Introduction
We present a case of a recurrent skull base meningioma after resection and salvage radiation therapy that highlights dose considerations and a unique pattern of recurrence, underscoring the importance of understanding regions at risk for recurrence during initial treatment planning.
Case presentation
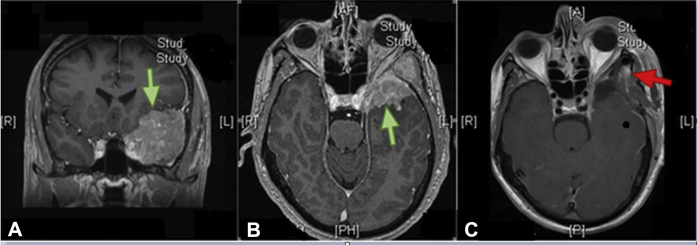
A 55-year-old, otherwise healthy, right-handed man presented with a 3-month history of declining visual acuity in the left eye and bifrontal morning headaches. Visual acuity in the left eye measured 20/150 but the right eye was preserved at 20/20. A magnetic resonance imagining (MRI) head scan revealed an extra-axial mass with an extension into the posterior left orbit and suspected involvement of the left cavernous sinus, superior orbital fissure, and optic nerve (Fig 1A,B).
Figure 1.
Initial presentation. T1-weighted enhanced magnetic resonance imaging head scans. (A and B) Preoperative. (C) Postoperative. The green arrows indicate preoperative gross tumor and the red arrow indicates postoperative gross residual disease.
The patient underwent a left pterional craniotomy with tumor resection and decompression of the optic nerve. Postoperative pathology revealed a World Health Organization grade 1 (benign) meningioma with low mitotic activity and Ki-67 and no necrosis or anaplastic features. A postoperative MRI scan revealed subtotal resection (Simpson grade 4) with residual tumor adjacent to the left cavernous sinus, measuring 4 mm by 10 mm (transverse by craniocaudal; Fig 1C). Visual acuity in the left eye improved to 20/25, and the right eye remained at 20/20. Given the low tumor grade, adjuvant treatment was deferred in favor of annual surveillance imaging.
First recurrence
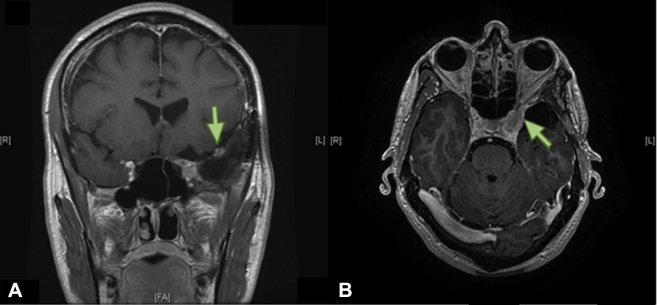
The patient was asymptomatic with stable imaging until the fourth-year surveillance MRI scan, which revealed growth of the residual tumor to 12 mm by 18 mm (Fig 2). Gamma Knife radiation surgery was contraindicated because of the proximity to the optic nerve. Subsequently, the patient was considered for fractionated radiation therapy. The patient had become symptomatic with diplopia on upward gaze and sensitivity to light. Salvage radiation therapy was delivered to a dose of 50.4 Gy (1.8 Gy/fraction) via intensity modulated radiation therapy (IMRT) with a thermoplastic mask for immobilization.
Figure 2.
First recurrence after surgical resection. T1-weighted magnetic resonance imaging head scans post-gadolinium. (A) Coronal view. (B) Axial view. Green arrow indicates gross recurrent tumor.
Computed tomography (CT) simulation images were fused with diagnostic MRI images to help with delineation. The macroscopic tumor on the fused CT and MRI images of the tumor recurrence was contoured as the gross tumor volume (GTV) with 1 cm added as a clinical target volume (CTV) margin and 0.5 cm added as a planning target volume margin. The preoperative MRI scan from the original surgery was used to aid in delineating the recurrent tumor in relation to the original tumor volume. Daily image guidance included a combination of cone beam CT and orthogonal electronic portal imaging. After treatment, there was clinical improvement with a partial resolution of gaze-dependent diplopia but with new deficits including left-sided tinnitus and left facial numbness in the V1 and V2 distributions.
Second recurrence
Surveillance MRI scans taken 8 months after radiation therapy revealed a slight reduction in tumor size. At 20 months, MRI scans showed stability. Six months before the next annual surveillance MRI scan, the patient presented with left facial pain in the V1 and V2 distributions. Development of trigeminal nerve injury was suspected, and pain control was initiated with a combination of carbamazepine, dexamethasone, and hydromorphone. A contrast-enhanced CT scan revealed no change in the residual lesion.
