Abstract
Purpose
To conduct phase 1 and 2 trials with photon intensity modulated radiation therapy and intensity modulated proton therapy (IMPT) arms to selectively escalate the retroperitoneal sarcoma preoperative radiation dose to tumor volume (clinical target volume [CTV] 2) that is judged to be at a high risk for positive margins and aim to reduce local recurrence. We report on the IMPT study arm in phase 1.
Methods and materials
Patients aged ≥18 years with primary or locally recurrent retroperitoneal sarcoma were treated with preoperative IMPT, 50.4 GyRBE in 28 fractions, to CTV1 (gross tumor volume and adjacent tissues at risk of subclinical disease) with a simultaneous integrated boost to CTV2 to doses of 60.2, 61.6, and 63.0 GyRBE in 28 fractions of 2.15, 2.20, and 2.25 GyRBE, respectively. The primary objective of the phase 1 study was to determine the maximum tolerated dose to CTV2, which will be further tested in the phase 2 study.
Results
Eleven patients showed increasing IMPT dose levels without acute dose limiting toxicities that prevented dose escalation to maximum tolerated dose. Acute toxicity was generally mild with no radiation interruptions. No unexpected perioperative morbidity was noted. Eight months postoperatively, one patient developed hydronephrosis that was treated by stent with ureter dissected off tumor and received 57.5 GyRBE. Retained ureter(s) was (were) subsequently constrained to 50.4 GyRBE without further problem. With an 18-month median follow-up, there were no local recurrences.
Conclusions
IMPT dose escalation to CTV2 to 63 GyRBE was achieved without acute dose limiting toxicities. The phase 2 study of IMPT will accrue patients to that dose. Parallel intensity modulated radiation therapy phase 1 arm is currently accruing at the initial dose level. Ureters that undergo a high dose radiation and/or surgery are at risk for late hydro-ureter. Future studies will constrain retained ureters to 50.4 GyRBE to avoid ureteral stricture.
Summary.
Selective preoperative intensity modulated radiation therapy dose escalation to the posterior retroperitoneal volume that is predicted to be at a high risk for positive margins has been investigated as a strategy to reduce the high local recurrence rate (approximately 50%) after preoperative radiation and surgery for retroperitoneal sarcoma. This prospective, phase 1 study reports further dose escalation to a planned target dose of 63 GyRBE in 28 fractions to this volume with intensity modulated, magnetically scanned protons.
Introduction
Soft tissue sarcomas are uncommon, with an estimated incidence of 11,930 cases in the United States in 20151; 15% is retroperitoneal sarcoma (RPS).2 The size of RPS, typically >10 cm, and complex retroperitoneal anatomy make treatment challenging.3 Wide surgical margins are not achievable, and narrowly negative margins are achieved in approximately 60% to 70% of patients,4 although local recurrence (LR) is frequent even with negative margins.5 Conventional external beam radiation therapy (EBRT) is problematic because the target dose often exceeds the tolerance levels of the small bowel, kidney, and liver. Many RPS deaths are attributed to uncontrolled locoregional disease, although intermediate- and high-grade RPS have significant rates of distant metastases (DM).6 Improved local tumor control (LC) may improve overall survival, which currently is approximately 50% at 5 years. RPS can recur after 5 years7, 8 as illustrated by the Toronto report of 5- and 10-year survival rates of 36% and 14%, respectively, and 5-year locoregional relapse-free rates of 28% at 5 years but only 9% at 10 years.7
RPS poses significant surgical challenges. Incomplete resections (residual tumor [R] 2) are not curable. However, there is a clear benefit with macroscopically complete resections (R0 or R1)8, 9, 10, which is standard-of-care for localized, operable RPS.11 Satisfactory margins may require en bloc removal of adjacent viscera.3 Approximately 30% to 40% of gross total RPS resections have microscopically (+) margins, although their prognostic significance is unclear and in part reflects the pathological margin assessment limitations that are related to complex and variable anatomy.5 Given these limitations, even patients with (−) margins are at risk for relapse, and prognostic factor studies do not demonstrate a clear association between margin status and LR.5, 8
Currently, there are no defined roles for adjuvant therapy outside of clinical trials. Although patients with high-grade RPS are at risk for distant recurrence, there is no level-one evidence that adjuvant chemotherapy mitigates this risk.12 Given the uncertain benefit of adjuvant chemotherapy for RPS, it remains investigational.12
There is no level-one evidence that postoperative radiation improves LC after R0/R1 resections.13 Many groups do not administer postoperative EBRT after R1 resection because they are concerned about the potential toxicity to fixed bowel in the tumor bed.14, 15 In a small trial conducted by the U.S. National Cancer Institute, 35 patients were randomized to receive either postoperative EBRT of 50 to 55 Gy (35-40 Gy to extended field + 15 Gy to boost field) or misonidazole plus electron intraoperative radiation therapy (IORT) and postoperative EBRT (35-40 Gy).16 The LR rate was 6 of 15 patients (40%) with IORT plus EBRT versus 16 of 20 patients (80%) with EBRT alone (P < .001). Significant radiation-related enteritis occurred in 10 of 20 patients who received only EBRT compared with 2 of 15 patients with IORT/EBRT (P < .05). Peripheral neuropathy rates were higher with IORT/EBRT, which was attributed to overlapping IORT fields and misonidazole. Given the small sample size, toxicity, and relatively high LR rates in both arms, the trial is not considered definitive and has not changed the standard of care. Nevertheless, several other reports of IORT plus EBRT suggest that high rates of LC with higher radiation doses are achievable with IORT.17, 18, 19 However, IORT, which is generally one 10 to 15 Gy fraction, can be associated with neural injury or ureteral stricture.20
Some retrospective reports suggest that EBRT improves LC11 or delays LR,7 but others report no value.8, 21 In general, these reports are primarily of postoperative EBRT, include small patient numbers, and contain little information on the treatment selection criteria.
