tumor stage) remains challenging particularly when considering black race. Recent analyses from Johns Hopkins found that black men classified as having very low-risk prostate cancer, technically eligible for active surveillance, were more likely to have adverse pathologic features at the time of radical prostatectomy,3 as well as larger dominant intraprostatic lesions (IPLs) with high prevalence of anterior foci.4 A higher risk of disease progression in black men after enrollment on active surveillance trials has similarly been confirmed in other cohorts with larger numbers of black men.5 There is increased interest in incorporating additional tools, such as multiparametric magnetic resonance imaging (mpMRI), into risk stratification paradigms given its ability to detect otherwise occult high-risk features.
Multiparametric MRI with diffusion-weighted imaging and dynamic contrast enhancement sequences allow for the identification of dominant IPLs.6 Prior diagnostic literature has demonstrated that IPL characteristics, such as their apparent diffusion coefficient,7 contrast agent transfer rate between blood and tissue,8 extracapsular extension,9 and seminal vesicle invasion,10 are associated with other known prognostic indicators such as the PSA, Gleason score, and risk group.9 Pathologic correlation with prostatectomy specimens has demonstrated the accuracy of these findings.11 Increasingly, a relationship between pre-radiation therapy MRI findings, such as IPL size, extracapsular extension, and seminal vesicle invasion, and long-term outcomes, such as biochemical relapse and metastases, has been demonstrated12, 13, 14, 15; however, existing nomograms used in predicting prostate cancer biochemical relapse after external-beam radiation therapy, such as the Kattan nomogram16 generally do not currently include these imaging parameters.
The increasing body of evidence that supports the use of mpMRI underscores the importance of performing research to quantify its dissemination. Although prostate mpMRI may have the potential to reduce prostate cancer disparities by improving risk stratification, it is unclear how often this technology is used across different clinical risk and demographic groups. Known cancer health disparities exist in imaging in other malignancies, such as breast,17 colon,18 and lung19 cancer, but have not been well explored or documented in prostate cancer, and health care resource utilization may additionally serve as a measure of access to care.20 Thus, the aim of our study was to determine whether disparities exist in mpMRI utilization across risk groups in men with prostate cancer undergoing definitive radiation therapy.
Methods and materials
We conducted an institutional review board–approved retrospective analysis of prostate cancer patients treated with definitive external beam radiation therapy with curative intent at a single academic institution between November 2005 and December 2013. Existing medical databases and electronic medical records were used to identify all patients (N = 705) treated with histologically confirmed, nonmetastatic adenocarcinoma of the prostate. We collected pretreatment demographic characteristics, including age, race, socioeconomic status (SES), distance in miles from home address to the radiation facility, and primary insurance, primary treating radiation oncologist, and treatment year. Racial groups included white, black, Asian, other, and unknown; Asian and unknown were combined with other for this analysis given their small numbers in the cohort. As a surrogate for SES, we used the federal definition of residing in poverty based on geocoded census tract.21 Distance was classified as <45 miles or ≥45 miles from the radiation treatment facility as a surrogate for patients residing locally or regionally versus those traveling more distantly. A total of 95% of patients received staging MRI at the same facility. Primary insurance type was classified as either private or nonprivate, which included Medicare and Medicaid. Pretreatment PSA, treatment-recorded clinical tumor stage (as assessed by digital rectal exam or imaging), Gleason score, D'Amico risk stratification group,22 percentage of positive biopsy cores, International Prostate Symptom Score (IPSS), date of prostate cancer diagnosis, treatment dates, and pretreatment usage of diagnostic prostate and/or pelvic mpMRI (including T2-weighted, diffusion-weighted, and/or dynamic contrast-enhanced sequences, 91% with endorectal coil), were retrospectively recorded through chart review. Patients were divided into comparison groups based on whether they did or did not undergo pretreatment diagnostic prostate mpMRI. Six patients with explicitly recorded contraindications to mpMRI were excluded from the analysis, leaving 609 patients assessed. During the treatment time frame, our institution-wide standard practice guideline encouraged a diagnostic MRI in all-risk prostate cancer patients without contraindications.