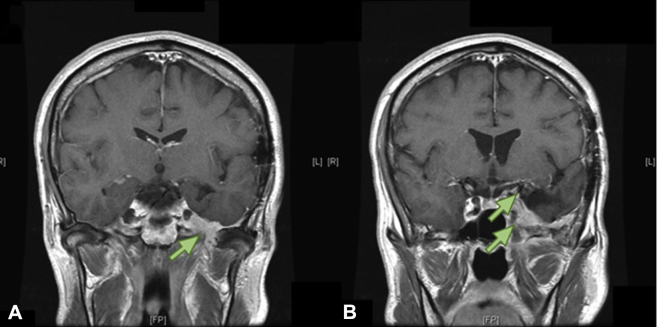
The next regularly scheduled surveillance MRI scan 3 months later revealed tumor enlargement with extensions along the floor of the middle cranial fossa and through the foramen ovale and foramen rotundum into the infratemporal fossa (Fig 3). The MRI scan that demonstrated recurrence was fused with the previous treatment plan, which revealed that the GTV was flush against the inner table of the skull base with the regrowth of tumor occurring both within and outside of the previous high-dose volumes (Fig 4). It was unclear whether these findings indicated radiation-resistant tumor or incomplete coverage of microscopic disease through the foramina at the skull base that was unrecognized in the original treatment plan.
Figure 3.
Recurrence after first course of radiation therapy. Coronal T1 weighted magnetic resonance imaging post gadolinium. Green arrows indicate gross recurrent disease both outside (A) and within (B) the original radiation treatment field.
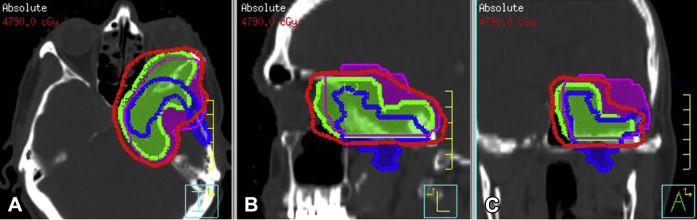
Figure 4.
Recurrent disease after salvage radiation therapy superimposed over the original treatment plan, demonstrating recurrence outside of the original target volumes. Non-contrast computed tomography images. (A-C) Axial, sagittal, and coronal views, respectively. Green is clinical tumor volume from time of first radiation; magenta is clinical tumor volume per Radiation Therapy Oncology Group 0539 recommendations; blue is gross tumor volume for recurrent disease after first radiation; and red is 95% isodose line from the first course of radiation treatment.
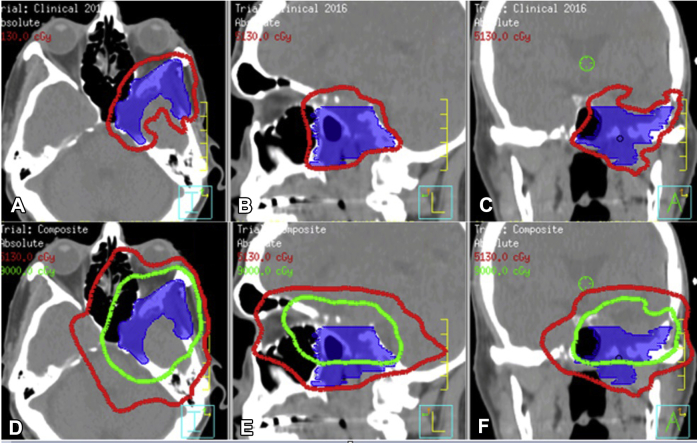
Two neurosurgical opinions were in consensus that a meaningful surgical decompression was not feasible. Because of the patient's progressive symptoms, repeat image guided IMRT to a dose of 54 Gy in 1.8 Gy/fraction was offered (Fig 5). With an anticipated cumulative dose greater than 100 Gy to the left cavernous sinus and posterior orbit, the patient was counseled regarding the risk of visual pathway injury, cranial nerve or brain injury, and hearing loss. The patient consented to retreatment. Planning details were similar to those of the first course of radiation therapy.
Figure 5.
Non-contrast computed tomography images. Blue is gross tumor volume for the recurrent tumor. (A-C) Retreatment plan for recurrence with the 95% isodose line in red. (D-F) Composite plan that sums the doses for the initial and retreatment plans with the 51 Gy (red) and 90 Gy (green) isodose lines indicated.
