Introduction
In 2016, a projected 30,000 to 40,000 Americans will be diagnosed with small cell lung cancer (SCLC),1 and approximately 10% to 20% of them will have brain metastasis at time of diagnosis.2 The intrinsic radiosensitivity and propensity of SCLC to metastasize intracranially provides the rationale for whole brain radiation therapy (WBRT), either prophylactically or therapeutically. Given the aggressive nature of SCLC, intracranial recurrence is not uncommon.3 Recurrent intracranial metastasis is typically treated with focal or whole brain reirradiation; however, given the added neurotoxicity risks of reirradiation, other options such as surgery or systemic therapy may be offered.
Toxicities of systemic therapy, such as myelosuppression, can complicate the management of SCLC. Despite the expanded differential diagnoses that often accompany immunocompromised patients, clinicians may fail to consider all possibilities when managing this patient population. We present the case of an immunocompromised patient with SCLC whose apparent metastatic brain recurrence was in actuality an uncommon infectious agent.
Case report
A 67-year-old male with a 30 pack-year smoking history presented with new-onset grand mal seizure. A brain magnetic resonance imaging (MRI) scan revealed 4 intracranial lesions; the largest a 1.5 cm × 1.8 cm ring enhancing lesion in the right temporal lobe with significant edema (Fig 1). The patient was treated with dexamethasone, levetiracetam, and a navigation-guided craniotomy with temporal lobe resection. A soft, tan-brown, 1.7 cm mass was visualized under the dura and totally resected without violating the surrounding vasculature via microdissector under high magnification. Pathology of the resected brain mass demonstrated metastatic, poorly differentiated neuroendocrine carcinoma with immunohistochemical positivity for PANCK, TTF1, CK7, chromogranin, synaptophysin, and CD56 (Fig 2). A positron emission tomography-computed tomography scan revealed a 2 cm mass in the right middle lobe yielding a diagnosis of extensive-stage SCLC, T1N1M1.
Figure 1.
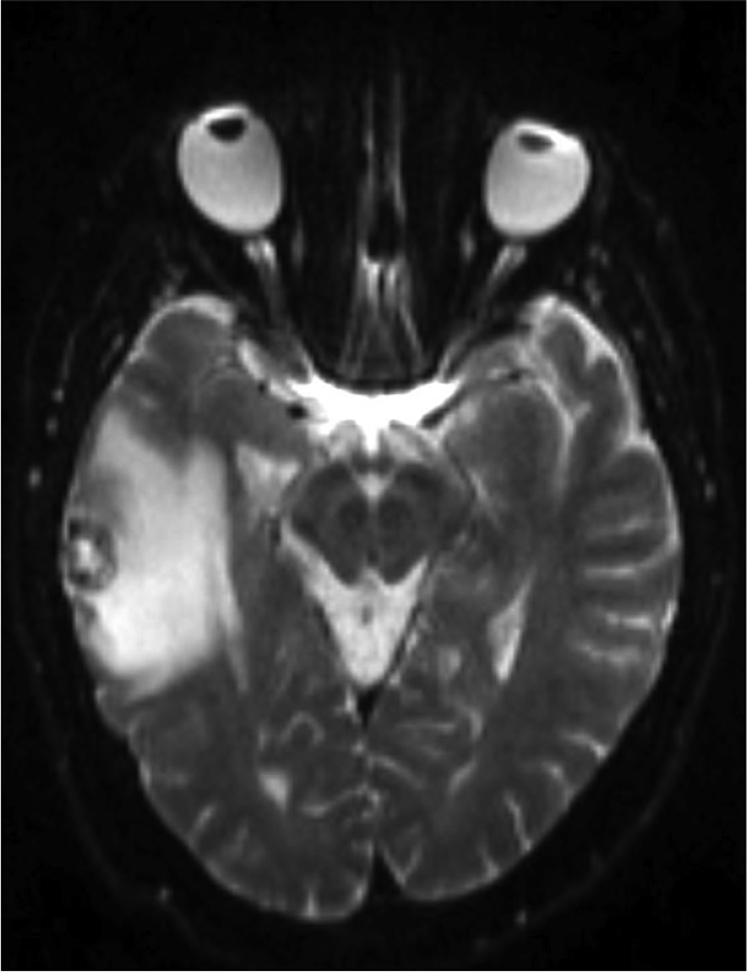
T2-weighted axial brain magnetic resonance imaging scan showing a hypointense lesion with surrounding edema in right temporal lobe.
