Abstract
Purpose
The most effective treatments in elderly patients with esophageal cancer remain a subject of debate. This multicenter phase 2 study was designed to evaluate the efficacy and toxicity of chemoradiation therapy (CRT) with docetaxel (DTX) in elderly patients with stage II/III (non-T4) esophageal cancer.
Methods and materials
Patients ≥70 years of age with clinical stage II/III esophageal cancer received DTX at a weekly dose of 10 mg/m2 during 6 consecutive weeks and concurrent radiation therapy (60 Gy in 30 fractions). The primary endpoint was the 2-year survival rate, and the required number of enrolled patients was 37.
Results
Between July 2008 and January 2011, 16 patients were enrolled. The study was prematurely closed because of slow accrual. Characteristics of the patients were as follows: median age, 77 years (range, 73-81); performance status 0/1, 4/12; and clinical stage IIA/IIB/III, 3/4/9. Of the 16 patients, 14 (87.5%) completed the CRT. The 2-year survival rate was 62.5% (90% confidence interval [CI], 42.5-82.5). The median survival time was 27.7 months (95% CI, 23.3-32.2 months) and the median progression-free survival was 15.2 months (95% CI, 5.4-25.0 months). Seven patients achieved complete response, resulting in a complete response rate of 43.8% (95% CI, 19.8-70.1). Grade 3 or higher acute toxicities included esophagitis (31.3%), anorexia (12.5%), leukopenia (6.3%), neutropenia (6.3%), thrombocytopenia (6.3%), mucositis (6.3%), and infection (6.3%). Grade 3 or higher late toxicities included esophagitis (12.5%), pleural effusion (12.5%), pneumonitis (6.3%), and pericardial effusion (6.3%).
Conclusions
CRT with DTX might be a treatment option for elderly patients with stage II/III esophageal cancer, particularly for patients who are medically unfit for surgery or cisplatin-containing therapy. However, further improvements of this therapy are required to decrease the incidence of esophagitis.
Introduction
Esophageal cancer is the sixth most common cause of cancer-related mortality worldwide.1 In Japan, esophageal cancer was responsible for 11,182 deaths in 2005, accounting for 3.4% of the total cancer death in the country. Of the 16,323 esophageal cancer cases in 2001 in Japan, 7585 (46.5%) were elderly patients older than age 70 years.2 Surgery is still the mainstay treatment for resectable esophageal cancer; however, because the indications for radical surgery in elderly patients are not well-defined,3 these patients are less likely to undergo surgery.4 Moreover, outcomes after esophagectomy in elderly patients are controversial.5, 6, 7 Chemoradiation therapy (CRT) is also a standard treatment for patients with localized esophageal cancer. The Radiation Therapy Oncology Group (RTOG) 85-01 trial demonstrated the superiority of CRT with fluorouracil (5-FU) and cisplatin (CDDP) over radiation therapy alone in patients with T1-3 N0-1 M0 esophageal cancer.8 In Japan, a phase 2 trial for evaluating CRT with 5-FU and CDDP for clinical stage II/III (non-T4) esophageal cancer showed promising efficacy with a complete response (CR) rate of 62.2% and 3-year survival rate of 44.7% (9). The 2-year survival rates were 36% in the CRT arm of the RTOG 85-01 trial and 52.6% in the Japanese trial, respectively. However, 23% of patients were older than 70 years in the RTOG 85-01 trial, and the eligibility for the Japanese trial had an age range of 20 to 70 years. A retrospective comparison of the outcomes of CRT with 5-FU and CDDP between elderly and nonelderly patients with stage II/III (non-T4) esophageal cancer demonstrated that elderly patients showed higher frequency of hematologic adverse events, poor compliance, and significantly inferior survival. The 2-year survival rate was 45% in elderly patients (≥71 years of age) compared with 55% in nonelderly patients (<70 years of age) (10). Another retrospective study concluded that elderly patients experienced substantial morbidity from CRT and poor long-term survival.9 Thus, the most effective treatment modalities in elderly patients with esophageal cancer remain a subject of debate, and new treatment options with lower toxicity and higher efficacy must be developed.
