Abstract
Selective internal radiation therapy has emerged as a well-accepted therapeutic for primary and metastatic hepatic malignancies. This therapeutic modality requires the combined efforts of multiple medical disciplines to ensure the safe delivery of yttrium-90 (90Y)-labeled microspheres. The development of this therapy followed decades of clinical research involving tumor vascularity and microsphere development. Today, it is essential that treating physicians have a thorough understanding of hepatic tumor vascularity and 90Y microsphere characteristics before undertaking this complex intervention. This review explores the contributions of early investigators of this therapy, as well as the development, US Food and Drug Administration approval, manufacturing process, and attributes of the 2 commercially available 90Y radiolabeled microsphere device to clarify the key physical differences between the products.
Introduction
Selective internal radiation therapy (SIRT) has become a widely employed brachytherapy for the treatment of primary and metastatic hepatic malignancies. During SIRT, millions of yttrium-90 (90Y)-radiolabeled microspheres are injected into the hepatic arteries preferentially depositing into tumors because of their increased vascularity, with the goal of delivering lethal doses of radiation to tumors but sublethal doses to normal parenchyma. The treatment algorithm for this therapy is complex and involves many different health care professionals with different areas of expertise including interventional radiologists, nuclear medicine physicians, radiation oncologists and physicists, and medical and surgical oncologists. A thorough understanding of the available products used in this therapy is important to all members of the treatment team to ensure successful outcomes and to limit treatment-related complications. Many treating physicians favor the use of 1 of the 2 commercially available 90Y microsphere products based on their fellowship training or the preferences at their institution and may be unfamiliar with the key characteristics of the alternative microsphere device. The following review will explore the contributions of early investigators of this therapy, the development, US Food and Drug Administration (FDA) approval, manufacturing process and attributes of the 2 commercially available 90Y-radiolabeled microsphere devices, and the potentially clinically relevant differences based on the products' physical properties.
Yttrium discovery and physical properties
In 1787, Carl Arrhenius discovered a mineral not previously identified in a mine near Ytterby, Sweden, and named it ytterbite. Analyses by Gadolin et al and Ekeberg et al found that the mineral was composed of several different elements including a previously unknown metal, which was subsequently named yttrium.1 90Y is produced for labeling of microspheres by the neutron bombardment of stable yttrium 89 but can also be developed by chemical separation from its parent isotope strontium 90.2 The decay of 90Y is primarily through β(−) emission of a high-speed electron to stable zirconium-90 with an average energy of 0.9367 MeV, a mean tissue penetration of 2.5 mm and a maximum of 11 mm, and a half-life of 2.67 days (64.2 hours). A small portion of decay, however, is through pair production, which has recently been used to assess 90Y microsphere distribution after SIRT with positron emission tomography (PET) imaging.2, 3 During deceleration of the high-energy electrons in the atomic electric field, continuous X-rays or bremsstrahlung (“braking” gamma radiation) are produced. Imaging of this bremsstrahlung radiation is currently the most common manner in which distribution of microspheres are determined following SIRT.4
Radioembolization: Early experience and development
1950s to 1960s
Beginning in the early 1950s and continuing through the 1980s, investigators discovered the key elements of hepatic tumor vascularity, which allowed for the development of hepatic artery–directed therapies. In 1951, Bierman et al demonstrated angiographically that liver tumors received their blood supply from the hepatic artery and not the portal vein.5 This was confirmed by Breedis and Young in 1954.6 Further study led to the conclusion that hepatic malignancies received greater than 80% of their blood supply from the hepatic artery while the normal liver parenchyma received less than one-third.7 These early studies allowed for future investigators to postulate that hepatic arterial-directed therapies may be an effective means of treating hepatic malignancies.
In the 1960s, there were concurrently several reports of hepatic radioembolization with 90Y. Early animal studies by Grady et al demonstrated the feasibility of treating tumors with 90Y and paved the way for human applications.8 Simon et al reported on 5 patients with hepatic neuroendocrine tumors and carcinoid syndrome who were treated with hepatic artery radioembolization using carbonized microspheres 15 microns in diameter embedded with 90Y. The authors discussed the key issues of the technical aspects of the therapy that are still relevant today including intratumoral lung shunts, selective catheterization, dosimetry, preferential deposition of microspheres in tumors, bremsstrahlung scans to evaluate radiation distribution and, most important, nontarget delivery. Unfortunately, 2 of the patients in the cohort developed significant gastric symptoms from unintentional irradiation of the stomach, which is not surprising given the unsophisticated catheters and angiographic techniques available at the time.9 Other reports described the use of 90Y in the treatment of lung cancers, osteogenic sarcomas, and other tumors using plastic and ceramic microspheres with different techniques and, unfortunately, overall poor results.10, 11
1970s to 1980s
The following decade brought further advances in the understanding of hepatic and tumor vascularity, which led the way for the development of techniques to treat liver tumors more effectively through the hepatic artery. Ackerman studied rat tumors and determined that, after tumors reached a diameter of 3 mm, they had developed an arterial supply.12 Taylor et al showed that colorectal metastases received most of their blood supply from the hepatic artery but when it was ligated, portal vein supply to tumors significantly increased, demonstrating what we now know to be the arterial portal communications that exist at the sinusoidal level.13 After colleagues attempted radioembolization through the portal vein with little success, Grady used a 15-micron resin 90Y microsphere injected intra-arterially and reported on 25 patients with metastatic colon cancer, 17 of whom had an “objective decrease” in tumor size. He suggested that those tumors with greater vascularity on angiography should respond more favorably to the treatment.14
In 1983, Stribley et al reported that after injecting 15 micron Cobalt-57 labeled microspheres into the hepatic arteries of rats with implanted salivary adenocarcinoma, the periphery of the tumor consistently demonstrated a blood flow of 3.9 times that of the normal liver parenchyma with a progressive decrease in blood flow toward the central part of tumors once a tumor diameter of 6 mm had been exceeded.15 This supports the premise that microsphere distribution during radioembolization is concentrated in the periphery of many tumors. In 1987, Meade et al evaluated the distribution of different-sized microspheres in experimental rat liver tumors and normal hepatic parenchyma and demonstrated that 15- and 32.5-micron diameter particles lodged preferentially in tumors versus normal parenchyma with a ratio of 3:1. Microspheres of 50 microns in diameter showed no preferential distribution into tumors with equal distribution demonstrated between normal parenchyma and tumors.16
This impacted future decisions regarding microsphere design. Mantravadi et al later noted that increased vascularity, absence of extrahepatic disease, and good performance status were determinants of a successful outcome following SIRT,17 concepts that have been demonstrated in numerous studies and are widely accepted today.18, 19, 20, 21
Toxicities related to radioembolization were reported in early studies, including elevation of liver enzymes, radiation pneumonitis, radiation hepatitis (renamed radiation-induced liver disease and recently radioembolization induced liver disease) and gastrointestinal ulceration.14, 22, 23, 24 Additionally, some patients in these early trials developed myelosuppression, leading to death secondary to leaching of yttrium from the microspheres and subsequent deposition in bone marrow.17 This leaching phenomenon led to decreased interest in SIRT and eventual discontinuation of existing microsphere manufacturing. Development of yttrium-binding techniques that would eliminate or significantly reduce leaching became a primary focus of advanced microsphere technology for both glass and resin platforms in the late 1980s and early 1990s.
