Abstract
Purpose
Maximum dose to the left anterior descending artery (LADmax) is an important physical constraint to reduce the risk of cardiovascular toxicity. We generated a simple algorithm to guide the positioning of the tangent fields to reliably maintain LADmax <10 Gy.
Methods and materials
Dosimetric plans from 146 consecutive women treated prone to the left breast enrolled in prospective protocols of accelerated whole breast radiation therapy, with a concomitant daily boost to the tumor bed (40.5 Gy/15 fraction to the whole breast and 48 Gy to the tumor bed), provided the training set for algorithm development. Scatter plots and correlation coefficients were used to describe the bivariate relationships between LADmax and several parameters: distance from the tumor cavity to the tangent field edge, cavity size, breast separation, field size, and distance from the tangent field. A logistic sigmoid curve was used to model the relationship of LADmax and the distance from the tangent field. Furthermore, we tested this prediction model on a validation data set of 53 consecutive similar patients.
Results
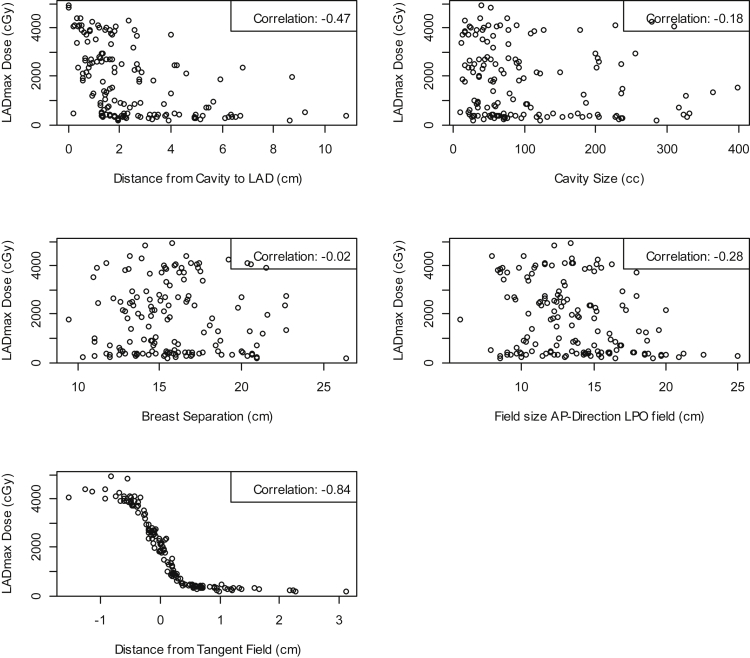
A lack of linear relationships between LADmax and distance from cavity to LAD (−0.47), cavity size (−0.18), breast separation (−0.02), or field size (−0.28) was observed. In contrast, distance from the tangent field was highly negatively correlated to LADmax (-0.84) and was used in the models to predict LADmax. From a logistic sigmoid model we selected a cut-point of 2.46 mm (95% confidence interval, 2.19-2.74 mm) greater than which LADmax is <10 Gy (95% confidence interval, 9.30-10.72 Gy) and LADmean is <3.3 Gy.
Conclusions
Placing the edge of the tangents at least 2.5 mm from the closest point of the contoured LAD is likely to assure LADmax is <10 Gy and LADmean is <3.3 Gy in patients treated with prone accelerated breast radiation therapy.