Statistical considerations
The primary objective was to compare demographic and clinical characteristics of prostate cancer patients who either did or did not undergo pretreatment diagnostic mpMRI at our institution. Descriptive statistics were computed for the overall cohort and then MRI versus non-MRI utilization groups were compared using the χ2 test for categorical variables and the Student t test for continuous variables. Age, PSA, distance, percentage of positive biopsy cores, and IPSS were analyzed as continuous variables, and the remainder as categorical. All P values were 2-sided. Univariable logistic regression was used to evaluate the relationship of clinical and demographic characteristics with MRI utilization as the outcome variable. Multivariable logistic regression models were constructed using variables significant in the univariable analysis. Multivariable analysis was stratified by risk group to best approximate clinical practice and decision making at the common point of MRI utilization. Odds ratios (OR) were reported with associated 95% confidence intervals (CI). A P value <.05 was considered statistically significant. Analyses were conducted using either STATA/MP 13 (College Station, TX) or SAS, version 9.4 (Cary, NC).
Results
Table 1 shows the overall cohort and comparison of characteristics for those who underwent MRI compared with those who did not. Demographically, patients who did not undergo MRI were significantly older (mean age 67 vs 71), and more likely to reside in poverty (36% vs 23%), live closer to the facility (<45 miles), have nonprivate primary insurance, and be of black race (all P values < .05). The percentages of whites, blacks, and the other race group undergoing MRI were 80% (343/427), 68% (156/231), and 85% (40/47), respectively. There was also increasing MRI utilization over time. Regarding clinical characteristics, patients undergoing MRI were more likely to have clinical stage T3 disease (11.7% vs 3.6%); the cohorts did not differ by PSA (mean 10.5 vs 9.3), Gleason score, risk group, percentage positive biopsy cores, or IPSS, respectively, for the cohort that had an MRI versus the cohort that did not.
Table 1.
Overall cohort characteristics and comparison of prostate multiparametric MRI vs non-MRI cohort
| Variable | Characteristic | Overall | No staging MRI | Staging MRI | P value |
|---|---|---|---|---|---|
| N (%) | 705 | 166 | 539 | ||
| Age (y) | Mean ± SD | 67.1 ± 7.9 | 70.7 ± 7.8 | 65.9 ± 7.6 | <.001 |
| Race | White | 427 (60.6) | 84 (50.6) | 343 (63.6) | <.001 |
| Black | 231 (32.8) | 75 (45.2) | 156 (28.9) | ||
| Other | 47 (6.7) | 7 (4.2) | 40 (7.4) | ||
| Poverty | No | 521 (73.9) | 106 (63.9) | 415 (77.0) | <.001 |
| Yes | 184 (26.1) | 60 (36.1) | 124 (23.0) | ||
| Distance (miles) | <45 | 556 (78.9) | 142 (85.5) | 414 (76.8) | .016 |
| ≥45 | 149 (21.1) | 24 (14.5) | 125 (23.2) | ||
| Primary insurance | Private | 243 (34.5) | 42 (25.3) | 201 (37.3) | .005 |
| Nonprivate | 462 (65.5) | 124 (74.7) | 338 (62.7) | ||
| Treatment year | 2005-2007 | 159 (22.6) | 77 (48.4) | 82 (51.6) | <.001 |
| 2008-2009 | 129 (18.3) | 49 (38.0) | 80 (62.0) | ||
| 2010-2013 | 417 (59.1) | 40 (9.6) | 377 (90.4) | ||
| PSA (ng/mL) | Mean ± SD | 9.6 ± 14.2 | 10.5 ± 15.3 | 9.3 ± 13.8 | .351 |
| Gleason score | 6 | 290 (41.1) | 65 (39.2) | 225 (41.7) | .439 |
| 7 | 325 (46.1) | 75 (45.2) | 250 (46.4) | ||
| 8 | 52 (7.4) | 13 (7.8) | 39 (7.2) | ||
| 9 | 38 (5.4) | 13 (7.8) | 25 (4.6) | ||
| Clinical T stage | T1 | 502 (71.2) | 122 (73.5) | 380 (70.5) | .006 |
| T2 | 134 (19.0) | 38 (22.9) | 96 (17.8) | ||
| T3 | 69 (9.8) | 6 (3.6) | 63 (11.7) | ||
| Risk group | High | 151 (21.4) | 40 (24.1) | 111 (20.6) | .578 |
| Intermediate | 308 (43.7) | 68 (41.0) | 240 (44.5) | ||
| Low | 246 (34.9) | 58 (34.9) | 188 (34.9) | ||
| Percent positive biopsy cores | Mean ± SD | 0.4 ± 0.3 | 0.4 ± 0.3 | 0.4 ± 0.3 | .348 |
| IPSS | Mean ± SD | 7.9 ± 6.5 | 8.2 ± 6.1 | 7.8 ± 6.6 | .590 |
IPSS, International Prostate Symptom Score; MRI, magnetic resonance imaging; PSA, prostate-specific antigen; SD, standard deviation.
