Abstract
Purpose
Many patients treated with stereotactic radiosurgery (SRS) alone as initial treatment require 1 or more subsequent salvage therapies. This study aimed to determine if commonly used salvage strategies are associated with differing risks of radiation necrosis (RN).
Methods and materials
All patients treated with upfront SRS alone for brain metastases at our institution were retrospectively analyzed. Salvage treatment details were obtained for brain failures. Patients who underwent repeat SRS to the same lesion were excluded. RN was determined based on pathological confirmation or advanced brain imaging consistent with RN in a symptomatic patient. Patients were grouped according to salvage treatment and rates of RN were compared via Fisher's exact tests.
Results
Of 284 patients treated with upfront SRS alone, 132 received salvage therapy and 44 received multiple salvage treatments. This included 31 repeat SRS alone, 58 whole brain radiation therapy (WBRT) alone, 28 SRS and WBRT, 7 surgery alone, and 8 surgery with adjuvant radiation. With a median follow-up of 10 months, the rate of RN among all patients was 3.17% (9/284), salvaged patients 4.55% (6/132), and never salvaged patients 1.97% (3/152). Receiving salvage therapy did not significantly increase RN risk (P = .31). Of the patients requiring salvage treatments, the highest RN rate was among patients that had both salvage SRS and WBRT (delivered as separate salvage therapies) (6/28, 21.42%). RN rate in this group was significantly higher than in those treated with repeat SRS alone (0/31), WBRT alone (0/58), surgery alone (0/7), and surgery with adjuvant radiation (0/8). Comparing salvage WBRT doses <30 Gy versus ≥30 Gy revealed no effect of dose on RN rate. Additionally, among patients who received multiple SRS treatments, number of treated lesions was not predictive of RN incidence.
Conclusion
Our results suggest that initial management approach for recurrent brain metastasis after upfront SRS does not affect the rate of RN. However, the risk of RN significantly increases when patients are treated with both repeat SRS and salvage WBRT. Methods to improve prediction of toxicity and optimize patient selection for salvage treatments are needed.
Introduction and rationale
Up to 40% of cancer patients develop brain metastases each year in the United States, causing significant morbidity and mortality.1 Much of the current literature focuses on treatment approaches for brain metastases at initial diagnosis. For many patients stereotactic radiosurgery (SRS) alone, deferring whole brain radiation therapy (WBRT), has become a preferred strategy for small (<4 cm) metastases and relatively favorable life expectancies.2, 3, 4
Phase 3 trials of SRS with or without WBRT for 1 to 3 metastases demonstrated that after SRS alone, local and distant brain failures are approximately 10% to 30% and 40% to 70%, respectively.5, 6, 7 As systemic therapies improve extracranial disease control, the number of patients who develop new and recurrent brain metastases is likely to increase. Currently, there is no consensus on the optimal treatment for patients with recurrent metastases. Options often include supportive care, repeat SRS, surgery, WBRT, or a combination of these approaches. The evidence regarding the safety and efficacy of the various salvage treatments is very limited.8 The 2012 American Society for Radiation Oncology brain metastases guidelines did not put forth any recommendations for management of recurrent brain metastases and identified this topic as an area warranting further research.9
Given that many patients are treated with additional radiation therapy in the setting of recurrent brain metastases, it is important to consider the benefits of treatment against the risks. One of the most severe side effects of brain radiation therapy is radiation necrosis (RN). The aim of this study was to analyze current salvage practices with respect to risk of developing RN for patients with recurrent brain metastases after initial therapy with SRS. We hypothesized that salvage SRS and/or WBRT under current prescriptive paradigms do not significantly increase the rates of RN.
Methods and materials
With institutional review board approval, we retrospectively obtained data from electronic medical records of patients treated with SRS alone for primary management of brain metastases between 2001 and 2012. Variables collected included patient age, gender, race, Karnofsky performance status (KPS), tumor histology, extracranial disease burden, as well as number and largest diameter of both initial and recurrent brain metastases. Radiation Therapy Oncology Group (RTOG) Recursive Partitioning Analysis scores were calculated for each patient.10 Treatment data including radiation modality, prescription doses, and schedules were also obtained.