Although both pre- and postoperative EBRT are options, preoperative radiation therapy is well tolerated and has potential advantages22, 23 that include (1) small bowel displacement from high dose volume by tumor, resulting in less toxicity; (2) better gross tumor delineation for radiation therapy planning; (3) the potential for higher dose delivery to portions of tumor; (4) potentially lower risk of intraperitoneal dissemination of a viable tumor at the time of surgery; and (5) possible higher biological effectiveness of preoperative radiation therapy. In a series from Toronto24, although the radiation volume was large (median 7.3 L), preoperative EBRT (median dose, 45 Gy) was associated with European Organization for Research and Treatment of Cancer (EORTC)/Radiation Therapy Oncology Group (RTOG) acute toxicity scores <2. In a phase 1 trial from MD Anderson, patients received preoperative EBRT in escalating doses in combination with low-dose infusional doxorubicin.25 At the maximum dose of 50.4 Gy, preoperative chemoradiation was well tolerated, and only 18% of participants had grade 3 or grade 4 nausea. Similarly, a recent series of 31 patients who were treated with 50 Gy preoperative radiation therapy at Brigham and Women’s Hospital reported only 3% with grade ≥3 acute gastrointestinal toxicity.26 The low toxicity level of preoperative EBRT likely relates to the tumor displacement of the bowel from the radiation target volume.
In reports on preoperative EBRT, outcomes appear improved compared with those expected from treatment with surgery alone.27 However, without level-one data, it is not possible to determine whether this improvement is real or stems from selection or other confounding factors. A randomized study of preoperative radiation therapy versus treatment with surgery alone conducted by the American College of Surgeons Oncology Group was terminated early because of poor accrual. A similar study by the EORTC is currently ongoing and accruing well and will hopefully define the role of preoperative EBRT (Clinical Trials identifier: NCT01344018).
In a dosimetric study, photon intensity modulated radiation therapy (IMRT) was superior to 3-dimensional conformal photons for RPS.28 Bossi et al29 delivered preoperative IMRT (50 Gy) to a limited clinical target volume that encompassed the posterior abdominal wall region at a higher risk for relapse in patients with retroperitoneal liposarcomas. At the 27-month median follow-up, 2 of 18 patients had LR.29 Tzeng et al30 safely delivered dose-painted, dose-escalated preoperative IMRT of 45 Gy to a standard-risk volume and 57.5 Gy to the high-risk posterior RPS margin where surgical margins were predicted to be positive. The actuarial risk of LR at 2 years was 20%.30
In an effort to further reduce LR, we hypothesized that further dose escalation to this high-risk margin was possible with advanced radiation therapy techniques and that protons, with no exit dose beyond the Bragg peak, might be an excellent tool for further dose escalation. Both magnetically scanned intensity modulated proton therapy (IMPT) and IMRT photons can selectively “dose paint” a simultaneous integrated boost to predicted high-risk tumor margins. Although protons have up to 60% lower integral dose31, IMRT might safely allow a similar dose escalation. Hence, we designed a phase 1 and phase 2 study to employ separate photon IMRT and proton IMPT arms and assess the safety and efficacy of selective dose escalation to this high-risk volume. We report on the phase 1 proton IMPT arm of the study.
Methods and materials
Study design
This multi-institutional phase 1 and phase 2 study of preoperative image guided IMPT or IMRT with simultaneously integrated boost (SIB) to the high-risk margin of RPS was approved by the institutional review boards of the participating institutions, and patients provided informed consent. The phase 1 study was designed to evaluate up to 4 dose levels. Once the maximum tolerated dose (MTD) was determined in phase 1, participants would enroll in the phase 2 arm to determine LR rate and potential merit of a larger phase 2R or phase 3 trial. We hypothesized that preoperative IMRT and/or IMPT at the MTD would reduce the 5-year LR rate from 50% (estimated from reported results24 with resection and conventional EBRT dose/fractionation) to 20% and that IMPT might result in fewer toxicities than IMRT.