Introduction
The fundamental law of radiobiology proposed by Bergonié and Tribondeau in 1906 postulated that highly differentiated organs with a low mitotic index are radioresistant and therefore described the heart as the quintessential radioresistant organ.1 However, multiple studies from the treatment of Hodgkin disease, breast cancer, and peptic ulcer disease have all refuted this claim and demonstrated a significant cardiac risk when portions of the heart are irradiated.2, 3, 4 Radiation-induced cardiotoxicity remains an ongoing area of concern, particularly in curable early-stage breast cancer, where the survival advantage conveyed by radiation therapy is mitigated by a significant increase in mortality from heart disease that is sustained 15 years following radiation therapy to the left breast, as demonstrated in a large Surveillance, Epidemiology, and End Results registry study.2, 5, 6 Because of its proximity to the anterior chest wall, the left anterior descending coronary artery (LAD) is often significantly exposed to breast radiation and is a major cause of this complication.7 Generally, the mean LAD dose (LADmean) is monitored as a surrogate predictor of cardiotoxicity.8, 9 However, a recent study of cardiac perfusion imaging with stress single photon emission computed tomography (CT) scans before and after comprehensive nodal irradiation in 32 women with left-sided breast cancer failed to detect a correlation between clinically significant defects and heart or LAD mean dose.10
The maximum LAD dose (LADmax) may be more clinically relevant, considering the classical pathophysiology of coronary heart disease, because occlusion of only 1 section of the LAD can result in symptomatic heart disease. Scant preclinical evidence on radiation dose and fractionation response of the LAD is available. A maximum single dose to the LAD >10 Gy has been shown to cause coronary artery sclerosis in a rodent preclinical model.11 In the clinical setting of fractionated radiation therapy, reducing LADmax to <10 Gy is likely to be an adequate precaution.
In the prone position, we routinely contour the LAD as an avoidance structure and design tangent fields to exclude the LAD from the primary beam. However, when developing radiation portals, one cannot reliably predict the exact dose structures outside of the primary beam will receive, because the dose may reflect multiple variables such as the breast size and shape as well as the tumor cavity size and location. Once dosimetric planning is optimized with intensity modulation or field-in-field design and the dose is calculated, even minor adjustments are time and labor intensive. We hypothesized that defining the closest distance of the LAD to the tangent fields could offer a reliable prediction of the LADmax, particularly with a prone setup, which is characterized by minimal intrafraction displacement of the chest wall and highly reproducible intrafraction positioning.12, 13
We used a dosimetry database of 146 consecutive left breast cancer patients as a training set to determine the minimum LAD distance from the edge of the tangent fields required to consistently assure a LADmax <10 Gy. We then prospectively collected the plans of 53 consecutive left breast cancer patients to create a validation set to test the validity of our model.
Methods and materials
Dosimetric plans from consecutive, early breast cancer patients enrolled in 2 institutional review board–approved prospective protocols (05-181 and 12-01299), who had undergone segmental mastectomy and were treated with standard tangent fields alone were reviewed. Patients in 05-181 were treated to the whole breast to a dose of 2.7 Gy with an additional daily boost to the postsurgical cavity of 0.5 Gy a day over 15 fractions (48 Gy total). Women in 12-01299 were treated to the whole breast to a dose of 2.7 Gy and randomized to an additional boost delivered to the postsurgical cavity of 0.5 Gy a day (daily arm, 48 Gy total) or 2 Gy on the Fridays before the weekend break (weekly arm, 46.5 Gy total).
Women treated to the left breast in the prone position were included in this training set and their LAD was contoured on noncontrast simulation CT scans. This series of 146 consecutive patients enabled adequate representation of anatomic variability and different postsurgical cavity size and location. Variables measured included: distance from the tumor cavity to the LAD defined as the shortest distance from the contoured tumor cavity to the LAD in the beams-eye view; tumor cavity volume; breast separation measured from the anterior edge of the latissimus dorsi to the medial sternum; and width of the left posterior oblique (LPO) tangent field in the anteroposterior (AP) direction. The maximum point dose to the LAD was identified for each individual treatment plan. The distance from the tangent field edge to the LADmax was measured using the Eclipse (Varian Medical Systems, Palo Alto, CA) treatment planning software (Fig 1). This was determined by first identifying the maximum point dose to the LAD on the simulation CT scan, and then measuring the perpendicular distance from this point to the field edge on the same CT slice.
Figure 1.
Example of measuring the distance from tangent field edge to left anterior descending (LAD) artery dose maximum (LADmax). The LAD artery is shown in green and the edges of the tangent fields are shown in yellow. The distance from field edge to LAD is 1.14 cm and the LADmax is 2.96 Gy.