Initial and repeat SRS without planned WBRT was generally offered for <5 brain metastases, each <4 cm, in patients with KPS ≥70, and life expectancies ≥3 months. If additional occult metastases were found at the time of SRS, ≥5 lesions were sometimes treated with SRS with or without WBRT. Patients who received SRS to the same lesion multiple times were excluded given previously demonstrated elevated risk of RN.11 WBRT was not combined with initial or salvage SRS but was used as a separate salvage therapy. Although there was no strict standard for use of salvage WBRT, it was generally used for patients with >4 active lesions, Eastern Cooperative Oncology Group performance scale ≥2, or predicted life expectancy of <3 months. Salvage surgery was typically reserved for patients with large (>4 cm) or severely symptomatic lesions.
SRS was performed using a Leksell Gamma Knife Model C (Elekta AB, Stockholm, Sweden). The target volume included the contrast-enhancing lesion with a 1 to 2 mm margin. Prescription doses were generally based on tumor size according to RTOG study 90-05.12 Prescription doses for salvage WBRT ranged from 20 to 37.5 Gy in 10 to 15 fractions.
Patients were seen in follow-up approximately 1 month after treatment and every 3 months thereafter. Magnetic resonance imaging scans were obtained at each scheduled visit. The primary outcome measure for this study was rate of RN. RN was either confirmed by pathology after surgical resection or defined as a symptomatic patient with magnetic resonance spectroscopy or perfusion results consistent with necrosis. Patients were grouped according to salvage treatment received. Potential variables affecting incidence of necrosis were compared via Fisher's exact tests. Two-sided P values <.05 were considered statistically significant.
Results
A total of 284 patients with 677 total brain metastases treated with initial SRS alone were included. The demographic data for these patients are presented in Table 1. No salvage treatments were delivered to 152 patients (53.5%), whereas 132 patients (46.5%) experienced in brain failure and received salvage therapy. Forty-four (15.5%) received multiple salvage treatments. Of the salvaged patients, 31 (23.5%) underwent 1 or more SRS treatments without WBRT, 58 (43.9%) WBRT alone, 28 (21.2%) SRS and WBRT delivered as separate salvage therapies, 7 (0.5%) underwent surgery alone, and 8 (0.6%) surgery with adjuvant radiation (5 adjuvant SRS, 3 adjuvant WBRT).
Table 1.
Patient demographics at initial SRS
| Variable | Category | All patients (N = 284) | Salvaged patients (N = 132) | Never salvaged patients (N = 152) |
|---|---|---|---|---|
| Sex | Male | 130 (46%) | 58 (44%) | 71 (47%) |
| Female | 154 (54%) | 74 (56%) | 81 (53%) | |
| Age at first SRS | Median | 61.6 | 59.7 | 63.5 |
| Histology | Non-small cell lung | 174 (61%) | 84 (64%) | 90 (59%) |
| Breast | 32 (12%) | 23 (17%) | 9 (6%) | |
| Melanoma | 20 (7%) | 8 (6%) | 12 (8%) | |
| Colon | 7 (2%) | 3 (2%) | 4 (3%) | |
| Other | 51 (18%) | 14 (11%) | 37 (24%) | |
| RPA class | 1 | 69 (25%) | 44 (33%) | 31 (20%) |
| 2 | 187 (66%) | 69 (52%) | 111 (73%) | |
| Unknown | 25 (9%) | 15 (11%) | 7 (5%) | |
| Number of lesions | Average | 3 | 3 | 2 |
| Range | 1-14 | 1-12 | 1-14 | |
| Maximum lesion size, cm3 | Average | 9.87 | 6.20 | 7.66 |
| Range | 0.1-93.29 | 0.27-93.29 | 0.27-64.45 | |
| Prescription dose (minimum dose delivered to largest met), Gy | Average | 18.63 | 18.37 | 18.91 |
| Range | 12-22 | 12-22 | 12-22 |
RPA, recursive partitioning analysis; SRS, stereotactic radiosurgery.