On univariable logistic regression (Table 2), all demographic variables assessed, except for the other race category, were associated with MRI utilization, including age (OR, 0.92; 95% CI, 0.89-0.94; P < .05), black race (OR, 0.51, 0.35, 0.73; P < .05), poverty (OR, 0.53, 0.36, 0.77; P < .05), distance (OR, 1.79; 1.11, 2.88; P < .05), and primary insurance type (OR, 0.57, 0.39, 0.84; P < .05). Only clinical stage T3 (OR, 3.37, 1.42, 7.97; P < .05) was associated with MRI utilization, whereas treatment year and the other clinical characteristics assessed—PSA, Gleason score, clinical stage, risk group, percent positive cores, and IPSS—were not.
Table 2.
Univariable association of demographic and clinical characteristics with staging MRI
| Independent variable | Characteristic | OR (95% CI) | P value |
|---|---|---|---|
| Age (y) | 0.92 (0.89-0.94) | <.001 | |
| Race | Black vs white | 0.51 (0.35-0.73) | <.001 |
| Other vs white | 1.40 (0.61-3.23) | .432 | |
| Poverty | Yes vs no | 0.53 (0.36-0.77) | <.001 |
| Distance (miles) | ≥45 vs <45 | 1.79 (1.11- 2.88) | .017 |
| Primary insurance | Nonprivate vs private | 0.57 (0.39-0.84) | .005 |
| Treatment Year | 2008-2009 vs 2005-2007 | 0.43 (−0.05 to 2.63) | .076 |
| 2010-2013 vs 2005-2007 | 1.40 (0.61-3.23) | .432 | |
| PSA (ng/mL) | 0.99 (0.98-1.01) | .354 | |
| Gleason score | 7 vs 6 | 0.96 (0.66-1.41) | .845 |
| 8 vs 6 | 0.87 (0.44-1.72) | .683 | |
| 9 vs 6 | 0.56 (0.27-1.15) | .112 | |
| Clinical T stage | T2 vs T1 | 0.81 (0.53-1.24) | .337 |
| T3 vs T1 | 3.37 (1.42-7.97) | .006 | |
| Risk group | Intermediate vs high | 1.27 (0.81-2.00) | .296 |
| Low vs high | 1.17 (0.73-1.86) | .514 | |
| Percent positive biopsy cores | 0.64 (0.25-1.63) | .348 | |
| IPSS | 0.99 (0.95-1.03) | .589 |
CI, confidence interval; IPSS, International Prostate Symptom Score; MRI, magnetic resonance imaging; OR, odds ratio; PSA, prostate-specific antigen.
The results of the multivariable logistic regression stratified by risk group are shown in Table 3. Age was associated with MRI utilization in all risk groups. Black versus white race (OR, 0.21, 0.08, 0.55; P < .05) and nonprivate versus private insurance (OR, 0.37, 0.16, 0.86; P < .05) were associated with MRI utilization in the low-risk group (Fig 2). Clinical stage T3 remained associated with MRI utilization in the high-risk group.
Table 3.