Figure 2.
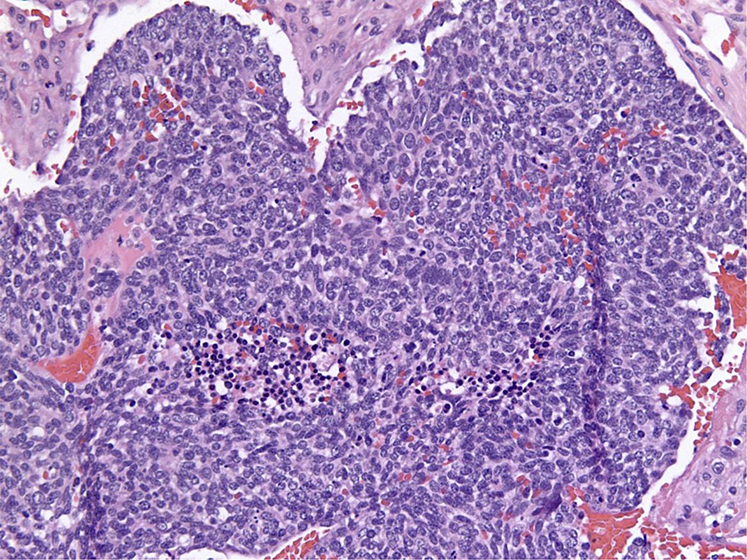
Dense sheets of malignant cells with scant cytoplasm, prominent nuclei, and absent nucleoli consistent with small cell carcinoma.
Whole brain radiation therapy at a dose of 30 Gy over 10 fractions was delivered followed by 4 cycles of carboplatin and etoposide, with 4 mg dexamethasone every 6 hours throughout treatment. A progressive and debilitating dizziness developed after the first round of chemotherapy and was exacerbated by attempted steroidal taper. Two days after completing round 3 (of 4) of chemotherapy and 52 days after completion of WBRT, the patient was hospitalized because of iatrogenic exogenous adrenal insufficiency. During this admission, an MRI scan of the brain showed a ring enhancing 0.6 cm left frontal-parietal lesion concerning for new metastasis.
To treat the presumed recurrence, palliative external beam radiation was limited to the left supratentorial and parietal areas of the brain at a dose of 25 Gy over 10 fractions, followed by the fourth and final round of chemotherapy. One week later, the patient developed a right-sided facial droop and expressive aphasia. Noncontrast CT of the head demonstrated a 2.3 cm × 2.3 cm left frontal mass within the previously irradiated field, causing a midline shift. An MRI scan with and without contrast confirmed a ring-enhancing mass with restricted diffusion and significant edema (Figs 3 and 4).
Figure 3.