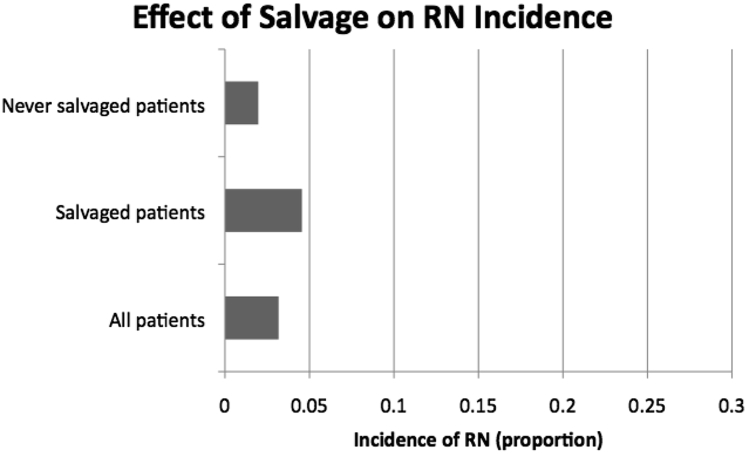
With a median follow-up of 10 months, the rate of RN among all patients was 3.17% (9/284), never salvaged patients 1.97% (3/152), and salvaged patients 4.55% (6/132). RN was confirmed by pathology in 7/9 (78%) of these patients, and suggested by symptomatology and magnetic resonance spectroscopy or perfusion in 2/9 (22%) patients. Receiving salvage therapy did not significantly increase risk of RN (P = .31). These data are depicted in Fig 1.
Figure 1.
Effect of salvage treatment on radiation necrosis (RN) incidence.
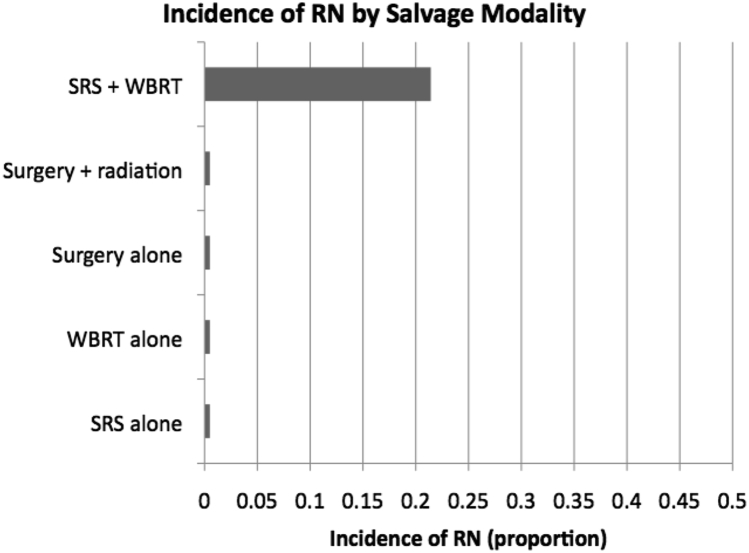
Subgroup analysis according to salvage approach revealed the highest rate of necrosis in patients that had both salvage SRS and salvage WBRT (6/28 or 21.42%). RN in this group was significantly more frequent than those receiving no salvage therapy (3/152, P < .001), salvage SRS alone (0/31, P < .001), and salvage WBRT alone (0/58, P < .001). The RN rate was not significantly greater than that in patients who underwent salvage surgery alone (0/7, P = .311), or salvage surgery with adjuvant radiation (0/8, P = .302), likely because of small sample sizes in these groups. RN incidence between patients that underwent salvage SRS alone and salvage WBRT alone was also not significantly different (P = 1.0). These data are graphed in Fig 2.
Figure 2.