Objectives
The primary objective of the phase 1 study was MTD determination, based mainly on acute toxicity. The primary objective of the phase 2 study is the assessment of LC with IMRT and/or IMPT at the MTD and surgery. Secondary objectives of the study are clinical response on post-IMRT and post-IMPT imaging, pathologic response (necrosis percentage and margin status), and survival with inclusion of an exploration of progression-free survival times relative to surrogate biological endpoints in tissue and blood.
Eligibility
Previously unirradiated patients aged ≥18 years with Eastern Cooperative Oncology Group Performance Status ≤1, normal liver and/or bone marrow function, and measurable, histologically proven primary (or locally recurrent after prior surgery) RPS were eligible. Patients with rhabdomyosarcoma, Ewing’s sarcoma, osteosarcoma, Kaposi’s sarcoma, angiosarcoma, aggressive fibromatosis, or dermatofibrosarcoma protuberans, chondrosarcoma other than extraskeletal chondrosarcoma, well-differentiated liposarcoma where the target could not be distinguished from normal retroperitoneal fat, multifocal intra-abdominal disease, or lymph node or distant metastases on the basis of abdominopelvic computed tomography (CT) or magnetic resonance imaging (MRI) and chest CT scans were excluded. Other exclusions were uncontrolled intercurrent or psychiatric illness that would limit compliance with the study requirements, other invasive malignancy (except skin basal and/or squamous cell) unless the patient was disease-free for ≥3 years, or HIV-positive status.
Eligible participants were judged resectable and medically fit for surgery by a surgical oncologist and had received no chemotherapy for >4 weeks with resolution of chemotherapy-related adverse events to Common Terminology Criteria for Adverse Events 4 grade <1.
Treatment plan
The dose escalation for the phase 1 study is outlined in Table 1. The maximum target CTV2 proton dose tested was 63 GyRBE/28 fractions of 2.25 GyRBE. This dose is estimated to be radiobiologically equivalent to approximately 66.6 GyRBE at 1.8 GyRBE/fraction using an acute sarcoma α/β ratio of 6, which is the appropriate dose range for margin positive sarcoma,32 and equivalent to approximately 69.8 GyRBE at 1.8 GyRBE/fraction using a neurologic late effect α/β ratio of 3, which approaches spinal nerve tolerance.33
Table 1.
Protocol schema
|
DLT, dose-limiting toxicity; fx, fraction; GyRBE, Gray radiobiological equivalent; MTD, maximum tolerated dose; PTV, planning target volume.
In the phase 2 arm, patients will receive the MTD from the phase 1 study. Phase 1 study participants received preoperative image guided intensity modulated radiation therapy (in separate IMRT or IMPT arms) with SIB to the high-risk margin followed by surgery 4 to 8 weeks later. If patients developed bowel obstruction during radiation therapy, they were referred for immediate surgery.
Starting dose on each phase 1 arm was level one. DLTs were counted separately within each arm so that DLTs observed in one arm would not preclude dose escalation in the other. Patients were evaluated for toxicities at baseline, during treatment, and through the 30-day postsurgery assessment. If none of the first 3 patients in a dose level cohort or arm experienced a DLT (Table 2), the dose for that arm was escalated to the next dose level. After phase 1 MTD determination, phase 2 would begin accrual at the MTD, planning 22 subsequent participants per arm.
Table 2.
Acute toxicity noted in study patients and definitions of dose-limiting toxicities
| Acute radiation-related toxicities in study patients | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Nausea | Vomiting | Anorexia | Reflux | Diarrhea | Constipation | Flatulence | Fatigue | Skin | Neutropenia |
| 0 | 3 | 6 | 7 | 10 | 9 | 9 | 10 | 2 | 2 | 10 |
| 1 | 5 | 4 | 2 | 1 | 2 | 2 | 1 | 6 | 9 | 1 |
| 2 | 3 | 1 | 2 | 0 | 0 | 0 | 0 | 3 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade | Dose-limiting toxicity definition |
|---|---|
| ≥3 | Non-hematologic or hematologic toxicity that requires >7-day interruption in therapy (excluding alopecia and nausea/vomiting not controlled by optimal supportive care) |
| ≥4 | Non-hematologic toxicity |
| ≥4 | Neutropenia or thrombocytopenia |
Radiation therapy guidelines
Daily image guidance, kilovoltage orthogonal, or cone beam imaging was required. The radiation therapy administered on each arm was either IMPT or IMRT; patients in the IMPT arm received up to 11 IMRT fractions as needed to avoid a treatment start delay if an immediate proton treatment slot was unavailable. The prescription dose covered 95% of the planning target volume (PTV), with >99% of the PTV receiving >97% of the prescription dose and ≤20% of the PTV receiving ≥110% of the prescription dose. Normal tissue constraints could limit the dose to portions of the target volumes at the discretion of the treating physician.