Late 1980s to 1990s
In the late 1980s and 1990s, preclinical dosimetry considerations and phase 1 trials emerged regarding both resin and glass microspheres. Burton and Gray and their colleagues at the University of Western Australia obtained tissue samples after resin 90Y administration showed a mean absorbed dose of tumor to liver of 6:1 (range, 0.4-45:1) and suggested that intra-arterial injection of angiotensin II would allow for greater flow to tumors because of its selective vasoconstrictive activity on normal hepatic microvasculature.25 An animal study in 1988 on the recently developed glass microsphere, TheraSphere (BTG International Group),26 was followed by an early human study by Herba, who reported on the treatment of 15 predominantly metastatic colorectal cancer (mCRC) patients, with 10 patients showing stable disease at 7 months after TheraSphere treatment and 5 showing progression. This was one of the first articles to discuss coil embolization of hepatic arteries to allow for redistribution and injection of technetium 99-labeled macroaggregated albumin to determine lung shunt and extrahepatic supply before radioembolization.27
Three years later, Anderson studied different sized microspheres (12.5, 25, and 40 microns) and their relative distribution between liver tumors and normal liver in a rat model similar to the study conducted by Meade described earlier.16, 28 He concluded that optimal ratios were achieved with the larger 40 micron particles over smaller particles. Tumor to normal liver distribution ratios for the three sized microspheres were 0.5, 1.4, and 1.8, important information, which further impacted microsphere design.28
FDA approval of resin microspheres
In the 1990s, Gray and his colleagues studied a resin microsphere labeled with 90Y and developed a technique for its administration, which they named SIRT. Following a laparotomy, a catheter was placed into the hepatic arteries, redistribution of blood performed using a vasoactive agent (angiotensin II) that caused vasoconstriction of normal hepatic parenchymal vessels but not tumor vessels, and the radiolabeled microspheres were then injected to a desired liver dose measured intraoperatively using a survey meter. In 1989, they reported on the first 10 patients with mCRC treated with SIRT, escalating the dose of radiation to the normal liver. Assuming a homogeneous distribution of microspheres, those patients who received greater than 30 Gy to the normal liver had a decrease in carcinoembryonic antigen (CEA) level greater than 50%.29 In 1992, the group reported on an additional 29 patients with all evaluable patients demonstrating a decrease in the CEA level at 3 months (mean 70% decrease).30 Based on these data, Gray and colleagues modified their technique, which allowed for attaching the hepatic arterial catheter to a subcutaneous port so patients could receive intra-arterial infusion of chemotherapy subsequent to SIRT. Following a retrospective analysis of 71 mCRC patients treated with SIRT and floxuridine administered through the arterial infusion catheter in 2000, which reported an 89% response rate of CEA,31 Gray and colleagues published the results of a randomized controlled trial involving 70 patients with mCRC. In this pivotal trial, patients who received a single session of 90Y-labeled resin microspheres plus monthly hepatic artery infusions of floxuridine showed a significantly increased objective tumor response (50% vs 24% [P = 0.03]) and improved median time to progression in the liver (12 vs 7.6 months [P = 0.04]) when compared with patients receiving only hepatic artery infusional floxuridine FUDR.32 Based on the results of these studies, the FDA granted premarket approval (PMA; the FDA process of scientific and regulatory review to evaluate the safety and effectiveness of class III medical devices. PMA approval is based on a determination by FDA that the PMA contains sufficient valid scientific evidence to assure that the device is safe and effective for its intended use(s)33 for SIR-Spheres in 2002 with an approved indication for the treatment of unresectable metastatic liver tumors from primary colorectal cancer with adjuvant intrahepatic artery chemotherapy of FUDR.34 SIR-Spheres microspheres are manufactured and distributed by Sirtex Medical Ltd. (North Sydney, Australia).