Information from the plans of 53 consecutive left-sided breast cancer patients (consented to protocol 12-01299) were then prospectively acquired to generate a validation set to test the model obtained from the training set, in an independent cohort. Patients were included from both the daily and weekly boost arm. LADmax dose values were multiplied by 1.03225 (48 Gy/46.5 Gy) in the weekly boost arm to normalize the total dose delivered to 48 Gy.
Statistical methods
We first provide a summary of the characteristics for the 146 patients included in the training set. For these 146 patients, scatter plots and correlation coefficients were used to describe the bivariate relationships between the LADmax and distance from boost cavity to LAD, cavity size, breast separation, field size in the AP direction of the LPO field, and distance from the tangent field, respectively. Distance from the tangent field (labeled as “D”) was used as the predictor of LADmax. A logistic sigmoid curve was used to model the training data to develop an equation to predict LADmax from D. In this procedure, the outcome LADmax was transformed and regression models were developed for the transformed LADmax as a function of a polynomial of D; the model (transformed from logistic sigmoid curve model) with the largest R2 was selected; this model was then back transformed to the logistic sigmoid curve form. In addition, the other recorded measurements (listed previously) were considered for inclusion in these models and the contributions were evaluated by examining the additional increments to the R2 value. Cross-validation methods were used to test the stability of this logistic sigmoid curve model on the training data. In an n-fold cross-validation procedure, we equally divided the training data into n subsets and fit the logistic sigmoid curve model on n-1 subsets, leaving 1 subset of data as the “testing set” to obtain the tested fitting error. By regarding each of the n subsets (n = 10, 15, 20) as the “testing set,” we obtained the average fitting error by using n-fold cross-validation. From this logistic sigmoid curve model, we identify the cut-point for D such that the LADmax is <10 Gy; 95% confidence intervals (CIs) are provided for both the cut-point and the corresponding LADmax. As a supportive analysis, we also fit the model for patients with D greater than the identified cut-point. We provide the square root of mean square error for the training data with D greater than the cut-point, and for the validation cohort.
Results
Table 1 provides a summary of the characteristics for the 146 patients included in the training set. Figure 2 provides the scatter plots and the correlation coefficients between LADmax and distance from cavity to LAD, cavity size, breast separation, field size AP-direction LPO field, and distance from the tangent field, respectively, for these 146 patients. From these scatter plots, we observe the lack of any direct relationships of LADmax with distance from the tumor cavity to LAD, cavity size, breast separation, and field size AP-direction LPO field. We note the low linear correlations between the LADmax and distance from tumor cavity to LAD (−0.47), cavity size (−0.18), breast separation (−0.02), and field size AP-direction LPO field (−0.28). However, the linear correlation between LADmax and distance from the tangent field indicates that these 2 variables are highly inversely correlated (−0.84). Moreover, we observe a reverse sigmoid shape relationship between LADmax and distance from the tangent field.
Table 1.
Training data: summary statistics for all measurements (n = 146)
| Variable | Mean | SD | Median | Range |
|---|---|---|---|---|
| Distance from tumor cavity to LAD (cm) | 2.47 | 2.00 | 1.78 | 0.01, 10.83 |
| Cavity size (mL) | 101.94 | 87.52 | 69.40 | 11.24, 398.40 |
| Breast separation (cm) | 15.76 | 2.95 | 15.45 | 9.45, 26.34 |
| Field size anteroposterior-direction left posterior oblique field (cm) | 13.52 | 3.41 | 13.15 | 5.80, 25.00 |
| Distance from the tangent field (cm) | 0.21 | 0.68 | 0.13 | −1.53, 3.11 |
| LADmax (Gy) | 17.90 | 14.35 | 14.32 | 1.72, 49.20 |
| LADmean (Gy) | 4.95 | 4.62 | 3.10 | 0.90, 25.50 |
LAD, left anterior descending artery; LADmax, left anterior descending artery dose maximum; LADmean, left anterior descending artery dose mean.