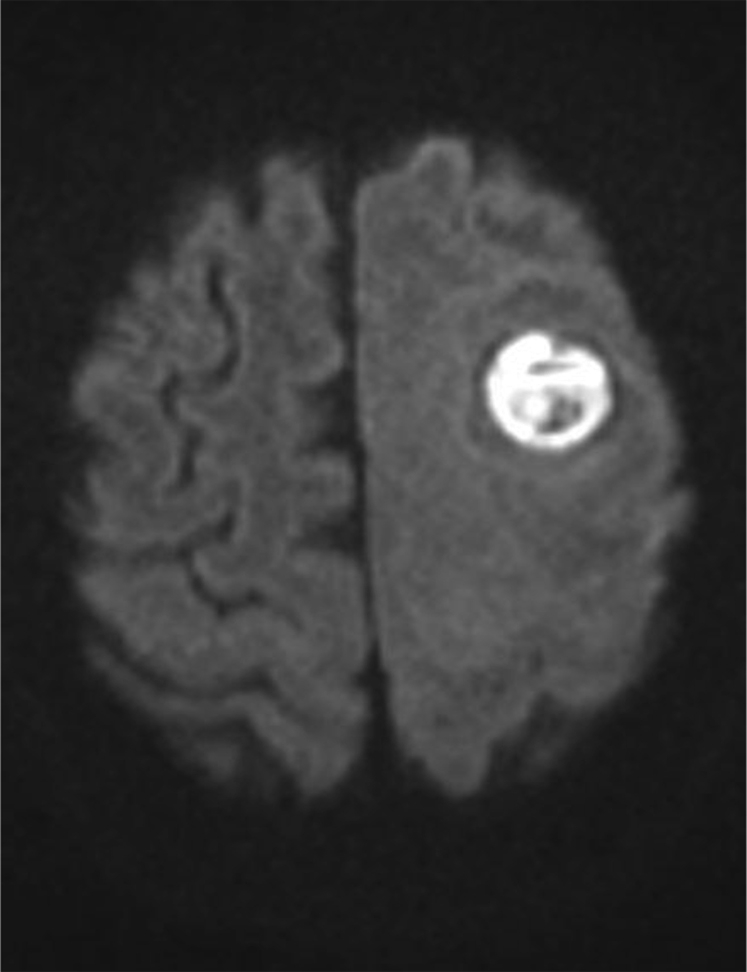
Diffusion weighted axial brain magnetic resonance imaging scan showing restricted diffusion in the left frontoparietal region.
Figure 4.
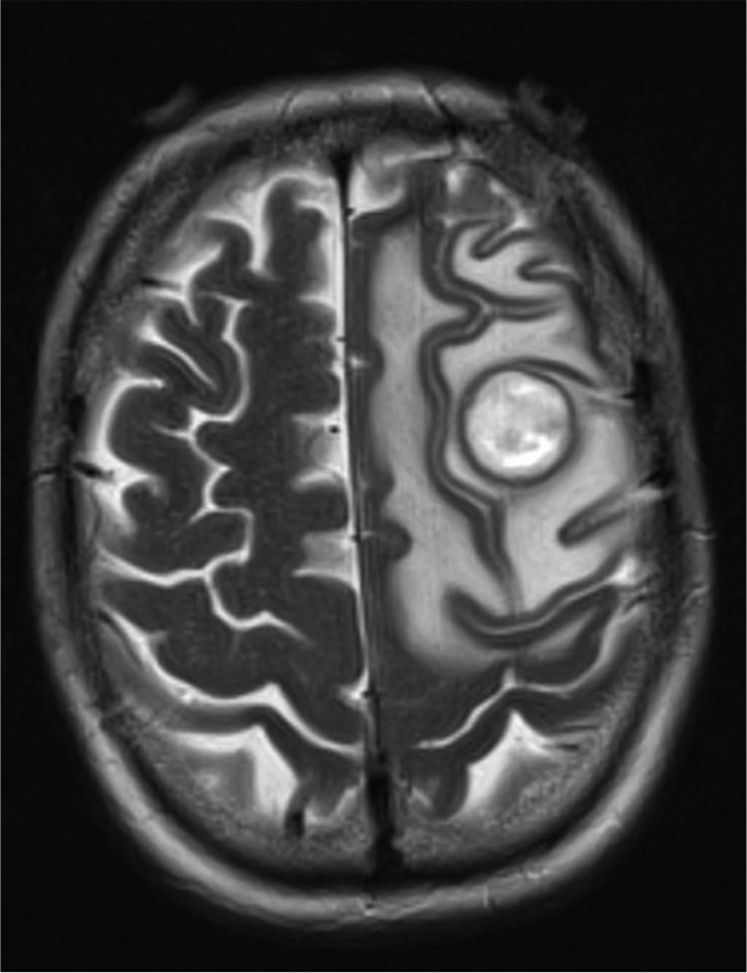
T2-weighted axial brain magnetic resonance imaging scan showing hypointense rim with hyperintense heterogenous core in the left frontoparietal region.