Humanitarian device exemption for glass microspheres
Following the development of the 90Y glass microsphere, TheraSphere, through collaboration between the University of Missouri and Theragenics Corporation (Atlanta, Georgia) in the early 1980s, several animal studies were conducted including a canine trial performed by Wollner and colleagues at the University of Michigan in 1988. Doses 12 times those anticipated for humans were administered, which were well-tolerated with minimal hepatic injury. Although observed damage increased with increasing dose, radiation exposure greater than 300 Gy did not cause total hepatic necrosis and the animals survived.26
A pilot trial for the use of glass microspheres in hepatocellular carcinoma (HCC)35 and 2 mixed neoplasia studies27, 36 evaluated more than 100 patients who received TheraSphere; the data from these trials supported the manufacturer's safety claims. These 3 trials led to the approval of TheraSphere in Canada in 1991 for the treatment of liver neoplasia.37
A fourth phase 2 trial, which enrolled 22 patients with HCC who were treated with glass microspheres with a planned dose of 100 Gy to the liver, was then conducted. Of the 22 enrolled, 20 patients were evaluated for therapeutic efficacy. The median survival was 378 days from the time of treatment with a 20% response rate.38 Based on the finding of this study, the FDA concluded that there was a probable benefit of 90Y glass microspheres for the treatment of HCC. In 1999, the FDA cited these 4 trials as the evidence leading to their granting of a humanitarian device exemption (HDE) for TheraSphere for use in patients with unresectable HCC.37 The FDA defines a human use device as one that is intended to benefit patients with any disease or condition that affects fewer than 4,000 individuals per year in the United States. An HDE, which in distinction to a PMA, does not require effectiveness data. Applicants of an HDE must demonstrate that the device does not “pose unreasonable or significant risk of illness or injury” and that the probable benefit of the device “outweighs the risk of injury or illness from the disease.” Institutional review board review and approval must be performed before the use of a human use device at a facility and ongoing review of its use performed at regular intervals.39 Currently, TheraSphere is manufactured by Nordion (Ottawa, Ontario Canada) and distributed by BTG International Group (London, United Kingdom).
Microsphere production and physical characteristics
SIR-Spheres
SIR-Spheres (Sirtex Medical) are composed of a proprietary biocompatible microsphere, coated with a partially cross-linked cation exchange polystyrene resin (Bio-Rad, Hercules, CA). 90Y is incorporated into the resin matrix (no nonintended radioactive impurities) through ion exchange of sodium for yttrium and at this point in the process is attached but not yet immobilized to the microspheres. 90Y is then immobilized to the microsphere following its precipitation as a phosphate salt.40, 41 Despite its immobilization, the manufacturer does not recommend exposing the microspheres to ionic solutions (ionic contrast, saline).42 The initial patent application for the resin microspheres indicated that 0.01% to 0.4% of 90Y is released from the microsphere after 20 minutes in water.41 The remainder is permanently bound to the resin microspheres and does not leach under physiological conditions.43 The specific gravity of resin microspheres is 1.6 g/dL (glass, 3.7 g/dL; blood, 1.05 g/dL), with a mean diameter of 32 μm.44, 45
Before April 2013, SIR-Spheres were manufactured for Sirtex by the Australian Radiologic Institute. Each batch produced included a set mass of resin, which yielded between 5 and 14 vials of microspheres containing 3 GBq of activity depending on the number of submitted orders. The batches producing 5 vials contained approximately 80 million spheres per vial (specific activity, 37.5 Bq/sphere; mean diameter, 25 μm) and those producing 14 vials contained approximately 40 million spheres per vial (specific activity, 75 Bq/sphere). There are currently three manufacturing sites all of which are owned by Sirtex Medical (Wilmington, MA; Singapore; Frankfurt).46 Production processes were changed such that each vial, regardless of the batch size, contains the same mass of resin resulting in approximately 40 million microspheres per vial (specific activity, 75 Bq/sphere) (G. Spindler, verbal communication, October 2016). An activity administration of 1 GBq, therefore, will consistently deliver approximately 13 million microspheres at the time of calibration. 90Y radiolabeled resin microspheres are ordered the week before administration and are delivered Mondays through Thursdays, allowing for administration Mondays through Fridays as same- or next-day treatments. The vial of microspheres is calibrated to be 3 GBq ± 10% at 6 PM the day following delivery to the institution and can be used up to 24 hours after calibration.42, 47
TheraSphere
The manufacturing process of TheraSphere involves mixing yttrium-89 (89Y) with ultrapure aluminum oxide and silicone dioxide, which is then melted in a furnace at 1500°C. After cooling, the 89Y embedded glass is crushed and passed through a flame sprayer, causing the glass particles to melt and “spheridize.” The spheres are filtered through sieves to select for those with a diameter of 20 to 30 microns. Neutron bombardment of the spheres then converts the embedded 89Y to 90Y. Because 90Y is embedded in the glass matrix, the possibility of leaching from the microspheres is minimal.48 Although 90Y dominates in the glass matrix, there are unintended radioactive substances present with long half-lives because of neutron bombardment of aluminum and silicone (88Y half-life 107 days, 154Europium half-life 8 years). The density of the glass microspheres is 3.6 g/dL, which is approximately 3 times that of blood (1.05 g/dL). The specific activity of the glass microsphere is approximately 2500 Bq per sphere at the time of calibration.44 There are 6 activity sizes available for delivery: 3, 5, 7, 10, 15, and 20 GBq. A 3-GBq vial contains approximately 1.2 million microspheres and a 20-GBq vial approximately 8 million. TheraSphere is supplied in 0.6 mL of sterile water in a 1.0-mL vial.49 They are dispensed Mondays, Wednesdays, and Thursdays and are calibrated for 12:00 PM (EST) the prior Sunday (vials dispensed Monday) or the following Sunday (vials dispensed Wednesday and Thursday). Activities over time for the different available vials are given in the following chart (Table 1).