Figure 2.
Scatter plots of left anterior descending (LAD) artery dose maximum (LADmax) versus distance from boost cavity to LAD, cavity size, breast separation, field size anteroposterior (AP)-direction left posterior oblique (LPO) field, and distance from the tangent field (training data).
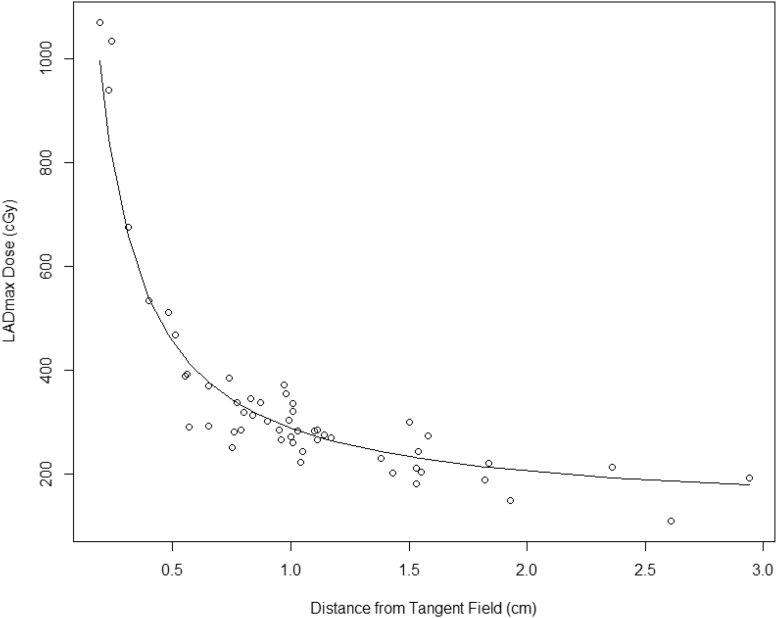
Next, we transformed LADmax to LADmax-trans as follows: logit(LADmax/5000) where logit(x) = x/(1−x), and performed linear regressions of LADmax-trans on polynomial terms of D. By selecting the largest R2 (0.95) from these linear regressions and back-transforming LADmax-trans to LADmax, we obtained the following equation to fit this sigmoid shape relationship (Fig 3):
| (1) |
Figure 3.
Left anterior descending artery dose maximum (LADmax) model for training data (n = 146).
Additional parameters were added to this model with only minimal change in R2 to 0.951 that indicated the model with D alone provides a parsimonious prediction model. The square root of mean square error for the training data was 2.41 Gy. Based on the large range of the outcome LADmax (maximum, 49.2 Gy), this error was relatively small (<5%). We also performed cross-validations to test the model fit. The average square root of mean square error was 2.82 Gy for 10-fold cross-validation; 2.61 Gy for 15-fold cross-validation; and 2.47 Gy for 20-fold cross-validation. The largest average error of 2.82 Gy was 5.8% of the largest LADmax and 15% of the mean of the LADmax, which indicate adequate fit for the model (Eq 1). From the model (Eq 1), for D greater than cut-point 2.46 mm (95% CI, 2.19-2.74 mm), then LADmax was less than 10 Gy (95% CI, 9.30-10.72 Gy). Thus, to obtain LADmax <10 Gy, in practice, the distance from the tangent field edge to the LAD should be >2.46 mm.
To predict LADmax from D (>2.46 mm), we refit the training data only for patients with D >2.46 mm (63 of 146 patients) and found the following relationship:
| (2) |
The square root of mean square error was 0.66 Gy (7% of the max of LADmax, 16% of the mean of LADmax) on the training data (63 of 146 patients) and 0.50 Gy (4% of the max of LADmax, 14% of the mean of LADmax) on the testing data (53 patients). Figures 4 and 5 show the fit for the original data and the test data, respectively.
Figure 4.
Left anterior descending artery dose maximum (LADmax) model fit for training data with distance from tangent field >2.46 mm (n = 53).
Figure 5.