Incidence of RN by salvage modality. RN, radiation necrosis; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
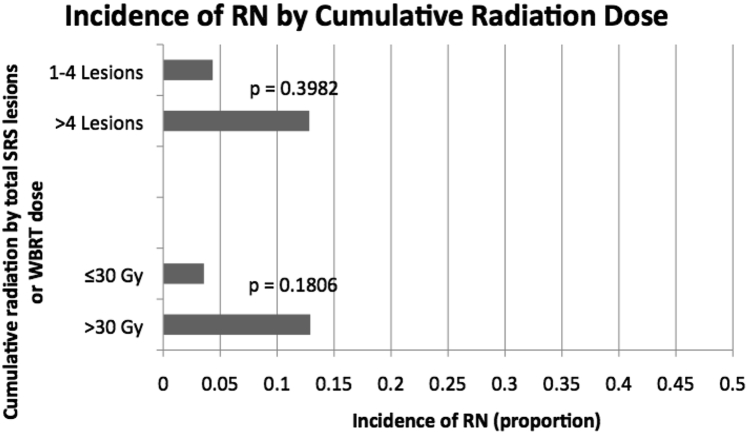
To assess whether dose of salvage WBRT contributed to rate of RN, patients that received doses of <30 Gy were compared with those that received ≥30 Gy. No significant effect of dose on incidence of RN was found (P = .181). Additionally, among patients who received multiple SRS treatments, number of treated lesions was not predictive of RN incidence (P = .3982). Size analysis in this population revealed no significant effect of volume of largest met <5 cm3 versus ≥5 cm3 on incidence of RN (P = .4168.) Patients who received SRS for >4 cumulative lesions had a risk of RN of 12.8%, whereas patients that were treated to ≤4 lesions had a risk of RN of 4.3%. These data are depicted in Fig 3. Primary and subgroup comparisons are given in Table 2.
Figure 3.
Risk of RN by cumulative dose. Abbreviations as in Fig 2.
Table 2.
Comparative analysis of salvage treatments and associations with risk of radiation necrosis
| Group | OR for RN with 95% CI | P value |
|---|---|---|
| Any salvage vs no salvage | 2.37 (0.56-9.65) | .31 |
| Salvage SRS vs any salvage | 2.32 (0.72-7.49) | .21 |
| Salvage WBRT vs any salvage | 1.59 (0.49-5.09) | .55 |
| Salvage surgery vs any salvage | 0.63 (0.03-11.69) | .76 |
| Any salvage vs salvage SRS + WBRT | 5.73 (1.69-19.38) | .0075 |
| Salvage SRS vs salvage SRS + salvage WBRT | 18.2 (0.98-339.79) | .05 |
| Salvage WBRT vs salvage SRS + salvage WBRT | 3.77 (1.11-12.85) | .04 |
| Salvage SRS alone vs salvage WBRT alone | 1.86 (0.034-95.86) | .31 |
| Subgroups | ||
| All salvage WBRT dose ≥30 vs <30 | 4 (0.69-23.23) | .18 |
| All SRS lesions >4 vs 1-4 | 3.24 (0.35-29.58) | .39 |
Bold font indicates P < .05.
CI, confidence interval; RN, radiation necrosis; OR, odds ratio; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
Discussion
Limited data exist to guide salvage treatment decisions for patients with recurrent brain metastases after initial management with SRS alone. Management is typically individualized and based on a variety of factors including patient KPS, extracranial disease status, prior treatment history, and recurrent lesion size, number, timing, and location.13 Given that many of these patients are treated with multiple courses of radiation therapy (ie, repeat SRS and/or salvage WBRT), risk of additional radiation-related toxicity must be considered.