Elderly patients sometimes show decreased renal function or poor nutrition and are not considered suitable for CDDP-containing therapy. Therefore, nonplatinum-based CRT could be an optional regimen for elderly patients. Anderson et al reported good results in 25 elderly patients (≥65 years of age) with esophageal cancer treated with 5-FU, mitomycin-C, and radiation therapy. The 2-year survival rate was 64% (95% confidence interval [CI], 45-83).10 Iyer et al evaluated erlotinib combined with radiation therapy in 17 elderly patients (>65 years of age) with esophageal cancer. Although this treatment was tolerable, the 1-year survival rate was 29% (95% CI, 11-51).11
Docetaxel (DTX) is an active agent for advanced esophageal cancer.12, 13 In addition to cytotoxic activity, DTX has a radiosensitizing activity through its ability to induce cell cycle blockade in G2-M.14, 15 Promising activity and manageable toxicity of CRT with DTX were reported in non-small cell lung cancer and head and neck cancer.16, 17, 18, 19 In a previous phase 1 study in Japan, CRT with weekly DTX was tolerable and effective in patients with localized esophageal cancer, and the recommended dose was determined to be DTX 10 mg/m2. CRT with DTX was expected to be a new treatment option in elderly patients who were medically unfit for surgery or CDDP-containing therapy; therefore, we conducted a multicenter phase 2 study of CRT with DTX in elderly patients to evaluate efficacy and toxicity.
Methods and materials
Patients
For this phase 2 trial, patients were recruited from 9 hospitals in Japan. Patients ≥70 years of age with histologically proven squamous cell carcinoma, adenocarcinoma, or adenosquamous cell carcinoma of the thoracic esophagus who were not considered suitable for CDDP-containing therapy were included. Clinical stage II/III tumors, excluding T4 tumors according to the International Union Against Cancer, 6th edition, were eligible. Staging was performed by computed tomography (CT) and endoscopic ultrasonography. Patients were required to be Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1, meet the following laboratory criteria within 14 days before registration: white blood cells ≥3000/mm3; platelet count ≥10×104/mm3; hemoglobin level ≥8.0 g/dL; aspartate aminotransferase and alanine aminotransferase ≤100 IU/L; total bilirubin ≤1.5 mg/dL; serum creatinine ≤2.0 mg/dL; and oxygen saturation ≥93%. Exclusion criteria were a history or current presence of other malignancies, previous chemotherapy, or radiation therapy. Written informed consent was obtained from all patients. The study was undertaken in accordance with the protocol and approved by the institutional review boards of the participating institutions.
Chemotherapy
All patients received DTX at a dose of 10 mg/m2 weekly during 6 consecutive weeks. DTX was administered through a 1-hour intravenous infusion on day 1 of each cycle.
The Common Terminology Criteria for Adverse Events (CTCAE), version 3.0, was used for evaluating toxicity. Chemotherapy was delayed because of toxicities until recovery to the following conditions: absolute neutrophil count ≥1000/mm3; platelet count ≥7.5×104/mm3; aspartate aminotransferase and alanine aminotransferase ≤ 100 IU/L; total bilirubin ≤2.5 mg/dL; serum creatinine ≤2.5 mg/dL; and grade 2 or lower nausea, vomiting, anorexia, esophagitis, diarrhea, or radiation pneumonitis.
Radiation therapy
Radiation therapy was delivered using megavoltage (≥6 MV) radiographs, with 2 Gy daily and 5 fractions per week, on the first day of chemotherapy. The total dose was set at 60 Gy in 30 fractions. Three-dimensional treatment planning was required. Target volumes were determined according to the images acquired from the CT. Primary tumor and metastatic lymph nodes were included in the gross tumor volume. The clinical target volume provided a 2-cm craniocaudal margin for the primary tumor. Planning target volume was defined as clinical target volume plus a 1 to 2 cm margin in the craniocaudal direction and a 0.5 to 1 cm margin in the lateral direction. Elective nodal irradiation was not performed. Tissue density inhomogeneity correction was used.