Left anterior descending artery dose maximum (LADmax) model fit for validation cohort (n = 53).
Test the association between LADmean, a commonly reported parameter, and distance, we collected LADmean dose on the initial 146 patients in the training set (eFigure A; available as supplementary material online only at www.practicalradonc.org). Based on our data, the LADmean was maintained at <3.3 Gy when the field edge was kept >2.46 mm.
Discussion
This study aimed to develop a clinical planning tool to provide a “rule of thumb” for the radiation oncologist when designing the tangent fields by providing a LADmax/LAD mean dose estimate before dosimetric plan optimization. This allows the physician to make minor adjustments to gantry angle and primary collimation to have a significant impact on the dose delivered to the LAD. This tool also permits a physician to perform a rapid mental cost-benefit analysis between target coverage and LADmax/LADmean before the treatment plan is calculated. Although the distance between the LAD and the tangent field edge is modifiable by the clinician, other factors inherent to the individual patient may also influence the maximum dose to the LAD. We identified some of the patient-specific factors that could be predictive of LADmax/LADmean as the size of the tumor cavity for the radiation boost, the distance from the tumor cavity to the tangent field edge, the breast separation, and the AP dimension of the tangent field: all of these parameters were found to have a low negative correlation with LADmax. In the series analyzed, the LADmax was consistently kept <10 Gy when the posterior edge of the tangents fields was situated >2.46 mm from the LADmax point, regardless of boost cavity size, boost cavity location, field size, or patient breast separation. Moreover, no patients had a LADmean >3.3 Gy when the edge of the tangent field was >2.46 mm.
Inter- and intraobserver variability in the contouring of the LAD could skew the interpretation of dosimetric and clinical results. We tried to limit variability by using a cardiac atlas to guide LAD organs-at-risk contouring14 and adopted a consistent method of measuring the distance from the tangent field edge to the LADmax. All women analyzed in this study were treated in the prone treatment position; a patient set-up that has been shown to reduce radiation dose to the heart and lungs,15, 16 and to drastically limit intra-fraction movement.12, 13 Prone positioning is becoming more widely available with nearly one-third of centers polled having this technology in their armamentarium in a recent survey.17 Furthermore, the number of centers adopting this technique will likely increase with recent publication of randomized data demonstrating improved cosmesis in large-breasted women treated in the prone compared with the supine position.18 Although there is some controversy regarding the benefit of prone technique to decrease coronary artery dose,19 we routinely treat the majority of women at our institution prone because of the demonstrable benefit in both cardiac volume exposed to radiation15 and LAD dose.20 The different results reported by other investigators likely reflect the use of prone techniques that interpose a wedge under the contralateral breast, forcing an axial rotation that enhances the displacement of the heart toward the chest wall when prone.21 This displacement can be avoided by assuring that the patient's sternum is on the prone breast board and the head is turned toward the treated breast; both measures result in maintaining the body on a horizontal plane parallel to ground.22
Other techniques, such as deep inspiratory breath hold,19, 20, 21 respiratory gating,22 and proton therapy23 have also been used to decrease the dose to the heart and lungs because even low doses of irradiation have been found to correlate with an increase in cardiovascular disease.24 Deep inspiratory breath hold has the benefit of maintaining patients in the supine position, often more comfortable for elderly or otherwise infirm patients, but it requires highly trained staff and a cooperative patient. Respiratory gating removes the stress component associated with breath hold but it significantly extends treatment duration.25 Because of their characteristic dose distribution, protons are a promising approach to assure cardiac sparing in breast cancer patients and an ongoing national prospective randomized trial is comparing proton versus photon radiation technique in women with nonmetastatic breast cancer (NCT02603341).