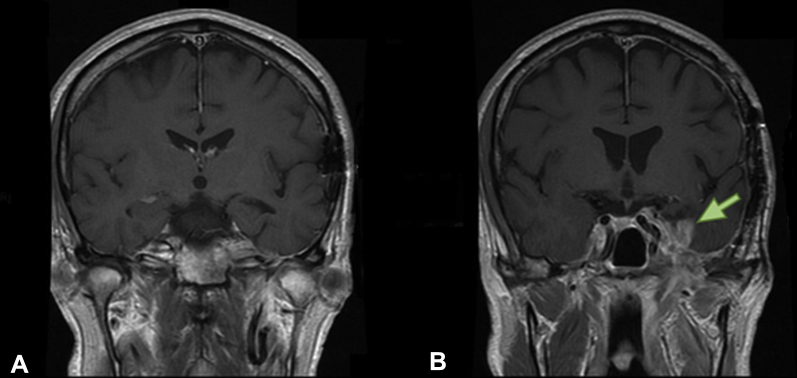
At the initial 8-week follow-up visit, improvement was noted in the patient's left-sided facial numbness and pain and a significant reduction in tumor size was found on MRI scan (Fig 6A). He could be weaned from dexamethasone and later discontinued hydromorphone and tegretol.
Figure 6.
Post-reirradiation. T1-weighted magnetic resonance imaging head scans post-gadolinium. (A) 8-week follow-up; (B) 6-month follow-up. Green arrow indicates new enhancement, possibly due to a radiation-related injury.
Unfortunately, at 6 months, the patient's trigeminal pain—consistent with a cranial nerve injury—returned, along with a significant decline in left visual acuity from 20/60 to detecting hand motions only. Opioid analgesics were reinstituted for pain control. Repeat imaging scans, however, demonstrated ongoing stability of the tumor with new enhancements in the medial temporal lobe consistent with radiation injury (Fig 6B). Given that radiation-related neuropathy is typically painless, there remained a concern for early tumor recurrence in the cavernous sinus that was not yet detected on serial imaging scans. Ongoing clinical and radiologic monitoring will be required to differentiate between radiation-induced toxicity and recurrence.
Discussion
We present a case of a skull base meningioma that recurred after initial surgery and salvage IMRT (50.4 Gy) and was subsequently reirradiated (54 Gy). This case highlights the issue of dose selection and understanding regions at risk for recurrence during treatment planning.
Meningiomas have a propensity for local infiltration, and the specific pattern of extension may be indicative of recurrence rates.1 Regardless, conventional target volumes typically cover the contrast-enhancing volume only. For example, typical radiation surgery plans add no CTV margin,2 and conventionally fractionated stereotactic plans include minimal or no CTV margin except in cases of atypical or high-grade tumors in which subclinical extension is more common.3 In our case, we speculate that the tumor regrowth after initial radiation therapy may be attributable to inadequate target coverage of microscopic disease at the skull base. In the original plan, the caudal border of the high-dose volume was flush against the skull base (Fig 4), which may have excluded microscopic disease that had extended below the skull base and possibly through the formina.
Previous reports have also suggested that regional multicentricity, the presence of microscopic foci of meningotheliomatous cell aggregates in regional dura, may also contribute to observed recurrence patterns even after an apparent complete resection.4 In addition, meningiomas may evolve over time into more aggressive variants, which have been associated with inferior salvage rates with delayed salvage radiation therapy after subtotal resection.5, 6
Initial radiation therapy was limited to 50.4 Gy because of the proximity of the tumor to the optic nerve. Despite the lower dose, this was unlikely to be a factor in the patient's subsequent recurrence. Previous reports have demonstrated the effectiveness of 50 to 50.4 Gy in treating benign meningiomas. A retrospective study treated 131 patients with benign meningiomas to 50 Gy in 30 to 33 fractions where the tumor involved or was adjacent to optic structures; the study reported 10-year local control rates of 100%, 100%, and 89% for tumors of the optic sheath, cavernous sinus, and skull base, respectively.7 A prospective observational study of 30 patients with menigiomas that caused visual impairment delivered a mean target dose of 50.4 Gy in 28 fractions, which improved visual symptoms in 40% of cases and achieved stability in the remainder.8 Of note, the recently reported Radiation Therapy Oncology Group (RTOG) 0539 protocol9 recommends a dose of 54 Gy for recurrent grade 1 tumors with a dose constraint of 50 Gy for optic nerves. An alternate approach for our case would have been to deliver a dose of 54 Gy to the original GTV and accept a lower dose (50 Gy) in regions of overlap between the target volumes and the optic nerve.