Assessment
Tumor response was assessed by CT and esophagogastroduodenoscopy (EGD), according to Response Evaluation Criteria in Solid Tumors, version 1.0.20 CT and EGD were carried out 4 weeks after treatment completion and 4, 6, 9, and 12 months after the beginning of the treatment in the first year. In the second and third years, follow-up examinations were conducted every 3 months and every 6 months thereafter. Primary tumor response was evaluated by EGD using the modified criteria of the 10th edition of Guidelines for Diagnosis and Treatment of Carcinoma of the Esophagus, issued by the Japanese Society for Esophageal Diseases.21 CR of the primary tumor was defined as the disappearance of the primary tumor lesion, ulceration, erosion, and the absence of malignant cells in biopsy specimens. CR of lymph node metastases was defined as the disappearance of all visible lymph node metastases on CT imaging. Overall CR was declared when CR at both the primary tumor and the lymph node was obtained without the appearance of a new lesion. Overall CR was confirmed by reassessment 4 or more weeks after the first assessment.
Acute toxicities were assessed weekly during CRT and 4 weeks after completion of CRT. Late toxicity was defined as an adverse event occurred more than 90 days after CRT initiation. Toxicities were evaluated based on the CTCAE, version 3.0.
Statistical methods
The primary endpoint of this phase 2 trial was the 2-year survival rate. Overall survival was defined as the time from the date of registration to that of death from any cause, and it was censored at the date of the last follow-up for survivors. The 2-year survival rate was estimated using the Kaplan-Meier method. A retrospective study showed the 2-year survival rate of 45% in elderly patients (≥71 years of age) treated with CRT with 5-FU and CDDP. We calculated the sample size expecting a 2-year survival rate of 50%, with a 30% threshold. With a power of 80% and 1-sided 5% significance, the required number of enrolled patients was 37. We finally planned to enroll 40 patients in total, including ineligible patients.
Secondary endpoints were progression-free survival (PFS), CR rate, and acute and late toxicities. PFS was defined as the time from the date of registration to that of disease progression or death from any cause, and it was censored at the date of the last visit for patients without progression.
Survival was estimated using Kaplan-Meier method. The 2-year survival rate, overall survival, PFS, and CR rate were calculated according to an intention-to-treat population. Statistical analyses were performed using R (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
Between July 2008 and January 2011, 16 patients were enrolled. The study was prematurely closed because of slow accrual. Baseline characteristics are summarized in Table 1. The median age was 77 years (range, 73-81). Four (25.0%) and 12 (75.0%) patients showed ECOG performance status of 0 and 1, respectively. Most patients (15 of 16 [93.8%]) had squamous cell carcinoma, whereas the remaining patient had adenocarcinoma. The clinical stages (International Union Against Cancer, 6th edition) were IIA for 3 patients (18.8%), IIB for 4 patients (25.0%), and III for 9 patients (56.3%).
Table 1.
Patient characteristics
| Characteristics | Number of patients (n = 16) | Percentage (%) |
|---|---|---|
| Age (y) | ||
| Median | 77 | |
| Range | 73-81 | |
| Sex | ||
| Male | 14 | 87.5 |
| Female | 2 | 12.5 |
| ECOG PS | ||
| 0 | 4 | 25.0 |
| 1 | 12 | 75.0 |
| Histology | ||
| Squamous cell carcinoma | 15 | 93.8 |
| Adenocarcinoma | 1 | 6.3 |
| Tumor location | ||
| Upper thorax | 2 | 12.5 |
| Middle thorax | 9 | 56.3 |
| Lower thorax | 5 | 31.3 |
| T factor | ||
| T1 | 4 | 25.0 |
| T2 | 1 | 6.3 |
| T3 | 11 | 68.8 |
| N factor | ||
| N0 | 3 | 18.8 |
| N1 | 13 | 81.3 |
| Stage (UICC 6th edition) | ||
| IIA | 3 | 18.8 |
| IIB | 4 | 25.0 |
| III | 9 | 56.3 |
| CCr (mL/min) | ||
| ≥60 | 4 | 25.0 |
| 50-60 | 6 | 37.5 |
| <50 | 6 | 37.5 |
CCr, creatinine clearance; ECOG PS = Eastern Cooperative Oncology Group performance status; UICC, International Union Against Cancer.