Table 1.
Estimated activities for TheraSphere vials dispensed Monday and calibrated the Sunday prior
| Available activities (GBq) | Microspheres (millions) | Activity at time of administration (GBq) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Monday | Tuesday | Wednesday | Thursday | Friday | Monday | Tuesday | Wednesday | Thursday | ||
| 3 | 1.2 | 2.7 | 2.1 | 1.6 | 1.2 | 0.95 | 0.43 | 0.3 | 0.2 | 0.17 |
| 5 | 2 | 4.5 | 3.5 | 2.7 | 2.1 | 1.6 | 0.7 | 0.5 | 0.4 | 0.29 |
| 7 | 2.8 | 6.3 | 4.8 | 3.7 | 2.9 | 2.2 | 1 | 0.7 | 0.5 | 0.40 |
| 10 | 4 | 9.0 | 6.9 | 5.3 | 4.1 | 3.2 | 1.5 | 1.0 | 0.76 | 0.57 |
| 15 | 6 | 13.5 | 10.4 | 8.0 | 6.2 | 4.8 | 2.2 | 1.5 | 1.1 | 0.86 |
| 20 | 8 | 18 | 13.8 | 10.7 | 8.2 | 6.3 | 2.9 | 2.0 | 1.5 | 1.15 |
A 1 GBq administration may represent between 1.2 million and 7 million microspheres depending on date of delivery. A Thursday administration would require a 3 GBq vial, or an extended shelf-life administration on the following Thursday would require 17.5 GBq.
Note that there is an option to perform radioembolization the week following delivery of glass microspheres to an institution, allowing for use up to 12 days following calibration (EX or extended shelf life). This method allows for the administration of more microspheres with a lower specific activity as the number of half-lives increases, with a potential added benefit of improved tumor coverage. With the use of the EX protocol, the specific activity may be reduced to as low as 150 Bq/sphere. Lewandowski et al treated 134 patients with extended shelf-life glass microspheres resulting in the delivery of approximately twice as many spheres as would have been delivered with a standard protocol. The occurrence of clinical toxicities were similar to previous cohorts using standard administration techniques with response rates reported at 48% (World Health Organization) and 57% (European Association for the Study of Liver) and a complete European Association for the Study of Liver response of 21%. The authors concluded that “the increase in number of microspheres administered theoretically resulted in better tumor distribution of the microspheres without an increase in adverse events.”50
Microsphere production and physical characteristics: Advantages and disadvantages
There are advantages and disadvantages of the 2 devices related to their physical characteristics and the manner in which they are supplied that have important clinical implications.
For the glass device, the sealed vial cannot be altered in any way and must be administered at a specific time during its decay based on the calculated activity for each patient. Although this does reduce radiation exposure to nuclear medicine staff and simplifies the steps involved in its administration, the inability to make changes to the treatment plan can be limiting. The ability to fractionate the activity of the delivered resin device allows for greater flexibility in timing of administration and changes to the treatment plan.44 This does, however, result in more exposure to the nuclear medicine staff.
Because the specific gravity of resin microspheres is close to that of blood they are more uniformly distributed within the blood stream and therefore potentially more uniformly distributed throughout the tumor vascular bed than the more than twice as heavy glass microspheres.44
Ibrahim showed, however, that there was no actual difference in tumor response between the anterior and posterior segments after administration of glass microspheres and concluded that the specific gravity of glass microspheres does not appear to impede their tumor distribution.51 Recently, Jernigan developed a surrogate hepatic arterial system, which included a tumor and injected glass and resin microspheres. He determined that the penetration depths of microspheres into the tumor, which are dependent on fluid drag, gravity, and pressure forces, were significantly greater for resin microsphere due to their larger size and lower specific gravity than for the small glass microspheres with higher specific gravity.52
Basciano, also using a flow model, determined that if microspheres were injected at velocities greater than the native arterial velocity, they would be diverted from the normal flow patterns in the low-resistance tumor vessels into higher resistance vessels not supplying tumors.53 Because of their higher specific gravity, glass microspheres must be injected more forcefully, which may lead to microspheres achieving velocities rates greater than the native artery velocity and subsequent suboptimal distribution into liver tumors.
One of the most contentious issues related to resin microspheres concerns flow reductions and stasis seen during some administration. This observation has led to the presumption that, because of their lower specific activity and therefore larger required number of spheres delivered during a treatment, the spheres have an embolic effect limiting the complete administration of the desired activity. Piana et al reported an overall incidence of stasis of 20% with the use of sterile water during SIR-Spheres administrations, with a 36.6% incidence seen during left lobe administrations, 15.4% during right lobe infusions, and only 4.5% of whole liver infusions.54 Despite these observations, an analysis of 680 SIR-Spheres administrations using sterile water showed that the median administered activity was approximately 92% of the drawn activity (prescribed: 1.2 ± 0.06 GBq; administered: 1.1 ± 0.06 GBq).55
Bilbao injected cold SIR-Spheres microspheres into the left lobe of pigs to stasis using sterile water and saw no microscopic evidence of vascular occlusion, ischemia, or infarction.56 Additionally, with the new production process resulting in fewer than 40 million resin microspheres per vial and the ability to deliver fewer spheres with higher specific activity the day before calibration, the number of spheres delivered for a given prescribed activity has decreased and is similar to the number of spheres administered during a TheraSphere EX treatment when the lower overall activity administered using the standardized body surface area (BSA) method is implemented.