Risk group stratified multivariable association of demographic and clinical characteristics with staging MRI
| Risk group | Variable | Characteristic | OR (95% CI) | P value |
|---|---|---|---|---|
| Low | Age | 0.89 (0.84-0.94) | <.001 | |
| Race | Black vs white | 0.21 (0.08-0.55) | .001 | |
| Other vs white | 0.22 (0.06-0.77) | .018 | ||
| Poverty | Yes vs no | 0.64 (0.25-1.64) | .353 | |
| Primary insurance | Nonprivate vs private | 0.37 (0.16-0.86) | .020 | |
| Distance (miles) | ≥45 vs <45 | 1.28 (0.48-3.44) | .623 | |
| Clinical T stage | T2 vs T1 | 0.51 (0.19-1.40) | .193 | |
| Intermediate | Age | 0.94 (0.90-0.97) | <.001 | |
| Race | Black vs white | 0.71 (0.29-1.73) | .454 | |
| Other vs white | 5.57 (0.71-43.8) | .103 | ||
| Poverty | Yes vs no | 0.98 (0.39-2.47) | .973 | |
| Primary insurance | Nonprivate vs private | 1.03 (0.55-1.93) | .930 | |
| Distance (miles) | ≥45 vs <45 | 1.26 (0.59, 2.71) | .551 | |
| Clinical T stage | T2 vs T1 | 1.38 (0.66-2.85) | .390 | |
| High | Age | 0.93 (0.88-0.98) | .013 | |
| Race | Black vs white | 0.99 (0.34-2.87) | .989 | |
| Other vs white | NE | NE | ||
| Poverty | Yes vs no | 0.92 (0.30-2.79) | .879 | |
| Primary insurance | Nonprivate vs private | 0.79 (0.31-2.01) | .623 | |
| Distance (miles) | ≥45 vs <45 | 1.03 (0.31-3.42) | .967 | |
| Clinical T stage | T2 vs T1 | 0.52 (0.20-1.34) | .176 | |
| T3 vs T1 | 3.75 (1.25-11.3) | .019 |
CI, confidence interval; MRI, magnetic resonance imaging; NE, not estimable due to small number of patients; OR, odds ratio.
Figure 2.
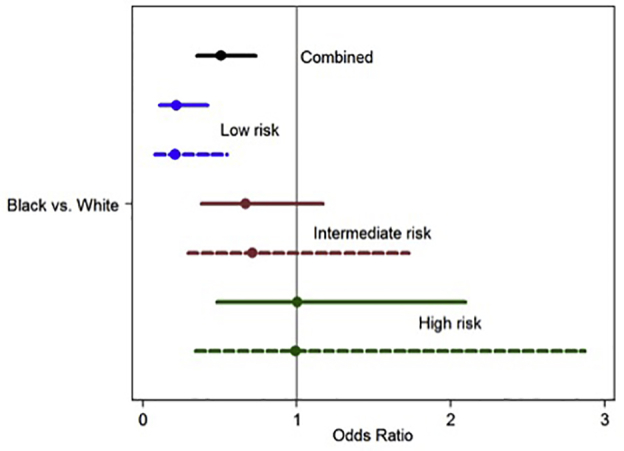
Plot of adjusted* (dashed line) and unadjusted (solid line) odds ratios of prostate magnetic resonance imaging utilization for black vs white men from the multivariable analysis stratified by risk-group. *Adjusted for age, poverty, primary insurance type, distance, and clinical stage.
Figure 1 shows MRI utilization by risk group and the variables significant on multivariable analysis—age, race, and insurance type. For age (Fig 1A), the percentages of men younger than age 65 compared with ≥65 undergoing MRI in the low-, intermediate-, and high-risk groups were, respectively, 91% versus 67%, 86% versus 73%, and 86% versus 68% (all P < .05). For race (Fig 1B), the percentages of whites and blacks undergoing MRI were 86% and 56%, in the low-risk group, 79% and72% in the intermediate-risk group, and 72% and 72% in the high-risk group, respectively. The percentages of patients with private insurance compared to nonprivate (Fig 1C) undergoing MRI in the low-, intermediate-, and high-risk groups were, respectively, 87% versus 71% (P < .05), 80% versus 76%, and 80% versus 70%.
Figure 1.