Treatment
Of the 16 enrolled patients, 14 (87.5%) completed the CRT according to the protocol. Two patients discontinued protocol treatment because of grade 3 esophagitis (n = 1) and patient refusal not related to any adverse event (n = 1). The patient who developed grade 3 esophagitis did not receive the last cycle of chemotherapy and 4 Gy of radiation therapy. The other patient received one cycle of chemotherapy and 10 Gy of radiation therapy before refusing to continue the treatment. After the evaluation of CRT, 2 patients among the non-CR cases received additional chemotherapy with DTX.
Efficacy
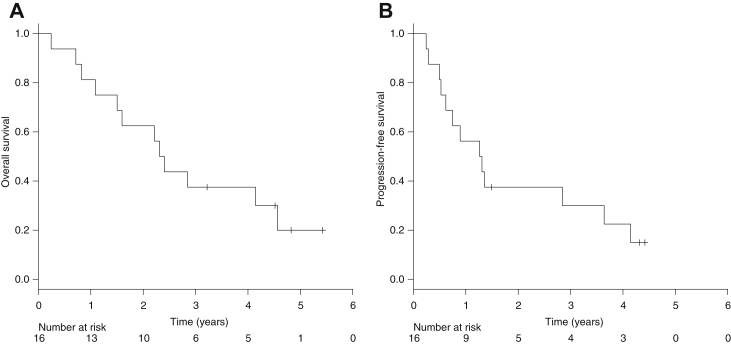
As of August 31, 2014, the median follow-up was 57.9 months. Twelve of 16 patients died at the time of analysis; 9 patients (75.0%) died as a result of progressive or recurrent disease, 3 patients died from other cause. Seven patients achieved CR, resulting in a CR rate of 43.8% (95% confidence interval [CI], 19.8-70.1). The median PFS was 15.2 months (95% CI, 5.4-25.0 months), and the median survival time was 27.7 months (95% CI, 23.3-32.2 months), respectively. The 2-year survival rate was 62.5% (90% CI, 42.5-82.5) (Fig 1).
Figure 1.
(A) Overall survival and (B) progression-free survival.
Toxicity
No treatment-related deaths were observed in any of the 16 patients. Toxicities occurring during CRT are shown in Table 2. Grade 3 or higher acute toxicities included esophagitis (31.3%), anorexia (12.5%), leukopenia (6.3%), neutropenia (6.3%), thrombocytopenia (6.3%), mucositis (6.3%), and infection (6.3%). Table 3 shows late toxicities associated with CRT. Grade 3 or higher late toxicities included esophagitis (12.5%), pleural effusion (12.5%), pneumonitis (6.3%), and pericardial effusion (6.3%).
Table 2.
Adverse events during CRT (CTCAE, version 3.0)
| Toxicity | Number of patients (n = 16) |
≥Grade 3 (%) | |||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Leukopenia | 7 | 3 | 1 | 0 | 6.3 |
| Neutropenia | 1 | 1 | 0 | 1 | 6.3 |
| Anemia | 13 | 1 | 0 | 0 | 0 |
| Thrombocytopenia | 9 | 0 | 1 | 0 | 6.3 |
| Creatinine | 5 | 2 | 0 | 0 | 0 |
| Anorexia | 4 | 6 | 2 | 0 | 12.5 |
| Nausea | 6 | 1 | 0 | 0 | 0 |
| Vomiting | 4 | 2 | 0 | 0 | 0 |
| Mucositis | 0 | 0 | 1 | 0 | 6.3 |
| Esophagitis | 3 | 6 | 5 | 0 | 31.3 |
| Diarrhea | 2 | 0 | 0 | 0 | 0 |
| Infection | 0 | 0 | 1 | 0 | 6.3 |
| Pneumonitis | 4 | 1 | 0 | 0 | 0 |
CRT, chemoradiation therapy; CTCAE, Common Terminology Criteria for Adverse Events.
Table 3.
Late adverse events (CTCAE, version 3.0)
| Toxicity | Number of patients (n = 16) |
≥Grade 3 (%) | |||
|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | ||
| Esophagitis | 4 | 4 | 2 | 0 | 12.5 |
| Pleural effusion | 4 | 0 | 2 | 0 | 12.5 |
| Pneumonitis | 10 | 1 | 1 | 0 | 6.3 |
| Pericardial effusion | 3 | 0 | 1 | 0 | 6.3 |
CTCAE, Common Terminology Criteria for Adverse Events.