The diluent initially used for the administration of SIR-Spheres was sterile water, chosen by the manufacturer as an extra safety precaution because it is nonionic. It is known that intravascular injection of sterile water, because of its hypotonicity relative to blood, causes vascular disturbances, which can lead to spasm and thrombosis.57, 58 Sterile water rapidly enters red blood cells with subsequent lysis and release of free hemoglobin. When the capacity of protective hemoglobin scavenging mechanisms has been saturated, levels of cell-free hemoglobin increases in plasma and reacts with nitric oxide, converting it to biologically inactive nitrate. Nitric oxide plays a major role in vascular homeostasis and has been shown to be a regulator of smooth muscle relaxation (maintaining vasodilatation) and vasomotor tone, platelet activation and aggregation, and thrombin generation. The end result of nitric oxide consumption is acute vasoconstriction and chronic proliferative vasculopathy.58, 59
Additionally, water also causes lysis of endothelial cells, which can expose basement membrane leading to platelet aggregation, and activation of the clotting cascade as well as initiating repair mechanisms, which subsequently lead to intimal hyperplasia and arterial narrowing.58
Because of the profound physiologic effect of sterile water on the vasculature, it was felt by many early clinicians performing SIRT with SIR-Spheres that water was the cause of changes in flow seen during resin microsphere administration because these changes were seen even after a few aliquots of microspheres.
Sirtex evaluated different diluents and determined that 5% dextrose in water (D5W) combined with nonionic contrast was compatible with SIR-Spheres and was therefore an acceptable alternative to sterile water. D5W is isotonic to plasma and is not associated with hemolysis, significant flow reductions, or stasis as seen with the use of sterile water. Its use during the administration of SIR-Spheres was approved by the FDA in October 2014.60 Ahmadzadehfar retrospectively evaluated 104 SIRT procedures in 78 patients using SIR-Spheres at the time of the switch from sterile water to D5W as the diluent (50 sterile water, 54 D5W). They found that the whole prescribed activity was administered in significantly more procedures with D5W than with sterile water (85% vs 22%), a statistically significant higher percentage of activity administered in the D5W group (96% vs 77.4%) and a statistically significant lower incidence of stasis (11% vs 28%). In addition, there was a significantly lower incidence of intraprocedural pain in the D5W group (1.8% vs 44%).61
There have been no clinical trials demonstrating superiority or inferiority of glass or resin microspheres in regards to changes in blood flow during radioembolization. Administration of resin microspheres is performed with real-time evaluation of blood flow, which is not possible with the current administration technique for glass microspheres. Sato et al, however, did report that the angiographic patency rate was 100% following the administration of glass microspheres in 30 patients.62 When reductions in blood flow are perceived during resin administration, they may improve after a short time (5 to 10 minutes). One explanation for this phenomenon is that the microspheres might initially aggregate in larger vessels, decreasing flow, but then over time distribute into smaller vessels leading to resumption of normal flow in the territory. Users of both products may choose 1 product over the other in certain patients because of concerns about the possibility of blood flow changes. A special situation involves the attempt to perform so-called “radiation segmentectomy” wherein a very large activity is delivered to a segment to destroy all tumor and normal hepatic parenchyma. If the region targeted is a small volume, then it is possibly preferable to delivery fewer microspheres with the highest specific activity (non-EX glass microspheres or resin microspheres administered on day of delivery to institution); however, this has not been supported by the literature to date.
Antireflux catheters are currently available and may impact particle distribution. At the present time, their role in radioembolization has yet to be determined.
Activity calculation and delivery
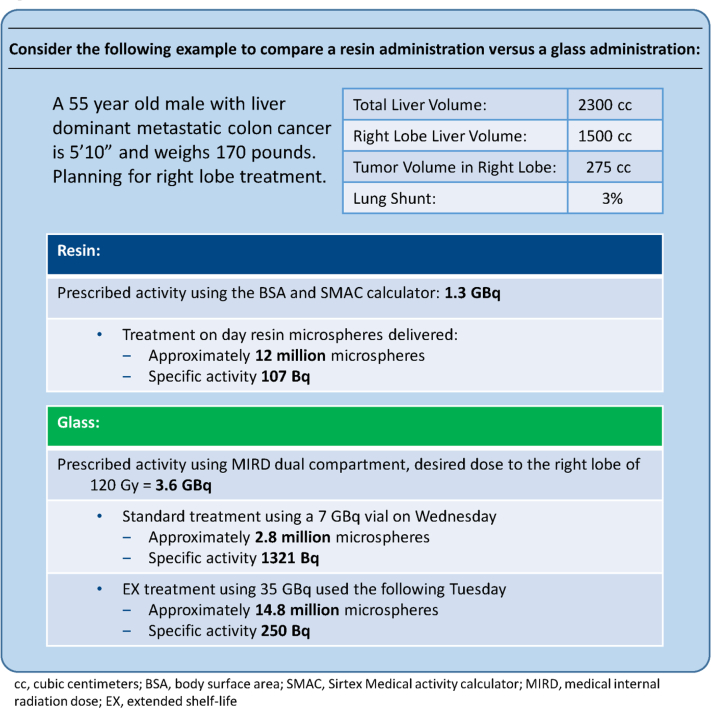
The methods involved in calculating activity to be administered and delivery of microspheres differ significantly between the glass and resin products. Calculated activities for glass are always higher than resin, but cannot be directly compared because of differences in specific activity between the 2 products. Fewer glass spheres with higher specific activity are required to achieve a desired administered activity; however, this results in decreased tumor coverage (Fig 1).
Figure 1.
Calculated activity and number of resin/glass microspheres that would be prescribed for a patient with liver dominant metastatic colon cancer who is 5'10” tall, weighs 170 pounds, and has a right lobe tumor (275 cc) with 3% lung shunt.