The present study investigated risk of RN, the most common and serious late complication after SRS. RN is permanent brain injury that manifests 3 months to several years after treatment. Although the exact pathogenesis of RN is unknown, it is thought to involve release of pro-inflammatory cytokines, vascular damage, and direct toxicity to neuroglia.14, 15 In the absence of invasive pathological confirmation, RN is often difficult to distinguish from temporary adverse radiation effects (ARE) and tumor progression. Both RN and tumor recurrence can cause progressive clinical deterioration and death.16 Advanced magnetic resonance imaging sequences, magnetic resonance spectroscopy, and magnetic resonance perfusion are most commonly used to help differentiate RN from recurrent tumor.17, 18, 19 Our group defined RN by pathology after resection or symptomatic ARE with magnetic resonance spectroscopy and/or perfusion consistent with RN. With this definition, the incidence of RN in our entire cohort of SRS treated patients was 3%. This is consistent with other studies which have demonstrated RN risk of approximately 5% after SRS for brain metastases.20, 21
The incidence of RN is directly related to total radiation dose, volume, and fraction size.22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 RTOG 90-05 established that for patients who had previously received irradiation for primary brain tumors or brain metastases, maximum tolerated dose of single-fraction SRS was 24 Gy, 18 Gy, and 15 Gy for tumors ≤20 mm, 21 to 30 mm, and 31 to 40 mm in maximum diameter, respectively, with larger tumor diameter predictive of greater central nervous system (CNS) toxicity.12 Incidence of RN in RTOG 90-05 was 5% at 6 months after SRS, and increased incrementally with time thereafter, up to 11% at 24 months.33 In the setting of SRS without WBRT, the volumes of CNS tissue that receives 10 and 12 Gy have been consistently shown to correlate with risk of RN.23, 28, 34 In addition to 10- and 12-Gy volumes, Sneed et al identified capecitabine/fluorouracil use within 1 month and prior SRS to the same lesion as other independent predictors of ARE after SRS. For locally recurrent metastases treated with repeat SRS, the risk of symptomatic ARE was approximately 20% at 1 year.35 A rate of 19% was seen for patients treated repeat SRS to the same lesion.11 We therefore excluded these patients from our study. We hypothesized, however, that other salvage practices under current prescriptive paradigms would not be associated with elevated risk of RN given that SRS doses used were established by RTOG 90-05, which, as previously noted, determined maximum tolerated SRS dose in previously irradiated patients.12
As predicted, we found that after initial SRS, treating recurrences with salvage SRS alone, WBRT alone, or surgery with adjuvant radiation were not associated with increased risk of RN. However, when repeat SRS was combined with WBRT, more than 20% of patients developed RN. This increased risk of RN is likely due to relatively higher radiation doses to larger volumes of normal brain tissue in these patients. Our results, thus, suggest that if a patient is felt to likely need salvage WBRT, combining this with additional SRS treatments might have risks that outweigh the benefits of repeat SRS. Therefore, it would be useful to estimate risk of regional recurrence and likelihood for future salvage WBRT before offering patients repeat SRS.
Multiple tools have been developed that predict for overall survival in patients with brain metastases.10, 36, 37, 38 The Graded Prognostic Assessment has recently been shown to also predict survival in the salvage SRS setting.39 Variables, such as patient age, KPS, cancer histology, number of metastases, and systemic disease status, which predict for regional brain failure and WBRT-free survival after initial SRS, are also likely useful in the context of salvage treatments.40, 41, 42, 43 In our study, only 30% (86/284) of patients treated with upfront SRS required salvage WBRT; however, a significant portion of patients (47.5%; 28/59) treated with repeat SRS eventually needed WBRT. Methods are needed to optimize patient selection for salvage SRS to avoid treating patients that will require early WBRT with its associated toxicities.
The interpretation of our results is limited by the retrospective nature and nonrandomized study design, which introduces multiple well-described biases in data collection and analysis.44 This could have led to the underestimation of incidence of RN in our patient population. Additionally, the generalizability of our results is dependent on how one defines RN. In patients without surgical removal of necrotic tissue, we had a high threshold to consider radiographic findings as consistent with RN. It is possible that some patients with RN were not identified because of loss of follow-up or because magnetic resonance spectroscopy or perfusion studies were simply not obtained. More accurate methods of distinguishing RN from temporary ARE or tumor progression is an area of active research.
Conclusion
Our results suggest that initial management approach for recurrent brain metastasis after upfront SRS alone does not affect the rate of subsequent RN. However, when patients are treated with repeat SRS and salvage WBRT, risk of RN significantly increases. When faced with recurrent brain metastases, patient and disease factors, as well as likelihood of requiring additional salvage treatment, are important considerations when estimating potential benefits and risks of therapy.
Footnotes
Conflicts of interest: None.