Salvage or second-line treatment
The pattern of failure is shown in Table 4. Eight patients had residual or progressive disease: 5 patients had residual or locoregional progressive disease only and 3 patients had distant metastasis. Two patients had locoregional recurrence after CR. Five patients who had residual or locoregional progressive disease received the following treatments: CRT with DTX in 3 patients, chemotherapy with 5-FU and CDDP in 1 patient, and best supportive care in 1 patient. Of the 3 patients with distant metastasis, 2 received palliative radiation therapy and 1 received chemotherapy with 5-FU and nedaplatin. Of the 2 patients with recurrent disease, 1 underwent salvage lymph node dissection and 1 received best supportive care.
Table 4.
Pattern of failure
| Pattern | Number of patients (n = 16) | Percentage |
|---|---|---|
| Alive without failure | 3 | 18.8 |
| Not examined | 1 | 6.3 |
| Any failure | 12 | 75.0 |
| Dead by other cause after CR | 2 | 12.5 |
| Residual or PD | 8 | 50.0 |
| Locoregional | 5 | 31.3 |
| Distant | 3 | 18.8 |
| Recurrence after CR | 2 | 12.5 |
| Locoregional | 2 | 12.5 |
| Distant | 0 | 0 |
CR, complete response; PD, progressive disease.
Discussion
The present study was designed to evaluate the efficacy and toxicity of CRT with DTX in elderly patients by enrolling 37 eligible patients during 2 years. However, the slow accrual led to premature closure, with only 16 patients enrolled. The main reason for this was that elderly and frail patients were not usually willing to receive the investigational treatment.
In our study, CRT with DTX in elderly patients with stage II/III esophageal cancer produced a 2-year survival rate of 62.5% (90% CI, 42.5-82.5), CR rate of 43.8% (95% CI, 19.8-70.1), and median survival time of 27.7 months (95% CI, 23.3-32.2 months). A retrospective study showed a 2-year survival rate of 45% in elderly patients treated with CRT with 5-FU and CDDP; thus, in this study, the expected 2-year survival rate was defined to be 50%, with a threshold of 30%. In spite of the immature patient accrual, the lower limit of 90% CI for the 2-year survival rate exceeded the threshold. Moreover, the 2-year survival rate was higher compared with previous studies that did not target elderly patients. The 2-year survival rates in the radiation therapy alone arm, CRT arm of the RTOG 85-01 trial, and the Japanese phase 2 trial of CRT with 5-FU and CDDP were 10%, 36%, and 52.6%, respectively.8, 22 Though we should pay attention to selection bias, CRT with DTX is expected to have a similar efficacy as CRT with 5-FU and CDDP, at least greater than radiation therapy alone, in elderly patients. On the other hand, the CR rate was lower compared with previous studies, probably because the rate of acute and late esophagitis was higher in this study, complicating the evaluation of a CR of the primary lesion. For CR evaluation, we used the criteria of the Japan Society of Esophageal Disease, which state the disappearance of esophagitis as a requirement to confirm CR.21 Actually, of 9 non-CR or not examined patients, 3 were not confirmed CR because of esophagitis with CR of lymph node metastases on CT imaging. Thus, the CR rate of this study might have been underestimated.
CRT with carboplatin and paclitaxel, another platinum-based regimen, have been used widely since the result of the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS) trial was reported.23 Because of its favorable tolerance, this regimen also seems to be suitable for elderly patients. However, the eligibility of the CROSS trial was younger than 75 years old, and the median age was 60 years. Moreover, carboplatin is not approved for esophageal cancer in Japan.