For radiation planning and/or treatment, patients were immobilized with arms elevated, and 4-dimensional CT simulation was performed. For the radiation therapy planning, gross tumor volume (GTV) was defined by CT or magnetic resonance imaging T1 plus contrast images. If 4-dimensional CT scans showed GTV motion, an iGTV was defined to capture motion. The average risk for CTV1 was an anatomically constrained 1 to 1.5 cm expansion on the GTV or iGTV with an edited reduction at bone, renal and hepatic interfaces (0 mm), bowel and air cavity (5 mm), and skin (3 mm).15 CTV1 expanded fully into the retroperitoneal muscles but not beyond the peritoneal compartment or intact fascia. For RPS that extended into the thigh through the inguinal canal, the inferior margin was 3 cm below the GTV and the thigh radial margin was 1.5 cm but not beyond the compartment, intact fascia, or bone.
The high-risk margin volume (CTV2) was delineated jointly by the radiation oncologist and surgeon and typically included tumor margin along the posterior retroperitoneal musculature, ipsilateral prevertebral space, major vessels, or organs to be left in situ after surgery. This included approximately 1.5 cm of the GTV that abuts the anticipated positive margin and expands 5 to 10 mm into the tissues at risk.34 A study patient with high-risk CTV2 is shown in Figure 1. The planning target volumes expanded CTV1 and CTV2 by 5 mm to reflect set-up uncertainty. Normal tissues were constrained per Table 3. The radiation treatment plan for one of the study patients is shown in Figure 2.
Figure 1.
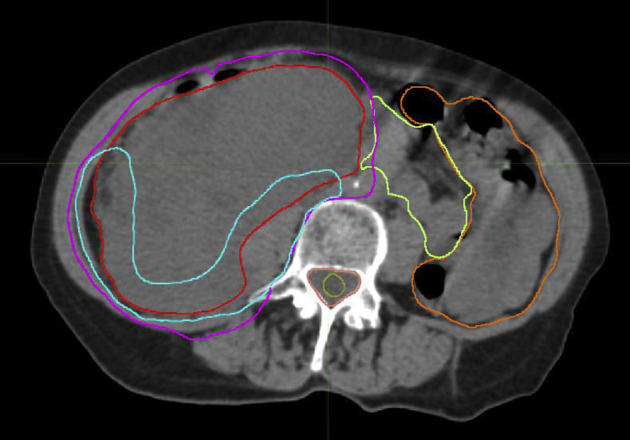
Target volumes in a patient with dedifferentiated liposarcoma: internal gross tumor volume (red), clinical target volume (CTV) 1 (magenta), and high risk CTV2 (turquoise).
Table 3.
Retroperitoneal sarcoma protocol normal tissue dose constraints
| Structure | Dose | Volume |
|---|---|---|
| Small bowel | V45 Gy | ≤50% |
| V55 Gy | ≤20 cm3 | |
| Max 55 Gy | Point dose | |
| Colon | V60 Gy | ≤20 cm3 |
| Max 60 Gy | Point dose | |
| Rectum | V60 Gy | ≤35% |
| V63 Gy | ≤30% | |
| Max 63 Gy | Point dose | |
| Stomach | V45 Gy | ≤100% |
| V50 Gy | ≤50% | |
| V55 Gy | ≤20 cm3 | |
| Max 55 Gy | Point dose | |
| Kidney (if 1 resected) | V20 Gy | ≤20% of retained kidney |
| Kidney (if both remain) | V18 Gy | ≤50%; mean dose < 15 Gy |
| Bladder | V60 Gy | ≤50% |
| V63 Gy | ≤25% | |
| Max 63 Gy | ||
| Liver | V25 Gy | ≤50% |
| V30 | ≤40% | |
| CTV2 Rx dose | ≤20% | |
| Spinal cord | V45 Gy | ≤95% |
| V50 Gy | ≤5% | |
| Max 50 Gy | ||
| Spinal nerves if applicable | 70.2 Gy equivalent (at 1.8 Gy/fx)∗ |
Point dose |
| Retained ureter(s) | Max 50.4 Gy | Point dose |
| Ureter to be resected | Max CTV2 Rx Dose | Point dose |
CTV, clinical target volume; GyRBE, Gray radiobiological equivalent.
D1 (d1 + α/β) = D2 (d2 + α/β), where α/β = 3, D1 is the total dose, d1 is the fraction size, d2 is 1.8 GyRBE, and D2 is the tolerance dose.
Figure 2.
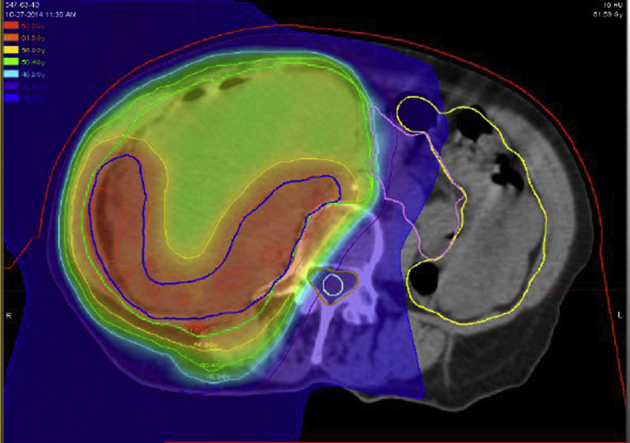
Radiation plan for patient with dedifferentiated liposarcoma. Clinical target volume (CTV) 1: 50.4 GyRBE and CTV2: 61.6 GyRBE treated with 11 intensity modulated radiation therapy and 17 intensity modulated proton therapy fractions.