Percentage prostate MRI utilization by (A) age, (B) race, and (C) insurance type and prostate cancer risk groups. *Represents significant difference; error bars represent the 95% confidence interval. Int, intermediate.
Discussion
In this urban, academic center cohort of nonmetastatic prostate cancer patients undergoing definitive radiation therapy, we explored whether clinical and demographic disparities exist in mpMRI utilization. We found that demographic characteristics, including age, race, poverty, and insurance type were associated with mpMRI utilization, whereas most clinical parameters, including PSA, Gleason score, and IPSS, were not. Patients not undergoing mpMRI were older and more likely to reside in poverty, live closer to the facility, have nonprivate primary insurance, and be of black race, indicating that demographic and not clinical characteristics most influenced mpMRI utilization. On multivariable analysis, age remained significant across all risk groups, and race and insurance type persisted only in the low-risk group.
Current National Cancer Comprehensive Network (NCCN) guidelines recommend cross-sectional imaging for all high-risk patients and also indicate that mpMRI may be used to risk-stratify patients who are considering active surveillance.23 American College of Radiology guidelines similarly recommend mpMRI in intermediate- and high-risk patients and indicate that MRI scans may be appropriate in low-risk patients considering active surveillance.24 Despite these staging guidelines and MRI's increasing role in aiding treatment selection and surgical and radiation planning,25 several studies have reported MRI use that is discordant with historical and present guidelines, including overutilization among low-risk patients and underutilization among high-risk patients.26, 27 Although we do not postulate a “correct” utilization rate, in our study we did interestingly find disparate utilization trends by age, insurance type, and race. For race specifically, blacks (Fig 1) and the other category—patients that were neither white nor black—were significantly less likely to undergo mpMRI than whites in the low-risk group, but there were no significant differences in the intermediate- and high-risk groups, reassuringly suggesting equitable utilization by race in these more advanced risk groups. However, considering the emerging evidence for mpMRI in active surveillance28 in the context of racial disparities in prostate cancer, a plausible opportunity emerges to use this technology to improve staging in black men and positively affect management decisions. The racial disparity observed in our study is therefore very concerning, given that black men in the low-risk group who have been found to have high rates of disease progression,5 might arguably benefit most from the additional information offered by the mpMRI. As this was ultimately a treatment cohort, it would be important to verify the analysis in an active surveillance cohort to determine whether the utilization disparities pervade.
Racial disparities in imaging utilization have been well-described in other malignancies,17, 18, 19 but only to a limited extent in prostate cancer. An analysis from the Surveillance, Epidemiology and End Results-Medicare linked database evaluating staging workup before and after implementation of NCCN, American Urological Association, and American College of Radiology guidelines in 96,986 men with incident prostate cancer from 1991 to 1994 compared with 1995 to 1999 found that, of men who met guideline criteria for staging, black men were less likely to undergo bone scan and pelvic imaging than white men diagnosed from 1991 to 1994, but this racial difference was not seen from 1995 to 1999, suggesting historical improvements in disparate imaging trends. This parity was not noted in our study for patients in the low-risk group. In contrast, a more recent Surveillance, Epidemiology and End Results-Medicare analysis of prostate cancer patients diagnosed between 2004 and 2007 found that race was significantly associated with nonadherence to imaging guidelines on multivariable models, with bone scan and pelvic computed tomography (CT)/MRI overuse associated with non-white race (reference, white race).29 This same study did, however, find underuse of bone scan and pelvic CT/MRI associated with patient age >79 years (reference age, 66-69), consistent with the age disparity found in our study. The age disparity seen in this previous report as well as our own study across risk groups was expectedly consistent with numerous studies in other malignancies demonstrating that older patients tend to have less rigorous staging workup than younger patients.30