References
- 1.Tabouret E., Chinot O., Metellus P. Recent trends in epidemiology of brain metastases: An overview. Anticancer Res. 2012;32:4655–4662. [PubMed] [Google Scholar]
- 2.Linskey M.E., Andrews D.W., Asher A.L. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown P.D., Asher A.L., Ballman K.V. NCCTG N0574 (alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1-3 brain metastases. J Clin Oncol. 2015;33 (suppl; abstr LBA4) [Google Scholar]
- 4.Sahgal A., Aoyama H., Kocher M. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: Individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2015;91:710–717. doi: 10.1016/j.ijrobp.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama H., Shirato H., Tago M. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 6.Chang E.L., Wefel J.S., Hess K.R. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 7.Kocher M., Soffietti R., Abacioglu U. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ammirati M., Cobbs C.S., Linskey M.E. The role of retreatment in the management of recurrent/progressive brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:85–96. doi: 10.1007/s11060-009-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsao M.N., Rades D., Wirth A. Radiotherapeutic and surgical management for newly diagnosed brain metastasis(es): An American Society for Radiation Oncology evidence-based guideline. Pract Radiat Oncol. 2012;2:210–225. doi: 10.1016/j.prro.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaspar L., Scott C., Rotman M. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 11.Chan J., Zhung J., Rava P. Re-treatment of brain metastases using stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2014;90:S323–S324. [Google Scholar]
- 12.Shaw E., Scott C., Souhami L. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 13.Robbins JR, Elson A, Buatti JM, et al. ACR Appropriateness Criteria® Follow-up and retreatment of brain metastases. Available at: http://www.acr.org/∼/media/ACR/Documents/AppCriteria/Oncology/FollowupAndRetreatmentBrainMetastases.pdf. American College of Radiology. Accessed March 14, 2016.
- 14.Nordal R.A., Wong C.S. Molecular targets in radiation-induced blood-brain barrier disruption. Int J Radiat Oncol Biol Phys. 2005;62:279. doi: 10.1016/j.ijrobp.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 15.Belka C., Budach W., Kortmann R.D., Bamberg M. Radiation induced CNS toxicity—molecular and cellular mechanisms. Br J Cancer. 2001;85:1233. doi: 10.1054/bjoc.2001.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langleben D.D., Segall G.M. PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med. 2000;41:1861–1867. [PubMed] [Google Scholar]
- 17.Weybright P., Sundgren P.C., Maly P. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. Am J Roentgenol. 2005;185:1471–1476. doi: 10.2214/AJR.04.0933. [DOI] [PubMed] [Google Scholar]
- 18.Chuang M.-T., Liu Y.-S., Tsai Y.-S. Differentiating radiation-induced necrosis from recurrent brain tumor using mr perfusion and spectroscopy: A meta-analysis. Plos One. 2016;11:e0141438. doi: 10.1371/journal.pone.0141438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barajas R.F., Chang J.S., Sneed P.K., Segal M.R., McDermott M.W., Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009;30:367–372. doi: 10.3174/ajnr.A1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valk P.E., Dillon W.P. Radiation injury of the brain. ANR. 1991;12:45–62. [PMC free article] [PubMed] [Google Scholar]
- 21.Petrovich Z., Yu C., Giannotta S.L., O’Day S., Apuzzo M.L.J. Survival and pattern of failure in brain metastasis treated with stereotactic gamma knife radiosurgery. J Neurosurg. 2002;97:499–506. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 22.Lawrence Y.R., Li X.A., el Naqa I. Radiation dose–volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–S27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Korytko T., Radivoyevitch T., Colussi V. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64:419–424. doi: 10.1016/j.ijrobp.2005.07.980. [DOI] [PubMed] [Google Scholar]
- 24.Varlotto J.M., Flickinger J.C., Niranjan A. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2003;57:452–464. doi: 10.1016/s0360-3016(03)00568-6. [DOI] [PubMed] [Google Scholar]
- 25.Friedman W.A., Bova F.J., Bollampally S. Analysis of factors predictive of success or complications in arteriovenous malformation radiosurgery. Neurosurgery. 2003;52:296–307. doi: 10.1227/01.neu.0000043692.51385.91. [DOI] [PubMed] [Google Scholar]