Participants were seen weekly during radiation therapy by a radiation oncologist who conducted a physical examination and assessed patients’ vital signs, weight, symptoms, and toxicity. Toxicity was scored using NCI Common Terminology Criteria for Adverse Events v.4. DLTs were assessed from the start of radiation therapy until 30 days after resection (Table 2); neither events that were unrelated to radiation nor tumor symptoms were DLTs. The relationship between adverse events and study treatment was scored as definite, probable, possible, unlikely, or unrelated.
Surgery and pathology
The surgical plan was macroscopically complete tumor resection (R0 or R1), and adjacent organs were partially or completely resected as indicated by the operative findings. No IORT was given. The high-risk margins were marked with sutures on the resection specimen. Resections were classified as wide (margin of >1 cm), close (margin of ≤1 cm), marginal (margin abutting tumor), or R2 (gross residual disease). Patients who underwent wide, close, or marginal resections were further scored as either microscopically R0, margin (−) with the margin width specified in mm, or as R1, margin (+).
Follow-up after radiation therapy and surgery
After completion of radiation therapy, patients underwent restaging chest and/or abdominal scans, bloodwork (complete blood count/differential and liver/renal function), and toxicity assessments. Postoperatively, patients were examined within 1 month of hospital discharge and again 4 months postoperatively for a clinical assessment of weight and performance status, bloodwork (complete blood count/differential and liver/renal function), toxicity assessments, and restaging scans. Thereafter, patients were seen at least twice per year until year 5 and annually until year 10 for oncologic surveillance with chest/abdominal imaging and a clinical assessment that includes performance status, disease status (LR ± DM), and toxicity.
Oncologic outcome
Clinical response was assessed in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) criteria at the time of preoperative reimaging. Pathologic response was scored as percent necrosis (0-24%, 25-49%, 50-74%, 75-99%, 100%). LR was defined as tumor regrowth within the primary site and marginal failure as tumor growth at the CTV1 margin. Peritoneal failure was defined as intraperitoneal tumor growth beyond the CTV1 margins.
Results
Eleven patients with primary, previously untreated RPS were assigned to the IMPT arm (Table 4) and sequentially to each dose level without acute DLTs to prevent dose escalation to the MTD. Although chemotherapy was permitted if completed ≥4 weeks before study entry, no patients actually received chemotherapy before the start of protocol radiation treatment. The first patient started radiation on December 3, 2012 and the last patient completed surgery on August 28, 2015. All patients completed radiation therapy to the prescribed dose; 4 patients who each accrued to dose levels 1 and 2 (one patient in each of these levels did not undergo surgery because of metastases on preoperative restaging) and 3 patients accrued to dose level 3. One patient received only IMPT; the other patients received 5 to 11 IMRT fractions and the remaining fractions with IMPT. Volumes for iGTV ranged from 262 to 3161 cm3 (median 2029), CTV1 ranged from 412 to 4594 cm3 (median 3121), and CTV2 ranged from 163 to 1567 cm3 (median 819). Acute toxicity during radiation was generally mild with no required treatment breaks (Table 2).
Table 4.
Patient information
| Dose Level | Age (y) | Sex | Histology | Grade | Initial Size(cm) | Preoperative Size (cm) | Organs resected | Margins (mm) | Necrosis/Fibrosis (%) | Follow-up (mo) | LC | DM | Status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | F | LMS | 2 | 17.7 | 17.1 | 2 | <1 | 25 | 34 | Yes | No | NED |
| 1 | 69 | M | LMS | 2 | 16.1 | 15.9 | 2 | 1 | 20 | 33 | Yes | Yes | AWD |
| 1 | 62 | M | Spindle | 2 | 19.8 | 22.9 | No op | No op | No op | 6 | NA | Yes | DOD |
| 1 | 55 | F | DDLS | 2 | 23.2 | 22.3 | 6 | +WDLS | 50 | 24 | Yes | No | NED |
| 2 | 54 | M | DDLS | 2 | 21.4 | 20.2 | 4 | 1 | 15 | 21 | Yes | No | NED |
| 2 | 67 | M | DDLS | 3 | 13.9 | 15.9 | No op | No op | No op | 6 | NA | Yes | DOD |
| 2 | 62 | F | LMS | 2 | 10.6 | 8.1 | 5 | + | 15 | 18 | Yes | Yes | AWD |
| 2 | 70 | F | DDLS | 2 | 14.0 | 16 | 2 | +WD/DDLS | 15 | 12 | Yes | No | NED |
| 3 | 66 | F | DDLS | 2 | 17.4 | 16 | 3 | +WD/DDLS | 5 | 6 | Yes | No | NED |
| 3 | 52 | M | DDLS | 2 | 26.3 | 27.8 | 3 | +WDLS | 50 | 4 | Yes | No | NED |
| 3 | 74 | M | WDLS | 1 | 14.6 | 12.7 | 0 | +WDLS | 0 | 1 | Yes | No | NED |
AWD, alive with disease; DDLS, dedifferentiated liposarcoma; DM, distant metastases; DOD, dead of disease; LC, local control; LMS, leiomyosarcoma; NED, no evidence of disease; op, operation; WDLS, well-differentiated liposarcoma.