The major treatment-related toxicity was esophagitis. Although hematologic or other nonhematologic toxicities were not frequently observed, the rates of esophagitis in both acute and late toxicities were higher compared with previous studies. Furthermore, grade 3 or higher esophagitis was reported as acute (31.3%) or late (12.5%) adverse events, probably because elderly patients tend to develop esophagitis or the combination of DTX and radiation therapy is highly toxic for the esophageal mucosa. In a retrospective study, esophagitis was relatively more common in elderly than in nonelderly patients.24 For the latter, previous studies of CRT with DTX in non-small cell lung cancer or head and neck cancer reported high frequency of esophagitis or mucositis.16, 17, 18, 19 It is generally considered that a reduction in the total radiation dose may decrease the incidence of esophagitis. Because in Japan a radiation dose of 60 Gy has been used in definitive CRT for esophageal cancer, the present study was performed with a total radiation dose of 60 Gy. However, the Intergroup 0123 trial demonstrated that a higher irradiation dose of 64.8 Gy offered no advantage in terms of survival and local control compared with the standard dose of 50.4 Gy.25 In addition to this, the Kitasato Digestive Disease and Oncology Group 0501-P2 trial, a phase 2 trial for evaluating CRT with DTX, CDDP, and 5-FU for advanced esophageal cancer with T4 or M1 lymphoma, showed that the incidence of grade 3 or higher acute esophagitis decreased (41.6% to 23.3%) and the CR rate improved (33.3% to 60.0%), by reducing the total radiation dose from 61.2 Gy to 50.4 Gy.26 Therefore, a total radiation dose of 50.4 Gy can be recommended for CRT with DTX.
A major limitation of this study is the small sample size. In addition, there might be patient selection biases because patients in relatively good conditions were enrolled, thus leading to longer survival rates. Although not planned, most patients had squamous cell carcinoma, with only 1 patient having adenocarcinoma.
Even in elderly patients, standard treatments for localized esophageal cancer are surgery and CRT with 5-FU and CDDP; therefore, CRT with DTX seems to be an optional treatment, particularly in patients who are medically unfit for surgery or CDDP-containing therapy. To validate the benefit of this regimen, a prospective randomized trial of CRT with DTX versus radiation therapy alone will be required in the future.
Conclusions
In conclusion, CRT with DTX might be a treatment option for elderly patients with stage II/III esophageal cancer, particularly for patients who are medically unfit for surgery or CDDP-containing therapy. However, further improvements of the treatment are required to decrease the incidence of esophagitis.
Footnotes
Conflicts of interest: None.
References
- 1.Parkin D.M., Bray F., Ferlay J. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.The Editorial Board of the Cancer Statistics in Japan. Cancer Statistics in Japan 2007 Foundation for Promotion of Cancer Research.
- 3.Bonavina L., Incarbone R., Saino G. Clinical outcome and survival after esophagectomy for carcinoma in elderly patients. Dis Esophagus. 2003;16:90–93. doi: 10.1046/j.1442-2050.2003.00300.x. [DOI] [PubMed] [Google Scholar]
- 4.Steyerberg E.W., Neville B., Weeks J.C. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: A population-based analysis of elderly patients. J Clin Oncol. 2007;25:2389–2396. doi: 10.1200/JCO.2006.09.7931. [DOI] [PubMed] [Google Scholar]
- 5.Kinugasa S., Tachibana M., Yoshimura H. Esophageal resection in elderly esophageal carcinoma patients: Improvement in postoperative complications. Ann Thorac Surg. 2001;71:414–418. doi: 10.1016/s0003-4975(00)02333-x. [DOI] [PubMed] [Google Scholar]
- 6.Sabel M.S., Smith J.L., Nava H.R. Esophageal resection for carcinoma in patients older than 70 years. Ann Surg Oncol. 2002;9:210–214. doi: 10.1007/BF02557376. [DOI] [PubMed] [Google Scholar]