A 3-compartment partition model based on medical internal radiation dose, macrodosimetry is the most accurate means to determine the dose administered to the liver tumor, the normal parenchyma, and the lung63; however, it is rarely used in clinical practice because of its complexity and difficulty in obtaining accurate tumor to normal uptake ratio following technetium 99-labeled macroaggregated albumin injections during mapping arteriograms. The tumor to normal uptake ratio is unreliable, inconsistent, and varies between tumors in a given patient and even within normal liver tissue. Because of difficulties employing this methodology, alternative activity determinants have been developed, which are predominantly used in clinical practice and accepted as viable surrogates.64
Resin 90Y microsphere activity calculation and delivery techniques
Originally, the empiric method was used to calculate treatment activity, which relied on the percentage of liver involvement by tumor alone as the determinant of activity. This was abandoned as it became obvious that it did not account for differences in liver volume amongst individuals. Subsequently, the BSA method was instituted and continues to be the principal method of activity determination for resin microspheres.64 The calculated activity using the BSA method uses the following formula:
The BSA correlates well with liver volume except at extremes of height and weight and in those instances where the liver is markedly enlarged by tumor.65
For a bilobar (or segmental) treatment, the calculated activity for the whole liver is multiplied by the percentage by volume of the lobe (or segment) treated. For example, in a 5'8”, 170-pound patient (BSA = 1.9 m2) with 20% liver involvement, the activity for the entire liver would be:
With right and left lobe volumes being 60% and 40% of the total liver volume, respectively, the activities for each lobe are:
The BSA model is widely used in clinical practice as well as in multiple studies including completed and ongoing randomized controlled trials (Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer [SIRFLOX], FOLFOX6m Plus SIR-Spheres Microspheres vs FOLFOX6m Alone in Patients With Liver Mets From Primary Colorectal Cancer [FOXFIRE], FOXFIRE Global). This methodology assumes even distribution of tumor throughout the liver and does not account for differences in tumor volume within treated lobes (or segments). To account for differences in tumor involvement in treatment regions, the following equation is recommended to ascertain the desired activity for a treatment region (L)42:
To assist in calculating prescribed activity, the Sirtex Medical Activity Calculator was developed. This calculator allows for determination of activity to be administered to a known liver volume containing a known volume of tumor. It also automatically reduces the activity if a lung dose of 25 Gy or greater is calculated using the formula66:
The Sirtex Medical Activity Calculator also allows for activity reductions for those patients with risk factors for the development of REILD including underlying liver disease (cirrhosis), small livers or small tumor burdens, and history of systemic chemotherapy. It is recommended that these patients receive no more than 0.8 GBq per liter of targeted liver.67
Resin 90Y microspheres administration
After the activity has been drawn into the supplied V vial, it is placed within a Lucite box and the Delivery Set containing needles and tubing properly flushed and connected to the V vial (see instructions for use). The authorized user then agitates the microspheres with D5W, delivering aliquots of activity through the tubing toward the microcatheter. The interventional radiologist then flushes the microspheres through the delivery microcatheter with a D5W/contrast mixture (typically 50/50, but up to the user's discretion). The delivery of microspheres occurs during real-time fluoroscopic evaluation to ensure that flow is maintained within the vessel and that the catheter remains in satisfactory position throughout the delivery. When the V vial appears clear, the authorized user pushes the remaining fluid from the V vial using an air-filled syringe.42
Glass 90Y microsphere activity calculation and delivery techniques
TheraSphere activity calculation is based on a 2-compartment partition model consisting of the lung and the liver and assumes uniform distribution of microspheres throughout the liver compartment. The percentage of liver replaced by tumor does not factor into the activity determination.40, 64
The activity to be administered is calculated after a desired dose to the liver has been chosen factoring in the lung shunt calculated from the technetium 99-labeled macroaggregated albumin study49
A dose of 100 to 140 Gy to the liver compartment is typically chosen to determine activity to be administered with lower doses chosen in patients with underlying liver disease from any cause.
Recently the TheraSphere interactive dose ordering calculator was developed to aid users in determining activity to be delivered based on desired dose and timing of administration.68
Glass 90Y microspheres administration
TheraSphere microspheres are supplied in 0.6 mL of sterile, pyrogen-free water contained in a 1.0 mL V-bottom vial secured within a clear Lucite vial shield. The vial is placed within a reusable Lucite delivery box. A 2-needle plunger system connected to the tubing assembly is inserted into the TheraSphere vial and connection made to the microcatheter. A 20-mL syringe is filled from the saline source and then injected into the TheraSphere containing vial at a constant rate of 20 mL per minute at a pressure not to exceed 30 psi. This high-pressure, bolus technique is required due to the high specific gravity of the microspheres, which will not be properly agitated and delivered otherwise. This should be repeated approximately 3 times to ensure thorough delivery of the activity.49 At no time during the administration is it possible to inject contrast to evaluate for changes in flow within the hepatic artery.44, 69
At the end of an administration using either SIR-Spheres or TheraSphere, all of the disposable tubing and catheters are placed in a container, which will be stored until there has been adequate decay. Patients typically have a posttreatment bremsstrahlung scan to localize the delivered activity.64
Current clinical practice
A thorough discussion of the current practice of hepatic radioembolization as it relates to clinical outcomes, results of clinical trials to date, and the status of ongoing randomized controlled trials is beyond the scope of this manuscript. It is important to note that no prospective randomized controlled study has been performed to date comparing outcomes between patients treated with glass or resin microspheres.