During the preoperative restaging, primary tumors were all stable in accordance with the RECIST criteria (Table 4). All 9 patients who were metastasis-free underwent R0 or R1 surgery, generally multiorgan and/or structure resections (Table 4). Pathology showed histologic necrosis and/or fibrosis that ranged from 0% to 50% (median 15%; Table 4). Surgical margins were (+) in 6 patients and negative in 3 patients with a margin width of ≤1mm. Upon review of the radiation treatment plans and the pathology, sites with (+) margins were included in the high dose CTV2 volumes, but there is some uncertainty in whether all areas where well-differentiated liposarcoma was present at a surgical margin were included in the boosted volume because of the difficulty in reliably distinguishing well-differentiated liposarcoma from uninvolved retroperitoneal fat on CT imaging. The median hospital stay was 8 days (range, 5-10 days). One patient with dedifferentiated liposarcoma who underwent nephroureterectomy, ileocolectomy, adrenalectomy, partial hepatectomy, cholecystectomy, and partial diaphragm developed an infrahepatic abscess 1 month postoperatively, which necessitated CT-guided percutaneous drainage, after which the patient subsequently completely recovered.
Thirteen months after radiation therapy, one patient with a history of vitamin D deficiency who received a level-one dose experienced an asymptomatic grade 1 compression fracture in the superior endplate of the L3 vertebral body where the anterior cortex received 60.2 Gy. One patient with an inferior vena cava leiomyosarcoma with both ureters draped over the tumor and who received a level-two dose was given 61.6 GyRBE to the high-risk CTV2 with both ureters constrained to 57.5 GyRBE on the basis of a previously published study of IMRT dose escalation.30 The patient underwent radical resection of the retroperitoneal leiomyosarcoma; right nephroureterectomy; dissection of the left ureter off tumor; and inferior vena cava, distal aorta, bilateral common iliac vein/artery resection with aorto-bifemoral arterial bypass reconstruction. She recovered well from surgery but developed left-sided hydronephrosis (grade 3) 8 months later, which was managed with a ureteral stent. Renal function subsequently recovered to a creatinine level of 1.2.
Once this toxicity was recognized, the protocol was modified to reduce the dose constraint to a retained ureter to 50.4 GyRBE without further ureteral problems. The maximum dose to the retained ureters among the remaining 8 patients who underwent surgery ranged from 0.5 to 56 GyRBE (median 23.5 GyRBE), and the dose to the ureter exceeded 50.4 GyRBE in 1 of these patients.
With an 18-month median follow-up (range, 1-34 months), no patient who underwent resection experienced LR, 7 remained disease-free, and 2 are alive with metastases (lung ± liver). Both patients who did not undergo surgery because of lung metastases during preoperative restaging received chemotherapy but died of the disease 6 months after the start of radiation therapy.
Discussion
In this phase 1 study of dose escalation to RPS tumor volumes that are at high risk for positive resection margins, we reached the planned target dose in the IMPT arm. Enrollment in the IMRT arm continues and will be reported separately. Radiation was acutely well tolerated by patients with grade ≤2 acute toxicity. Patients without interval development of metastases underwent radical, generally multiorgan, resections without unanticipated acute perioperative morbidity. Because of the risk of normal tissue toxicity in the volumes receiving the CTV2 dose, it is important to emphasize that the reported results are based on a very close collaboration between the surgical and radiation oncologists to define the high-risk volume and determine which organs and/or structures should be resected. In addition, the radiation treatment plans were carefully reviewed to ensure that the protocol for normal tissue constraints were met to minimize treatment toxicity risk.
To date, one grade 3 late hydronephrosis occurred in a retained ureter that was constrained to 57.5 GyRBE and dissected off tumor. Hydronephrosis resolved and renal function recovered with ureteral stenting. Hydronephrosis is uncommon after EBRT of 50.4 Gy35 but has been reported after administration of higher doses. Shaw et al20 noted hydronephrosis in 10 of 16 ureters that were initially non-obstructed after treatment with 50.4 Gy EBRT, surgery, and IORT (more commonly with IORT doses ≥12.5 Gy). Tzeng et al30 reported no ureteral complications after treatment with 57.5 Gy to CTV2. Because RPS is often lateralized and surgery frequently includes nephrectomy on the affected side where a CTV2 boost would be given, hydronephrosis would not likely be seen unless the retained contralateral ureter were also included in CTV2. This scenario is uncommon and only relevant for bulky midline tumors. After the hydronephrosis in our patient, to err on the side of caution, we modified the protocol dose constraint for retained ureters to 50.4 Gy. No cases of hydronephrosis were seen in the highest dose level cohort with the ureter dose constrained as such.