For meningiomas, target volume delineation is controversial. For example, the inclusion of the entire dural tail within the prescription dose has been debated.10 Furthermore, hyperosteotic bone may be reactive in nature or may harbor infiltrating tumor cells. In RTOG 0539, recurrent grade 1 and 2 meningiomas received 54 Gy, with 5-year local control rates of 85%. Target volumes were defined by the tumor bed on the postoperative MRI scan, including any nodular enhancement but excluding cerebral edema and the dural tail. A CTV margin of 1.0 cm was recommended except along natural barriers (eg, skull base) where a 0.5 cm margin was permitted, provided there was no evidence of bone invasion.9 For reference, an RTOG-style GTV and CTV are included in Fig 4. This demonstrates that enhanced coverage supero-laterally would have been provided, but the inferior border may still have been undertreated. Fusion of preoperative imaging scans with planning CT scans at the time of salvage radiation therapy may be helpful in determining the extent of contact between the original tumor and fixed structures such as the base of the skull to better define regions at risk for recurrence.
Bearing in mind these uncertainties, we recommend including adjacent foramina at the skull base in target volumes, which may have been implicitly included in traditional, nonconformal radiation therapy approaches but require explicit coverage with modern conformal techniques. This is likely a worthwhile endeavor for potential reduction in recurrence risk with minimal added toxicity.
Conclusion
Radiation therapy provides an effective solution to the anatomical challenges of skull base meningiomas where complete resection is not possible but treatment planning requires consideration of areas at risk for recurrence. In particular, in the postoperative salvage setting, the original tumor bed and points of contact along the base of the skull should be encompassed by target volumes. Successful tumor control through reirradiation of recurrent grade 1 meningioma is possible, albeit with the risk of radiation-induced toxicity.
References
- 1.Bloss H.G., Proescholdt M.A., Mayer C., Schreyer A.G., Brawanski A. Growth pattern analysis of sphenoid wing meningiomas. Acta Neurochir (Wien) 2010;152:99–103. doi: 10.1007/s00701-009-0556-2. discussion 103. [DOI] [PubMed] [Google Scholar]
- 2.Baumert B.G., Rutten I., Dehing-Oberije C. A pathology-based substrate for target definition in radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2006;66:187–194. doi: 10.1016/j.ijrobp.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 3.Debus J., Kocagoncu K.O., Hoss A., Wenz F., Wannenmacher M. Fractionated stereotactic radiotherapy (FSRT) for optic glioma. Int J Radiat Oncol Biol Phys. 1999;44:243–248. doi: 10.1016/s0360-3016(98)00559-8. [DOI] [PubMed] [Google Scholar]
- 4.Borovich B., Doron Y. Recurrence of intracranial meningiomas: The role played by regional multicentricity. J Neurosurg. 1986;64:58–63. doi: 10.3171/jns.1986.64.1.0058. [DOI] [PubMed] [Google Scholar]
- 5.Pourel N., Auque J., Bracard S. Efficacy of external fractionated radiation therapy in the treatment of meningiomas: A 20-year experience. Radiother Oncol. 2001;61:65–70. doi: 10.1016/s0167-8140(01)00391-7. [DOI] [PubMed] [Google Scholar]
- 6.Condra K.S., Buatti J.M., Mendenhall W.M., Friedman W.A., Marcus R.B., Jr., Rhoton A.L. Benign meningiomas: Primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427–436. doi: 10.1016/s0360-3016(97)00317-9. [DOI] [PubMed] [Google Scholar]
- 7.Solda F., Wharram B., De Ieso P.B., Bonner J., Ashley S., Brada M. Long-term efficacy of fractionated radiotherapy for benign meningiomas. Radiother Oncol. 2013;109:330–334. doi: 10.1016/j.radonc.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Milker-Zabel S., Zabel-du Bois A., Huber P., Schlegel W., Debus J. Intensity-modulated radiotherapy for complex-shaped meningioma of the skull base: Long-term experience of a single institution. Int J Radiat Oncol Biol Phys. 2007;68:858–863. doi: 10.1016/j.ijrobp.2006.12.073. [DOI] [PubMed] [Google Scholar]
- 9.Rogers L., Zhang P., Vogelbaum M.A. Intermediate-risk meningioma: Initial outcomes from NRG Oncology/RTOG-0539. Int J Radiat Oncol Biol Phys. 2015;93:S139–S140. [Google Scholar]
- 10.Rogers L., Jensen R., Perry A. Chasing your dural tail: Factors predicting local tumor control after gamma knife stereotactic radiosurgery for benign intracranial meningiomas: In regard to DiBiase et al. (Int J Radiat Oncol Biol Phys 2004;60:1515-1519) Int J Radiat Oncol Biol Phys. 2005;62:616–618. doi: 10.1016/j.ijrobp.2005.02.026. [DOI] [PubMed] [Google Scholar]