Controversy exists about which specific cardiac dosimetric parameter best correlates with patient outcomes. Darby et al adopted the mean dose to the entire heart as the most predictive measure of major coronary events.6 In contrast, other investigators consider LAD dose more relevant, with debate over whether the mean or maximum dose is most predictive. Investigators at Duke University studied single photon emission tomography myocardial perfusion imaging in 69 patients who had undergone radiation for left-sided breast cancer and found perfusion defects in the LAD distribution, indicating a likely mechanism of cardiac damage.26 The dose parameter used in this study was percent of the left ventricle receiving >50% of the prescription dose with no reporting of specific LAD dose parameters. In a Swedish series of 199 patients, LAD stenosis measured by angiography was more common in left-sided than right-sided breast cancer patients who had undergone radiation therapy as part of their management.27 Interestingly, Aznar et al found that in 7 of 24 evaluated patients undergoing radiation for left-sided breast cancer, the volume of heart irradiated was within predefined dose constraints, but a high dose was still delivered to the LAD.28 Alternatively, in 1 patient in this study, the LAD dose was low, but the V20 Gy was elevated at 19%. This paradoxical effect warrants accurate contouring and dose monitoring of the LAD in addition to classic volumetric heart constraints. Despite this evidence, no published dose constraint for the LAD exists, either for LADmean or LADmax.
We chose a cut-point of 10 Gy (representing 4.8% of the total prescribed dose) based on preclinical data from using a single dose of 10 Gy.11 This threshold is arbitrary because it reflects the lack of robust human data correlating LAD dose with outcomes. In addition, preexisting individual risk is likely to determine late morbidity of heart radiation therapy; even minimal artery exposure could accelerate atherosclerosis in the genetically susceptible individual. We have modeled this concept and found that the effect of radiation exposure on the risk of future cardiac disease appears to be multiplicative, with the highest absolute radiation exposure risks corresponding to the highest baseline cardiac risk.29
We found that placing the tangent fields at a minimum distance of 2.46 mm from the closest point of the contoured LAD limits the LADmax to 10 Gy and LADmean to 3.3 Gy. Because our patients were planned and treated prone, a prospective validation in left breast cancer patients treated supine should be conducted and include an assessment of intrafraction movement of the chest wall. Respiration movements during the delivery of treatment supine likely modify the distance of LAD from the field.
Another limitation of this study is the unconventional dose and fractionation scheme used, but radiobiological modeling confirmed by long term clinical data30, 31, 32, 33 have demonstrated equivalence to the standard fractionation of 46 to 50 Gy to the breast with a sequential boost of 14 or 10 Gy to the tumor cavity, over a total of 6 weeks. Radiation Therapy Oncology Group 1005 is comparing accelerated radiation with a concomitant boost versus standard fractionation with a sequential boost. The dose and fractionation in the accelerated treatment arm are comparable to those of the regimen used in this report. In any event, the model presented (Eq 1) can easily be applied to any dose and fractionation.
In conclusion, we believe the simple tool presented could be of use in the daily challenge of converging safety and efficiency to ensure the best protection from long-term cardiovascular effects in irradiated left breast cancer patients.
Footnotes
Sources of support: This work was supported in part by National Cancer Institute CCSG P30 CA016087 (J.D.G.).
Conflicts of interest: None.
Supplementary material for this article (http://dx.doi.org/10.1016/j.prro.2016.08.001) can be found at www.practicalradonc.org.
Supplementary Data
Scatter plot of left anterior descending (LAD) artery mean versus distance from tangent field (training data, n = 146).