With an 18-month median follow-up, no patient who underwent resection experienced an LR. Additional follow-up is needed to assess treatment efficacy. DM, however, have developed in 4 of 11 patients and 2 of 9 patients after resection, underscoring the risk for systemic metastases with intermediate or high grade RPS.
The phase 2 study of the IMPT arm is now open at a CTV2 dose of 63 GyRBE with a plan to enroll 22 patients for study. This will hopefully provide additional information about the efficacy and safety of this approach and may permit some comparison with outcomes that are achieved with standard preoperative radiation doses of 50.4 Gy ± IORT. The phase 1 study of the IMRT arm (opened after the IMPT arm) is currently enrolling patients to the dose level 1 and will assess the feasibility of similar IMRT dose escalations and some toxicity comparison with IMPT.
Conclusions
IMPT dose escalation to high-risk CTV2 with a dose of 63 GyRBE was achieved without acute DLT. The phase 2 study of IMPT will enroll for this dose. A parallel phase 1 study of the IMRT arm is currently enrolling patients at the initial dose level. Ureters that undergo high dose radiation and/or surgery are at risk for late hydroureter; however, no ureteral problems were noted when the ureters were constrained to 50.4 Gy. Future studies will constrain retained ureters to 50.4 GyRBE to avoid ureteral stricture.
Footnotes
Sources of support: Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center and NCI grant # 2U19CA021239-35.
Conflicts of interest: None.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence W., Jr., Donegal W.L., Natarajan N., Mettlin C., Beart R., Winchester D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann Surg. 1987;205:349–359. doi: 10.1097/00000658-198704000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gronchi A., Lo Vulo S., Fiore M. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009;27:24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 4.Bonvalot S., Rivoire M., Castaing M. Primary retroperitoneal sarcomas: A multivariate analysis of surgical factors associated with local control. J Clin Oncol. 2009;27:31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 5.Stojadinovic A., Leung D.H., Hoos A., Jacques D.P., Lewis J.J., Brennan M.F. Analysis of the prognostic significance of microscopic margins in 2,084 localized primary adult soft tissue sarcomas. Ann Surg. 2002;235:424–434. doi: 10.1097/00000658-200203000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan M.C., Brennan M.F., Kuk D. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surg. 2016;263:593–600. doi: 10.1097/SLA.0000000000001149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catton C.N., O'Sullivan B., Kotwall C., Cummings B., Hao Y., Fornasier V. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 1994;29:1005–1010. doi: 10.1016/0360-3016(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 8.Lewis J.J., Leung D., Woodruff J.M., Brennan M.F. Retroperitoneal soft-tissue sarcoma: Analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998;228:355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bevilacqua R.G., Rogatko A., Hajdu S.I., Brennan M.F. Prognostic factors in primary retroperitoneal soft-tissue sarcomas. Arch Surg. 1991;126:328–334. doi: 10.1001/archsurg.1991.01410270072012. [DOI] [PubMed] [Google Scholar]
- 10.Singer S., Antonescu C.R., Riedel E., Brennan M.F. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003;238:358–370. doi: 10.1097/01.sla.0000086542.11899.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoeckle E., Coindre J.M., Bonvalot S. Prognostic factors in retroperitoneal sarcoma: A multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer. 2001;92:359–368. doi: 10.1002/1097-0142(20010715)92:2<359::aid-cncr1331>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 12.Miura J.T., Charlson J., Gamblin T.C. Impact of chemotherapy on survival in surgically resected retroperitoneal sarcoma. Eur J Surg Oncol. 2015;41:1386–1392. doi: 10.1016/j.ejso.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 13.Nussbaum D.P., Speicher P.J., Gulack B.C. Long-term oncologic outcomes after neoadjuvant radiation therapy for retroperitoneal sarcomas. Ann Surg. 2015;262:163–170. doi: 10.1097/SLA.0000000000000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbeau L., Kantor G., Stoeckle E. Surgical resection and radiotherapy for primary retroperitoneal soft tissue sarcoma. Radiother Oncol. 2002;65:137–143. doi: 10.1016/s0167-8140(02)00283-9. [DOI] [PubMed] [Google Scholar]
- 15.Baldini E.H., Wang D., Haas R.L. Treatment guidelines for preoperative radiation therapy for retroperitoneal sarcoma: Preliminary consensus of an international expert panel. Int J Radiat Oncol Biol Phys. 2015;92:602–612. doi: 10.1016/j.ijrobp.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Sindelar W.F., Kinsella T.J., Chen P.W. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch Surg. 1993;128:402–410. doi: 10.1001/archsurg.1993.01420160040005. [DOI] [PubMed] [Google Scholar]