The reason behind these age and racial disparities is likely multifactorial, including SES, insurance status, provider biases, and access to care. However, in a single academic institution experience with standard therapeutic regimens, one might expect the impact of these demographic factors to be lessened or eliminated, as has been shown in a secondary analysis of black men enrolled on prostate radiation therapy cooperative group trials.31 This was not the case in our study. Finally, we did also find that private insurance type was associated with mpMRI utilization in the low-risk group. Being uninsured or having nonprivate insurance, such as Medicaid, has been consistently associated with poor health outcomes.32, 33 Furthermore, patient insurance status has been shown to affect clinical decisions, both directly (ie, if the patient cannot afford the service) and indirectly by contributing to provider biases and preconceived notions that affect decision-making.32, 34, 35
A limitation of our study is that it represents a radiation therapy cohort and whether such disparities in mpMRI utilization exist in other prostate cancer management cohorts (eg, active surveillance, surgical, metastatic) remains to be explored. Additionally, we did not record whether patients not obtaining mpMRI had alternative cross-sectional imaging, such as CT, given that mpMRI is superior to CT in identifying intraprostatic lesions and adverse tumor characteristics. Indeed, the association of clinical T3 stage on multivariable analysis and MRI utilization in our study potentially speaks to the superior local staging and anticipated tumor upstaging by mpMRI. We also intentionally excluded provider-level characteristics because we were retrospectively unable to capture the “true” ordering physician and it is unclear (however unlikely) that disparate patterns of mpMRI utilization may have systematically reflected different referral origins (ie, radiation oncology vs urology vs medical oncology vs primary care), despite institution wide preference for MRI as baseline staging. Similarly, it is unknown whether providers may have ordered the MRI scans, but they were not obtained by the patient due to financial, psychosocial, logistical, and other barriers. We did not exclude patients with explicit contraindication to MRI (3 of 166 not undergoing MRI) given the potential likelihood that other patients had absolute and relative contraindications that were not documented in the available records over the study period; it is uncertain how this may differ by demographics or risk group in this prostate cancer radiation therapy cohort. Regarding insurance status, because primary insurance type was recorded at the time of treatment, at which time all patients would have acquired insurance, insurance status at time of diagnosis was unknown, which may or may not have influenced mpMRI utilization. However, there is evidence that patients with either Medicaid or who are uninsured have similarly poorer cancer outcomes.36, 37 Lastly, as a surrogate for SES, we used the federal definition of residing in poverty (>20%) based on geocoded census tract coded as a binary outcome, which differed significantly at baseline, but not in multivariable analysis. Similar results were seen with the percent residing in poverty analyzed as a continuous variable (results not shown). Nonetheless, other SES surrogates such as median income or education level might have yielded an association with SES and helped to parse the complex association between race and SES.
Conclusions
In this urban, academic center cohort, prostate cancer patients that were older, regardless of risk group, and that were black or with nonprivate insurance in the low-risk group, were less likely to undergo staging MRI. Collectively, these data raise areas for further practice review, analysis, and improvement, and should be undertaken on a wider scale as we embrace active surveillance, given the dearth of data on the safety and applicability of this strategy to black and older men. Given the known disproportionate burden of prostate cancer morbidity and mortality, further research should investigate whether MRI can help reduce prostate cancer disparities and is equitably used across all demographic groups.
Footnotes
Sources of support: This work was supported in part by the University of Pennsylvania Abramson Cancer Center Core Grant (P30CA016520) to W.-T.H. and X.W.
Conflicts of interest: None.