References
- 1.Bergonie J.T., Tribondeau L. De quelques resultats de la radiotherapie et essai de fixation d'une technique rationnelle. CR Séances Acad Sci. 1906;143:983–985. [Google Scholar]
- 2.Clarke M., Collins R., Darby S. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 3.Hancock S.L., Donaldson S.S., Hoppe R.T. Cardiac disease following treatment of Hodgkin's disease in children and adolescents. J Clin Oncol. 1993;11:1208–1215. doi: 10.1200/JCO.1993.11.7.1208. [DOI] [PubMed] [Google Scholar]
- 4.Carr Z.A., Land C.E., Kleinerman R.A. Coronary heart disease after radiotherapy for peptic ulcer disease. Int J Radiat Oncol Biol Phys. 2005;61:842–850. doi: 10.1016/j.ijrobp.2004.07.708. [DOI] [PubMed] [Google Scholar]
- 5.Darby S.C., McGale P., Taylor C.W., Peto R. Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5. [DOI] [PubMed] [Google Scholar]
- 6.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 7.Sardaro A., Petruzzelli M.F., D'Errico M.P., Grimaldi L., Pili G., Portaluri M. Radiation-induced cardiac damage in early left breast cancer patients: Risk factors, biological mechanisms, radiobiology, and dosimetric constraints. Radiother Oncol. 2012;103:133–142. doi: 10.1016/j.radonc.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Correa C.R., Das I.J., Litt H.I. Association between tangential beam treatment parameters and cardiac abnormalities after definitive radiation treatment for left-sided breast cancer. Int J Radiat Oncol Biol Phys. 2008;72:508–516. doi: 10.1016/j.ijrobp.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 9.Jagsi R., Griffith K.A., Koelling T., Roberts R., Pierce L.J. Rates of myocardial infarction and coronary artery disease and risk factors in patients treated with radiation therapy for early-stage breast cancer. Cancer. 2007;109:650–657. doi: 10.1002/cncr.22452. [DOI] [PubMed] [Google Scholar]
- 10.Chung E., Corbett J.R., Moran J.M. Is there a dose-response relationship for heart disease with low-dose radiation therapy? Int J Radiat Oncol Biol Phys. 2013;85:959–964. doi: 10.1016/j.ijrobp.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker J.E., Fish B.L., Su J. 10 Gy total body irradiation increases risk of coronary sclerosis, degeneration of heart structure and function in a rat model. Int J Radiat Biol. 2009;85:1089–1100. doi: 10.3109/09553000903264473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jozsef G., DeWyngaert J.K., Becker S.J., Lymberis S., Formenti S.C. Prospective study of cone-beam computed tomography image-guided radiotherapy for prone accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2011;81:568–574. doi: 10.1016/j.ijrobp.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Mitchell J., Formenti S.C., DeWyngaert J.K. Interfraction and intrafraction setup variability for prone breast radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:1571–1577. doi: 10.1016/j.ijrobp.2009.07.1683. [DOI] [PubMed] [Google Scholar]
- 14.Feng M., Moran J.M., Koelling T. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys. 2011;79:10–18. doi: 10.1016/j.ijrobp.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Formenti S.C., DeWyngaert J.K., Jozsef G., Goldberg J.D. Prone vs supine positioning for breast cancer radiotherapy. JAMA. 2012;308:861–863. doi: 10.1001/2012.jama.10759. [DOI] [PubMed] [Google Scholar]
- 16.Lymberis S.C., de Wyngaert J.K., Parhar P. Prospective assessment of optimal individual position (prone versus supine) for breast radiotherapy: Volumetric and dosimetric correlations in 100 patients. Int J Radiat Oncol Biol Phys. 2012;84:902–909. doi: 10.1016/j.ijrobp.2012.01.040. [DOI] [PubMed] [Google Scholar]
- 17.Dundas K.L., Pogson E.M., Barumalai V. Australian survey on current practices for breast radiotherapy. J Med Imaging Radiat Oncol. 2015;59:736–742. doi: 10.1111/1754-9485.12348. [DOI] [PubMed] [Google Scholar]