- 17.Gieschen H.L., Spiro I.J., Suit H.D. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int J Radiat Oncol Biol Phys. 2001;50:127–131. doi: 10.1016/s0360-3016(00)01589-3. [DOI] [PubMed] [Google Scholar]
- 18.Yoon S.S., Chen Y.L., Kirsch D.G. Proton-beam, intensity-modulated, and/or intraoperative electron radiation therapy combined with aggressive anterior surgical resection for retroperitoneal sarcomas. Ann Surg Oncol. 2010;17:1515–1529. doi: 10.1245/s10434-010-0935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stucky C.C., Wasif N., Ashman J.B., Pockaj B.A., Gunderson L.L., Gray R.J. Excellent local control with preoperative radiation therapy, surgical resection, and intra-operative electron radiation therapy for retroperitoneal sarcoma. J Surg Oncol. 2014;109:798–803. doi: 10.1002/jso.23576. [DOI] [PubMed] [Google Scholar]
- 20.Shaw E.G., Gunderson L.L., Martin J.K., Beart R.W., Nagorney D.M., Podratz K.C. Peripheral nerve and ureteral tolerance to intraoperative radiation therapy: Clinical and dose-response analysis. Radiother Oncol. 1990;18:247–255. doi: 10.1016/0167-8140(90)90060-a. [DOI] [PubMed] [Google Scholar]
- 21.Karakousis C.P., Velez A.F., Gerstenbluth R., Driscoll D.L. Resectability and survival in retroperitoneal sarcomas. Ann Surg Oncol. 1996;3:150–158. doi: 10.1007/BF02305794. [DOI] [PubMed] [Google Scholar]
- 22.Pisters P.W., O'Sullivan B. Retroperitoneal sarcomas: Combined modality treatment approaches. Curr Opin Oncol. 2002;14:400–405. doi: 10.1097/00001622-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen O.S., O'Sullivan B. Retroperitoneal soft tissue sarcomas: A treatment challenge and a call for randomized trials. Radiother Oncol. 2002;65:133–136. doi: 10.1016/s0167-8140(02)00365-1. [DOI] [PubMed] [Google Scholar]
- 24.Jones J.J., Catton C.N., O’Sullivan B. Initial results of a trial of preoperative external-beam radiation therapy and postoperative brachytherapy for retroperitoneal sarcoma. Ann Surg Oncol. 2002;9:346–354. doi: 10.1007/BF02573869. [DOI] [PubMed] [Google Scholar]
- 25.Pisters P.W., Ballo M.T., Fenstermacher M.J. Phase I trial of preoperative concurrent doxorubicin and radiation therapy, surgical resection, and intraoperative electron-beam radiation therapy for patients with localized retroperitoneal sarcoma. J Clin Oncol. 2003;21:3092–3097. doi: 10.1200/JCO.2003.01.143. [DOI] [PubMed] [Google Scholar]
- 26.Mak K.S., Mannarino E.G., Lee L.K. Bowel bag dose-volume parameters and acute gastrointestinal (GI) toxicity during preoperative radiation therapy (RT) for eetroperitoneal sarcoma (RPS) Int J Radiat Oncol Biol Phys. 2014;90:S117–S118. doi: 10.1016/j.prro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Kelly K.J., Yoon S.S., Kuk D., Qin L.X., Dukleska K., Chang K.K. Comparison of perioperative radiation therapy and surgery versus surgery alone in 204 patients with primary retroperitoneal sarcoma: A retrospective 2-institution study. Ann Surg. 2015;262:156–162. doi: 10.1097/SLA.0000000000001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koshy M., Landry J.C., Lawson J.D. Potential for toxicity reduction using intensity modulated radiation therapy (IMRT) for retroperitoneal sarcoma. Int J Radiat Oncol Biol Phys. 2003;57:S448–S449. [Google Scholar]
- 29.Bossi A., De Wever I., Van Limbergen E., Verstraelen B. Intensity modulated radiation-therapy for preoperative posterior abdominal wall irradiation of retroperitoneal liposarcomas. Int J Radiat Oncol Biol Phys. 2007;67:164–170. doi: 10.1016/j.ijrobp.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 30.Tzeng C.W., Fiveash J.B., Popple R.A. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer. 2006;107:371–379. doi: 10.1002/cncr.22005. [DOI] [PubMed] [Google Scholar]
- 31.Goitein M. Springer; New York, NY: 2008. Radiation oncology: A physicist's eye view. [Google Scholar]
- 32.DeLaney T.F., Kepka L., Goldberg S.I. Radiation therapy for control of soft-tissue sarcomas resected with positive margins. Int J Radiat Oncol Biol Phys. 2007;67:1460–1469. doi: 10.1016/j.ijrobp.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 33.DeLaney T.F., Liebsch N.J., Pedlow F.X. Phase II study of high dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74:732–739. doi: 10.1016/j.ijrobp.2008.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baldini E.H., Bosch W., Kane J.M., 3rd Retroperitoneal sarcoma (RPS) high risk gross tumor volume boost (HR GTV Boost) contour delineation agreement among NRG sarcoma radiation and surgical oncologists. Ann Surg Oncol. 2015;22:2846–2852. doi: 10.1245/s10434-015-4633-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marks L.B., Carroll P.R., Dugan T.C., Anscher M.S. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1257–1280. doi: 10.1016/0360-3016(94)00431-J. [DOI] [PubMed] [Google Scholar]