Given the recent repeat radiation, neurologic symptoms, immunocompromised state, and presence of restricted diffusion on MRI scan, metastasis was considered less likely in favor of a brain abscess. Subsequently, Burr hole craniotomy with aspiration of the lesion produced a purulent specimen, of which Gram stain showed “fine filamentous beaded bending” Gram-positive rods, and ultimately grew Nocardia nova (Fig 5).
Figure 5.
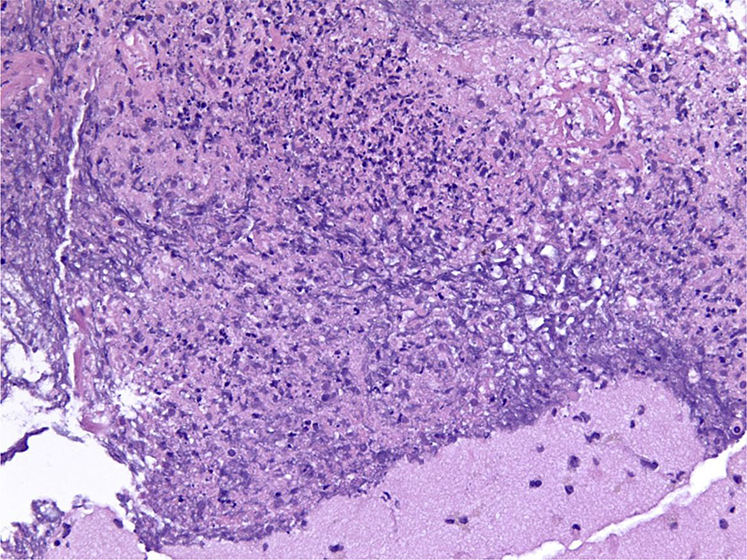
Gram-positive rods contained within organized purulent matter consistent with abscess.
The patient was treated with trimethoprim-sulfamethoxazole and intravenous meropenem for 6 weeks. Over the next 3 months, the patient required 2 additional craniotomies with aspiration after repeat brain MRI scans showed abscess reaccumulation in the previous location. At last follow-up, the patient remains on trimethoprim-sulfamethoxazole and meropenem, with minimal improvement of his right-sided weakness and dizziness.
Discussion
A cerebral abscess in the setting of brain metastasis is an unexpected phenomenon. Ring-enhancing lesions in a SCLC patient with intracranial metastasis likely represents recurrence. However, a broad differential is imperative for appropriate management. Given that this patient was treated with WBRT to 30 Gy, followed by 25 Gy to the left cerebral hemisphere within 2 months for a presumed recurrence, the likelihood of local recurrence should be carefully weighed against alternative diagnoses. The differential for contrast-enhancing intracranial lesions (Table 1) is comprehensive; but in the context of clinical history and examination, laboratory data, and discerning neuroimaging features, the list can be modified appropriately. New metastasis, recurrence, lymphoma, abscess, and radiation necrosis were all possible in this case, and given the recent radiation and concern for radionecrosis, surgery was a reasonable therapeutic option that ultimately diagnosed an N. novo brain abscess. Although radiation necrosis was strongly considered, the clinical picture of an immunosuppressed patient, in conjunction with characteristic MRI findings (circular, continuous, thin-walled border without irregularities) were more suggestive of an abscess. Furthermore, the interval of time from last radiation treatment was shorter than expected if radiation necrosis was the etiology, although radiographic pseudoprogression certainly would fall within the elapsed timeframe.6
Table 1.