Appropriate patient selection is paramount to ensuring minimal adverse events and maximum beneficial outcome. Bilirubin level is 1 of the most important parameters for assessing appropriateness of radioembolization.64 Great caution should be taken in treating patients with bilirubin levels of 2.0 or greater; however, it may be safe in those instances where minimal normal hepatic parenchyma is included in the targeted volume such as segmental treatments. Some reports indicate that a bilirubin level <1.2 is associated with improved survival following radioembolization.70 Eastern Cooperative Oncology Group status ≤2, minimal or stable extrahepatic disease, albumin level >3.0 g/dL, and excessive tumor burden with limited hepatic reserve are additional important indicators of tolerability of the procedure. Regular assessment of these parameters both before and following radioembolization is recommended.68, 69 Eastern Cooperative Oncology Group status ≤2, minimal or stable extrahepatic disease, albumin level >3.0 g/dL, and excessive tumor burden with limited hepatic reserve are additional important indicators of tolerability of the procedure. Regular assessment of these parameters both before and following radioembolization is recommended.71, 72
Although the FDA-approved indication for SIR-Spheres is for unresectable hepatic malignancies from primary colorectal cancer and the HDE indication for TheraSphere is for unresectable HCC,40, 49 both devices have been used to treat hepatic metastases from other primary tumor types, including intrahepatic cholangiocarcinoma, neuroendocrine, breast, melanoma, renal cell, lung, and pancreatic neoplasms.73, 74, 75, 76, 77, 78, 79, 80, 81, 82
Ongoing clinical studies
After 2 randomized trials demonstrated the safety and promising efficacy of SIRT in conjunction with oxaliplatin- and fluoropyrimidine-based chemotherapy regimens in patients with mCRC,83, 84 randomized controlled trials were initiated to determine if radioembolization conferred added benefit when administered with FOLFOX or 5FU - Leucovorin - Oxaliplatin in the first line. The recently reported SIRFLOX Trial compared patients treated in the first line who received FOLFOX6 ± bevacizumab alone or with radioembolization using 90Y resin microspheres. Although there was no statistical difference in overall progression, patients receiving radioembolization experienced a 7.9-month longer median progression-free survival in the liver than patients receiving standard therapy (P = .002).85
The results of ongoing FOXFIRE and FOXFIRE Global trials (also evaluating 90Y-resin SIRT plus FOLFOX), will be combined with the SIRFLOX data with a primary outcome of improved overall survival.85, 86
A second-line trial of 90Y glass microspheres in mCRC patients with safety and efficacy endpoints is also under way. In this trial, patients will receive second-line chemotherapy with or without radioembolization.87
Several randomized controlled trials evaluating radioembolization for HCC are ongoing including studies of glass (STOP – HCC: Efficacy Evaluation of TheraSphere in Patients With Inoperable Liver Cancer, YES-P: Treat Inoperable Liver Cancer With Blockage of the Portal Vein) and resin (SIRveNIB: Study to Compare Selective Internal Radiation Therapy [SIRT] Versus Sorafenib in Locally Advanced Hepatocellular Carcinoma, SARAH: SorAfenib Versus Radioembolization in Advanced Hepatocellular Carcinoma, SORAMIC: Sorafenib and Micro-Therapy Guided by Primovist Enhanced MRI in Patients with Inoperable Liver Cancer) 90Y microspheres (Table 2).88, 89, 90, 91, 92 Additional prospective studies are being conducted with SIRT treatment for patients with metastatic uveal melanoma, breast cancer, and renal cell carcinoma as well.93, 94
Table 2.
Current randomized clinical trials of 90Y microspheres for primary or secondary liver tumors
| Trial | Location | Intervention arms | Primary endpoint | n |
|---|---|---|---|---|
| mCRC (first line) | ||||
| SIRFLOX85 | Global | FOLFOX ± bevacizumab FOLFOX ± bevacizumab + SIR-Spheres |
PFS | 530 |
| FOXFIRE86 | UK | FOLFOX ± biological agent FOLFOX ± biological agent + SIR-Spheres |
OS | 320 |
| FOXFIRE Global95 | Global | FOLFOX ± bevacizumab FOLFOX ± bevacizumab + SIR-Spheres |
OS | 200 |
| mCRC (first line after PR/SD following 3-6 months of induction chemotherapy) | ||||
| SIR-step96 | Belgium | 5FU/LV ± biological agent 5FU/LV ± biological agent + SIR-Spheres |
TTP | 162 |
| mCRC (second line) | ||||
| EPOCH87 | Global | Standard of care chemotherapy Standard of care chemotherapy + TheraSphere |
PFS | 340 |
| HCC | ||||
| SIRveNIB90 | Global | Sorafenib SIRT with SIR-Spheres |
OS | 360 |
| SARAH92 | France | Sorafenib SIRT with SIR-Spheres |
OS | 496 |
| SORAMIC91 | Germany | RFA ± Sorafenib Sorafenib ± SIRT with SIR-Spheres |
TTR/OS | 665 |
| STOP-HCC88 | Global | Sorafenib Sorafenib + SIRT with TheraSphere |
OS | 390 |
| DOSISPHERES-0197 | France | Standard dosimetry SIRT with TheraSphere Optimized dosimetry SIRT with TheraSphere |
ORR | 210 |
| PREMIERE98 | US | TACE SIRT with TheraSphere |
TTP | 124 |
| TRACE99 | Belgium | TACE-DEB SIRT with TheraSphere |
TTP | 140 |
| HCC with PVT | ||||
| YES-P89 | Global | Sorafenib SIRT with TheraSphere |
OS | 328 |
| ICC | ||||
| NCT01798147100 | Germany | TACE-DEB SIRT with SIR-Spheres |
PFS | 24 |
DEB, drug-eluting beads; FOLFOX, leucovorin + fluorouracil + oxaliplatin; 5FU, fluorouracil; HCC, hepatocellular carcinoma; ICC, intrahepatic cholangiocarcinoma; LV, leucovorin; mCRC, metastatic colorectal cancer; PVT, portal vein thrombosis; RFA; radiofrequency ablation; SIRT, selective internal radiation therapy; TACE, transarterial chemoembolization; TTR, time to recurrence.