- 26.Barker F.G., II, Butler W.E., Lyons S. Dose-volume prediction of radiation-related complications after proton beam radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 2003;99:254–263. doi: 10.3171/jns.2003.99.2.0254. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura J.L., Verhey L.J., Smith V. Dose conformity of gamma knife radiosurgery and risk factors for complications. Int J Radiat Oncol Biol Phys. 2001;51:1313–1319. doi: 10.1016/s0360-3016(01)01757-6. [DOI] [PubMed] [Google Scholar]
- 28.Chin L.S., Ma L., DiBiase S. Radiation necrosis following gamma knife surgery: A case-controlled comparison of treatment parameters and long-term clinical follow up. J Neurosurg. 2001;94:899–904. doi: 10.3171/jns.2001.94.6.0899. [DOI] [PubMed] [Google Scholar]
- 29.Miyawaki L., Dowd C., Wara W. Five year results of LINAC radiosurgery for arteriovenous malformations: Outcome for large AVMS. Int J Radiat Oncol Biol Phys. 1999;44:1089–1106. doi: 10.1016/s0360-3016(99)00102-9. [DOI] [PubMed] [Google Scholar]
- 30.Flickinger J.C., Kondziolka D., Pollock B.E. Complications from arteriovenous malformation radiosurgery: Multivariate analysis and risk modeling. Int J Radiat Oncol Biol Phys. 1997;38:485–490. doi: 10.1016/s0360-3016(97)89481-3. [DOI] [PubMed] [Google Scholar]
- 31.Voges J., Treuer H., Sturm V. Risk analysis of linear accelerator radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36:1055–1063. doi: 10.1016/s0360-3016(96)00422-1. [DOI] [PubMed] [Google Scholar]
- 32.Lax I., Karlsson B. Prediction of complications in gamma knife radiosurgery of arteriovenous malformation. Acta Oncol. 1996;35:49–55. doi: 10.3109/02841869609098479. [DOI] [PubMed] [Google Scholar]
- 33.Lee A.W., Kwong D.L., Leung S.F. Factors affecting risk of symptomatic temporal lobe necrosis: Significance of fractional dose and treatment time. Int J Radiat Oncol Biol Phys. 2002;53:75–85. doi: 10.1016/s0360-3016(02)02711-6. [DOI] [PubMed] [Google Scholar]
- 34.Minniti G., Clarke E., Lanzetta G. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sneed P.K., Mendez J., Vemer-van den Hoek J.G.M. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J Neurosurg. 2015;123:373–386. doi: 10.3171/2014.10.JNS141610. [DOI] [PubMed] [Google Scholar]
- 36.Sperduto P.W., Chao S.T., Sneed P.K. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto M., Sato Y., Serizawa T. Subclassification of recursive partitioning analysis class II patients with brain metastases treated radiosurgically. Int J Radiat Oncol Biol Phys. 2012;83:1399–1405. doi: 10.1016/j.ijrobp.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 38.Weltman E., Salvajoli J.V., Brandt R.A. Radiosurgery for brain metastases: A score index for predicting prognosis. Int J Radiat Oncol Biol Phys. 2000;46:1155–1161. doi: 10.1016/s0360-3016(99)00549-0. [DOI] [PubMed] [Google Scholar]
- 39.Shultz D.B., Modlin L.A., Jayachandran P. Repeat courses of stereotactic radiosurgery (SRS), deferring whole-brain irradiation, for new brain metastases after initial SRS. Int J Radiat Oncol Biol Phys. 2015;92:993–999. doi: 10.1016/j.ijrobp.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 40.Gorovets D., Ayala-Peacock D., Tybor D. Multi-institutional nomogram predicting survival free from salvage whole-brain radiation after radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2015;93:E77. doi: 10.1016/j.ijrobp.2016.09.043. [DOI] [PubMed] [Google Scholar]
- 41.Ayala-Peacock D.N., Peiffer A.M., Lucas J.T. A nomogram for predicting distant brain failure in patients treated with gamma knife stereotactic radiosurgery without whole brain radiation therapy. Neurol Oncol. 2014;16:1283–1288. doi: 10.1093/neuonc/nou018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodrigues G., Warner A., Zindler J., Slotman B., Lagerwaard F. A clinical nomogram and recursive partitioning analysis to determine the risk of regional failure after radiosurgery alone for brain metastases. Radiother Oncol. 2014;111:52–58. doi: 10.1016/j.radonc.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Press R.H., Prabhu R.S., Nickleach D.C. Novel risk stratification score for predicting early distant brain failure and salvage whole-brain radiotherapy after stereotactic radiosurgery for brain metastases. Cancer. 2015;121:3836–3843. doi: 10.1002/cncr.29590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buring J.E. Lippincott Williams & Wilkins; New York City, NY: 1987. Epidemiology in Medicine. [Google Scholar]