| Enhancement border | Hyperintense T1 signal of peripheral rim | Hyperintense T2 signal of peripheral rim | Hyperintense on diffusion-weighted imaging | Hyperintense on apparent diffusion coefficient | |
|---|---|---|---|---|---|
| Metastasis | Thick, irregular | ✓ | Variable | ||
| Abscess | Thin, regular | ✓ | ✓ | ||
| Glioblastoma | Thick, irregular | ✓ | Iso/hyperintense | Iso/hyperintense | |
| Infarct | Gyriform | ✓ | ✓ | ||
| Contusion | Variable | Iso/hyperintense | ✓ | ✓ | Variable |
| Demyelination | Discontinuous | ✓ | ✓ | ✓ | |
| Radiation necrosis | Discontinuous | ✓ | ✓ | ✓ | |
| Lymphoma | Thick, irregular | Iso/hyperintense | ✓ | Hypointense/Iso |
Nocardiosis of the central nervous system is a rare cause of cerebral abscesses, accounting for only approximately 2% or 30 to 50 annual cases.7 The subject in this case carried several risk factors for Nocardia brain abscess, including long-term corticosteroid therapy, chemotherapy-induced leukopenia, and previous brain pathology. Clinical diagnosis is difficult because the presentation mimics other brain pathology and classic signs and symptoms of infection (ie, fever) are often hidden in immunocompromised patients. Retrospective reviews indicate that the median time to diagnosis of intracranial Nocardia is 30 days.8 Decreasing the time to diagnosis is paramount because the mortality associated with Nocardia-related abscesses is greater than 3 times that from other bacterial etiologies.9 If the initial appearance of the 0.6 cm left frontoparietal lesion is assumed to be the start of the Nocardia abscess in this case, the time to diagnosis was 48 days.
Identification of Nocardia-related intracranial abscess is particularly challenging in the presence of prior brain tumors or radiation therapy, given similarities in presentation. With the advent and increased utilization of magnetic resonance techniques such as spectroscopy, noninvasive measures of detection may be on the horizon.10 However, biopsy or aspiration remains the most reliable way to differentiate one diagnosis from the next. The morbidity of such procedures in already sick patients can present a dilemma for clinicians. Nonetheless, in select cases, when a brain lesion is anatomically accessible and the clinical picture is ambiguous or clouded by prior treatment and risk factors for infection, a biopsy should be strongly considered. This case exemplifies how the application and careful navigation through a broad differential may uncover a rare disease entity.
Footnotes
Conflicts of interest: None.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Doyle T.J. Brain metastasis in the natural history of small-cell lung cancer 1972-1979. Cancer. 1982;50:752–754. doi: 10.1002/1097-0142(19820815)50:4<752::aid-cncr2820500421>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 3.Olson A., Wegner R., Rwigema J.C., Heron D., Burton S., Mintz A. Clinical outcomes of reirradiation of brain metastases from small cell lung cancer with Cyberknife stereotactic radiosurgery. J Cancer Res Therapeutics. 2012;8:411–416. doi: 10.4103/0973-1482.103522. [DOI] [PubMed] [Google Scholar]
- 4.Garg R. Multiple ring-enhancing lesions of the brain. J Postgrad Med. 2010;56:307–316. doi: 10.4103/0022-3859.70939. [DOI] [PubMed] [Google Scholar]
- 5.Zhou J., Tryggestad E., Wen Z. Differentiation between glioma and radiation necrosis using molecular magnetic resonance imaging of endogenous proteins and peptides. Nature. 2011;17:130–134. doi: 10.1038/nm.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan Y.L., Leung S.F., King A.D., Choi P.H., Metreweli C. Late radiation injury to the temporal lobes: Morphologic evaluation at MR imaging. Radiology. 1999;213:800–807. doi: 10.1148/radiology.213.3.r99dc07800. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y.C., Wang T.L., Hsu Y.H. Clinical pathway in the treatment of nocardial brain abscesses following systemic infections. Case Rep Neurol Med. 2014;2014:587934. doi: 10.1155/2014/584934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matulionyte R., Rohner P., Uçkay I., Lew D., Garbino J. Secular trends of nocardia infection over 15 years in a tertiary care hospital. J Clin Pathol. 2004;57:807–812. doi: 10.1136/jcp.2004.016923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Threlkeld S.C., Hooper D.C. Update on management of patients with Nocardia infection. Curr Clin Top Infect Dis. 1997;17:1–23. [PubMed] [Google Scholar]
- 10.Chuang M.-T., Liu Y.-S., Tsai Y.-S., Chen Y.-C., Wang C.K. Differentiating radiation-induced necrosis from recurrent brain tumor using MR perfusion and spectroscopy: A meta-analysis. PLoS One. 2016;11:e0141438. doi: 10.1371/journal.pone.0141438. [DOI] [PMC free article] [PubMed] [Google Scholar]