References
- 1.Chornokur G., Dalton K., Borysova M.E., Kumar N.B. Disparities at presentation, diagnosis, treatment, and survival in African American men, affected by prostate cancer. Prostate. 2011;71:985–997. doi: 10.1002/pros.21314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bock C.H., Powell I., Kittles R.A., Hsing A.W., Carpten J. Racial disparities in prostate cancer incidence, biochemical recurrence, and mortality. Prostate Cancer. 2011;2011:716178. doi: 10.1155/2011/716178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sundi D., Ross A.E., Humphreys E.B. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: Should active surveillance still be an option for them? J Clin Oncol. 2013;31:2991–2997. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sundi D., Kryvenko O.N., Cartref H.B. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black american men. J Urol. 2014;191:60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iremashvili V., Soloway M.S., Rosenberg D.L. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. Urology. 2012;187:1594–1599. doi: 10.1016/j.juro.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 6.D'Amico A.V., Schnall M., Whittington R. Endorectal coil magnetic resonance imaging identifies locally advanced prostate cancer in select patients with clinically localized disease. Urology. 1998;51:449–454. doi: 10.1016/s0090-4295(97)00630-4. [DOI] [PubMed] [Google Scholar]
- 7.Woodfield C.A., Tung G.A., Grand D.J., Pezzullo J.A., Machan J.T., Renzulli J.F., 2nd Diffusion-weighted MRI of peripheral zone prostate cancer: Comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am J Roentgenol. 2010;194:W316–W322. doi: 10.2214/AJR.09.2651. [DOI] [PubMed] [Google Scholar]
- 8.Peng Y., Jiang Y., Yang C. Quantitative analysis of multiparametric prostate MR images: Differentiation between prostate cancer and normal tissue and correlation with Gleason score—a computer-aided diagnosis development study. Radiology. 2013;267:787–796. doi: 10.1148/radiol.13121454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoeks C.M., Barentsz J.O., Hambrock T. Prostate cancer: Multiparametric MR imaging for detection, localization, and staging. Radiology. 2011;261:46–66. doi: 10.1148/radiol.11091822. [DOI] [PubMed] [Google Scholar]
- 10.Wang L., Hricak H., Kattan M.W. Prediction of seminal vesicle invasion in prostate cancer: Incremental value of adding endorectal MR imaging to the Kattan nomogram. Radiology. 2007;242:182–188. doi: 10.1148/radiol.2421051254. [DOI] [PubMed] [Google Scholar]
- 11.Labanaris A.P., Zugor V., Smiszek R., Nützel R., Kühn R., Engelhard K. Guided e-MRI prostate biopsy can solve the discordance between Gleason score biopsy and radical prostatectomy pathology. Magn Reson Imaging. 2010;28:943–946. doi: 10.1016/j.mri.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 12.Riaz N., Afaq A., Akin O. Pretreatment endorectal coil magnetic resonance imaging findings predict biochemical tumor control in prostate cancer patients treated with combination brachytherapy and external-beam radiotherapy. Int J Radiat Oncol Biol Phys. 2012;84:707–711. doi: 10.1016/j.ijrobp.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 13.McKenna D.A., Coakley F.V., Westphalen A.C. Prostate cancer: Role of pretreatment MR in predicting outcome after external-beam radiation therapy—initial experience. Radiology. 2008;247:141–146. doi: 10.1148/radiol.2471061982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joseph T., McKenna D.A., Westphalen A.C. Pretreatment endorectal magnetic resonance imaging and magnetic resonance spectroscopic imaging features of prostate cancer as predictors of response to external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:665–671. doi: 10.1016/j.ijrobp.2008.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchsjäger M.H., Pucar D., Zelefsky M.J. Predicting post-external beam radiation therapy PSA relapse of prostate cancer using pretreatment MRI. Int J Radiat Oncol Biol Phys. 2010;78:743–750. doi: 10.1016/j.ijrobp.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westphalen A.C., Koff W.J., Coakley F.V. Prostate cancer: Prediction of biochemical failure after external-beam radiation therapy—Kattan nomogram and endorectal MR imaging estimation of tumor volume. Radiology. 2011;261:477–486. doi: 10.1148/radiol.11110457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sommer C.A., Stitzenberg K.B., Tolleson-Rinehart S., Carpenter W.R., Carey T.S. Breast MRI utilization in older patients with newly diagnosed breast cancer. J Surg Res. 2011;170:77–83. doi: 10.1016/j.jss.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimou A., Syrigos K.N., Saif M.W. Disparities in colorectal cancer in African-Americans vs Whites: Before and after diagnosis. World J Gastroenterol. 2009;15:3734–3743. doi: 10.3748/wjg.15.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farjah F., Flum D.R., Ramsey S.D., Heagerty P.J., Symons R.G., Wood D.E. Multi-modality mediastinal staging for lung cancer among medicare beneficiaries. J Thorac Oncol. 2009;4:355–363. doi: 10.1097/JTO.0b013e318197f4d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Healthcare Quality & Disparities Reports. June 2015. Agency for Healthcare Research and Quality, Rockville, MD. Available at: http://www.ahrq.gov/research/findings/nhqrdr/index.html. Accessed January 4, 2016.