- 7.Law S., Wong K.-H., Kwok K.-F. Predictive factors for postoperative pulmonary complications and mortality after esophagectomy for cancer. Ann Surg. 2004;240:791–800. doi: 10.1097/01.sla.0000143123.24556.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper J.S., Guo M.D., Herskovic A. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 9.Mak R.H., Mamon H.J., Ryan D.P. Toxicity and outcomes after chemoradiation for esophageal cancer in patients age 75 or older. Dis Esophagus. 2010;23:316–323. doi: 10.1111/j.1442-2050.2009.01014.x. [DOI] [PubMed] [Google Scholar]
- 10.Anderson S.E., Minsky B.D., Bains M. Combined modality chemoradiation in elderly oesophageal cancer patients. Br J Cancer. 2007;96:1823–1827. doi: 10.1038/sj.bjc.6603821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer R., Chhatrala R., Shefter T. Erlotinib and radiation therapy for elderly patients with esophageal cancer - clinical and correlative results from a prospective multicenter phase 2 trial. Oncology. 2013;85:53–58. doi: 10.1159/000351617. [DOI] [PubMed] [Google Scholar]
- 12.Einzig A.I., Neuberg D., Remick S.C. Phase II trial of docetaxel (Taxotere) in patients with adenocarcinoma of the upper gastrointestinal tract previously untreated with cytotoxic chemotherapy: The Eastern Cooperative Oncology Group (ECOG) results of protocol E1293. Med Oncol. 1996;13:87–93. doi: 10.1007/BF02993858. [DOI] [PubMed] [Google Scholar]
- 13.Rigas J.R., Dragnev K.H., Bubis J.A. Docetaxel in the treatment of esophageal cancer. Semin Oncol. 2005;32:S39–S51. doi: 10.1053/j.seminoncol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Hennequin C., Giocanti N., Favaudon V. Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res. 1996;56:1842–1850. [PubMed] [Google Scholar]
- 15.Dunne A.L., Mothersill C., Robson T. Radiosensitization of colon cancer cell lines by docetaxel: Mechanisms of action. Oncol Res. 2004;14:447–454. doi: 10.3727/0965040041791455. [DOI] [PubMed] [Google Scholar]
- 16.Mauer A.M., Masters G.A., Haraf D.J. Phase I study of docetaxel with concomitant thoracic radiation therapy. J Clin Oncol. 1998;16:159–164. doi: 10.1200/JCO.1998.16.1.159. [DOI] [PubMed] [Google Scholar]
- 17.Koukourakis M.I., Kourousis C., Kamilaki M. Weekly docetaxel and concomitant boost radiotherapy for non-small cell lung cancer. A phase I/II dose escalation trial. Eur J Cancer. 1998;34:838–844. doi: 10.1016/s0959-8049(97)10101-0. [DOI] [PubMed] [Google Scholar]
- 18.Calais G., Bardet E., Sire C. Radiotherapy with concomitant weekly docetaxel for stages III/IV oropharynx carcinoma. Results of the 98-02 GORTEC phase II trial. Int J Radiat Oncol Biol Phys. 2004;58:161–166. doi: 10.1016/s0360-3016(03)01370-1. [DOI] [PubMed] [Google Scholar]
- 19.Fujii M., Tsukuda M., Satake B. Phase I/II trial of weekly docetaxel and concomitant radiotherapy for squamous cell carcinoma of the head and neck. Int J Clin Oncol. 2004;9:107–112. doi: 10.1007/s10147-003-0375-z. [DOI] [PubMed] [Google Scholar]
- 20.Therasse P., Arbuck S.G., Eisenhauer E.A. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 21.Kuwano H., Nishimura Y., Ohtsu A. Guidelines for diagnosis and treatment of carcinoma of the esophagus; April 2007 edition: Part I. Edited by the Japan Esophageal Society. Esophagus. 2008;5:61–73. doi: 10.1007/s10388-014-0465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kato K., Muro K., Minashi K. Phase II study of chemoradiotherapy with 5-fluorouracil and cisplatin for stage II-III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906) Int J Radiat Oncol Biol Phys. 2011;81:684–690. doi: 10.1016/j.ijrobp.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 23.van Hagen P., Hulshof M.C.C.M., van Lanschot J.J.B. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 24.Takeuchi S., Ohtsu A., Doi T. A retrospective study of definitive chemoradiotherapy for elderly patients with esophageal cancer. Am J Clin Oncol. 2007;30:607–611. doi: 10.1097/COC.0b013e3180ca7c84. [DOI] [PubMed] [Google Scholar]
- 25.Minsky B.D., Pajak T.F., Ginsberg R.J. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–1174. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 26.Higuchi K., Komori S., Tanabe S. Definitive chemoradiation therapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) in advanced esophageal cancer: A phase 2 trial (KDOG 0501-P2) Int J Radiat Oncol Biol Phys. 2014;89:872–879. doi: 10.1016/j.ijrobp.2014.03.030. [DOI] [PubMed] [Google Scholar]