- 18.Veldeman L., Schiettecatte K., De Sutter C. The 2-year cosmetic outcome of a randomized trial comparing prone and supine whole-breast irradiation in large-breasted women. Int J Radiat Oncol Biol Phys. 2016;95:1210–1217. doi: 10.1016/j.ijrobp.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Sixel K.E., Aznar M.C., Ung Y.C. Deep inspiration breath hold to reduce irradiated heart volume in breast cancer patients. Int J Radiat Oncol Biol Phys. 2001;49:199–204. doi: 10.1016/s0360-3016(00)01455-3. [DOI] [PubMed] [Google Scholar]
- 20.Register S., Takita C., Reis I., Zhao W., Amestoy W., Wright J. Deep inspiration breath-hold technique for left-sided breast cancer: An analysis of predictors for organ-at-risk sparing. Med Dosim. 2015;40:89–95. doi: 10.1016/j.meddos.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Zellars R., Bravo P.E., Tryggestad E. SPECT analysis of cardiac perfusion changes after whole-breast/chest wall radiation therapy with or without active breathing coordinator: Results of a randomized phase 3 trial. Int J Radiat Oncol Biol Phys. 2014;88:778–785. doi: 10.1016/j.ijrobp.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Giraud P., Djadi-Prat J., Morelle M. Contribution of respiratory gating techniques for optimization of breast cancer radiotherapy. Cancer Invest. 2012;30:323–330. doi: 10.3109/07357907.2012.657818. [DOI] [PubMed] [Google Scholar]
- 23.Lin L.L., Vennarini S., Dimofte A. Proton beam versus photon beam dose to the heart and left anterior descending artery for left-sided breast cancer. Acta Oncol. 2015;54:1–8. doi: 10.3109/0284186X.2015.1011756. [DOI] [PubMed] [Google Scholar]
- 24.Little M.P., Azizova T.V., Bazyka D. Systematic review and meta-analysis of circulatory disease from exposure to low-level ionizing radiation and estimates of potential population mortality risks. Environ Health Perspect. 2012;120:1503–1511. doi: 10.1289/ehp.1204982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui G., Housley D.J., Chen F., Mehta V.K., Shepard D.M. Delivery efficiency of an Elekta linac under gated operation. J Appl Clin Med Phys. 2014;15:4713. doi: 10.1120/jacmp.v15i5.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lind P.A., Pagnanelli R., Marks L.B. Myocardial perfusion changes in patients irradiated for left-sided breast cancer and correlation with coronary artery distribution. Int J Radiat Oncol Biol Phys. 2003;55:914–920. doi: 10.1016/s0360-3016(02)04156-1. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson G., Holmberg L., Garmo H. Distribution of coronary artery stenosis after radiation for breast cancer. J Clin Oncol. 2012;30:380–386. doi: 10.1200/JCO.2011.34.5900. [DOI] [PubMed] [Google Scholar]
- 28.Aznar M.C., Korreman S.S., Pedersen A.N., Persson G.F., Josipovic M., Specht L. Evaluation of dose to cardiac structures during breast irradiation. Br J Radiol. 2011;84:743–774. doi: 10.1259/bjr/12497075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenner D.J., Shuryak I., Jozsef G., Dewyngaert K.J., Formenti S.C. Risk and risk reduction of major coronary events associated with contemporary breast radiotherapy. JAMA Intern Med. 2014;174:158–160. doi: 10.1001/jamainternmed.2013.11790. [DOI] [PubMed] [Google Scholar]
- 30.Bentzen S.M., Shuryak I., Jozsef G., Dewyngaert K.J., Formenti S.C. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Osa E.O., DeWyngaert K., Roses D. Prone breast intensity modulated radiation therapy: 5-year results. Int J Radiat Oncol Biol Phys. 2014;89:899–906. doi: 10.1016/j.ijrobp.2014.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper B.T., Formenti-Ujlaki G.F., Li X. Prospective randomized trial of prone accelerated intensity modulated breast radiation therapy with a daily versus weekly boost to the tumor bed. Int J Radiat Oncol Biol Phys. 2016;95:571–578. doi: 10.1016/j.ijrobp.2015.12.373. [DOI] [PubMed] [Google Scholar]
- 33.Finkel M.A., Cooper B.T., Li X., Fenton-Kerimian M., Goldberg J.D., Formenti S.C. Quality of life in women undergoing breast irradiation in a randomized, controlled clinical trial evaluating different tumor bed boost fractionations. Int J Radiat Oncol Biol Phys. 2016;95:579–589. doi: 10.1016/j.ijrobp.2016.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plot of left anterior descending (LAD) artery mean versus distance from tangent field (training data, n = 146).