- 21.Krieger N., Chen J.T., Waterman P.D., Soobader M.J., Subramanian S.V., Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: Does the choice of area-based measure and geographic level matter? The Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 22.D'Amico A.V., Whittington R., Malkowicz S. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 23.NCCN Guidelines Version 1. 2016 Prostate Cancer. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 4, 2016.
- 24.ACR Appropriateness Criteria® prostate cancer—pretreatment detection, staging and surveillance. Available at: http://www.guideline.gov/content.aspx?id=43879. Accessed January 4, 2016. [DOI] [PubMed]
- 25.Taneja S.S. Re: prostate cancer imaging trends after a nationwide effort to discourage inappropriate prostate cancer imaging. J Urol. 2014;191:1287–1288. doi: 10.1016/j.juro.2014.02.081. [DOI] [PubMed] [Google Scholar]
- 26.Makarov D.V., Hu E.Y., Walter D. Appropriateness of prostate cancer imaging among veterans in a delivery system without incentives for overutilization. Health Serv Res. 2016;51:1021–1051. doi: 10.1111/1475-6773.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjurlin M.A., Rosenkrantz A.B., Beltran L.S., Raad R.A., Taneja S.S. Imaging and evaluation of patients with high-risk prostate cancer. Nat Rev Urol. 2015;12:617–628. doi: 10.1038/nrurol.2015.242. [DOI] [PubMed] [Google Scholar]
- 28.Shukla-dave A., Hricak H., Akin O. Preoperative nomograms incorporating magnetic resonance imaging and spectroscopy for prediction of insignificant prostate cancer. BJU Int. 2012;109:1315–1322. doi: 10.1111/j.1464-410X.2011.10612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falchook A.D., Hendrix L.H., Chen R.C. Guideline-discordant use of imaging during work-up of newly diagnosed prostate cancer. J Oncol Pract. 2015;11:e239–e246. doi: 10.1200/JOP.2014.001818. [DOI] [PubMed] [Google Scholar]
- 30.Gould M.K., Schultz E.M., Wagner T.H. Disparities in lung cancer staging with positron emission tomography in the Cancer Care Outcomes Research and Surveillance (CanCORS) study. J Thorac Oncol. 2011;6:875–883. doi: 10.1097/JTO.0b013e31821671b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roach M., 3rd, Lu J., Pilepich M.V., Asbell S.O., Mohiuddin M., Grignon D. Race and survival of men treated for prostate cancer on radiation therapy oncology group phase III randomized trials. J Urol. 2003;169:245–250. doi: 10.1016/S0022-5347(05)64078-5. [DOI] [PubMed] [Google Scholar]
- 32.McWilliams J.M. Health consequences of uninsurance among adults in the United States: Recent evidence and implications. Milbank Q. 2009;87:443–494. doi: 10.1111/j.1468-0009.2009.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mcdavid K., Tucker T.C., Sloggett A., Coleman M.P. Cancer survival in Kentucky and health insurance coverage. Arch Intern Med. 2003;163:2135–2144. doi: 10.1001/archinte.163.18.2135. [DOI] [PubMed] [Google Scholar]
- 34.Meyers D.S., Mishori R., McCann J., Delgado J., O'Malley A.S., Fryer E. Primary care physicians' perceptions of the effect of insurance status on clinical decision making. Ann Fam Med. 2006;4:399–402. doi: 10.1370/afm.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mort E.A., Edwards J.N., Emmons D.W., Convery K., Blumenthal D. Physician response to patient insurance status in ambulatory care clinical decision-making. Implications for quality of care. Med Care. 1996;34:783–797. doi: 10.1097/00005650-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Amini A., Rusthoven C.G., Waxweiler T.V. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309–316. doi: 10.1016/j.jaad.2015.09.054. [DOI] [PubMed] [Google Scholar]
- 37.Fossati N., Nguyen D.P., Trinh Q.D. The Impact of insurance status on tumor characteristics and treatment selection in contemporary patients with prostate cancer. J Natl Compr Canc Netw. 2015;13:1351–1358. doi: 10.6004/jnccn.2015.0164. [DOI] [PubMed] [Google Scholar]