Choice of use of the two devices
As previously mentioned, there has been no randomized control trial performed to determine which of the commercially available 90Y-labeled microspheres is clinically superior. Deciding which device should be used is an institutional choice and depends on the training of the treating physicians, the local reimbursement policies, and the ability to schedule a particular time in the interventional radiology suite to deliver the therapy. Although treating physicians can be trained to use either device, his or her preference may reflect the fellowship training or prior experience with the devices. State and local insurance determinations of payment for these services will also impact the choice because some states will not reimburse for materials that are on a clinical trial, defined as an institutional review board–approved study. A thorough understanding of the properties of the 2 devices enables treating physicians to choose a product that best suits their patients' needs.
Conclusion
Selective internal radiation therapy of hepatic malignancies is a groundbreaking therapeutic modality that requires the combined efforts of multiple medical disciplines to ensure the safe delivery of 90Y-labeled microspheres. The development of this therapy followed decades of clinical research involving tumor vascularity and microsphere development. The 2 commercially available microspheres differ in many respects, but to date, no randomized controlled trials have been conducted to determine if these differences have clinical consequences in their currently approved indications. As SIRT expands into combination therapies and into patient populations beyond HCC and mCRC, clinicians may be able to take advantage of the individual attributes of resin and glass 90Y microspheres to improve patient outcomes.
Acknowledgments
The authors thank Eubio Medical Communications for editorial support (funding provided by Sirtex Medical Inc.). Special thanks to Dr. Grant Spindler at Sirtex Medical Inc. for helpful discussions over the course of writing this review.
Footnotes
Conflicts of interest: M.A.W. and D.M.C. have received proctoring fees from Sirtex Medical Inc. D.M.L. has received consultant fees and a research grant from Sirtex Medical Inc. and has served on an advisory board and as a clinical trial investigator for BTG International. J.F.Z. has no conflicts of interest to disclose.
References
- 1.Enghag P. Wiley-VCH Verlag GmbH & Co KGaA; Weinheim, Germany: 2004. Scandium, yttrium, lanthanum and the 14 lanthanides – rare earth metals (REMs). Encyclopedia of the Elements; pp. 373–492. [Google Scholar]
- 2.Walker L.A. Radioactive yttrium 90: A review of its properties, biological behavior, and clinical uses. Acta Radiol. 1964;2:302–314. doi: 10.1080/02841866409134063. [DOI] [PubMed] [Google Scholar]
- 3.D'Arienzo M. Emission of β+ particles via internal pair production in the 0+ – 0+ transition of 90Zr: Historical background and current applications in nuclear medicine imaging. Atoms. 2013;1:2–12. [Google Scholar]
- 4.Simon N., Feitelberg S. Scanning bremsstrahlung of yttrium-90 microspheres injected intra-arterially. Radiology. 1967;88:719–724. doi: 10.1148/88.4.719. [DOI] [PubMed] [Google Scholar]
- 5.Bierman H.R., Byron R.L., Jr., Kelley K.H., Grady A. Studies on the blood supply of tumors in man. III. Vascular patterns of the liver by hepatic arteriography in vivo. J Natl Cancer Inst. 1951;12:107–131. [PubMed] [Google Scholar]
- 6.Breedis C., Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. [PMC free article] [PubMed] [Google Scholar]
- 7.Dieterich S., Ford E., Pavord D., Zeng J. Elsevier; Philadelphia, PA: 2016. Practical radiation oncology physics: A companion to Gunderson & Tepper's Clinical radiation oncology. [Google Scholar]
- 8.Grady E.D., Sale W., Nicolson W.P., Jr., Rollins L.C. Intra-arterial radioisotopes to treat cancer. Am Surg. 1960;26:678–684. [PubMed] [Google Scholar]
- 9.Simon N., Warner R.R., Baron M.G., Rudavsky A.Z. Intra-arterial irradiation of carcinoid tumors of the liver. AJR Am J Roentgenol. 1968;102:552–561. doi: 10.2214/ajr.102.3.552. [DOI] [PubMed] [Google Scholar]
- 10.Ariel I.M., Pack G.T. The treatment of cancer metastases in the lung by means of radiating microspheres. Thoraxchir Vask Chir. 1966;14:286–307. doi: 10.1055/s-0028-1101262. [DOI] [PubMed] [Google Scholar]
- 11.Simon N., Siffert R., Baron M.G., Mitty H.A., Rudavsky A. Preoperative irradiation of osteogenic sarcoma with intra-arterially injected yttrium-90 microsphers. Cancer. 1968;21:453–455. doi: 10.1002/1097-0142(196803)21:3<453::aid-cncr2820210315>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 12.Ackerman N.B. The blood supply of experimental liver metastases. IV. Changes in vascularity with increasing tumor growth. Surgery. 1974;75:589–596. [PubMed] [Google Scholar]
- 13.Taylor I., Bennett R., Sherriff S. The blood supply of colorectal liver metastases. Br J Cancer. 1979;38:749–756. doi: 10.1038/bjc.1978.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grady E.D. Internal radiation therapy of hepatic cancer. Dis Colon Rectum. 1979;22:371–375. doi: 10.1007/BF02586901. [DOI] [PubMed] [Google Scholar]
- 15.Stribley K.V., Gray B.N., Chmiel R.L., Heggie J.C., Bennett R.C. Internal radiotherapy for hepatic metastases II: The blood supply to hepatic metastases. J Surg Res. 1983;34:25–32. doi: 10.1016/0022-4804(83)90018-5. [DOI] [PubMed] [Google Scholar]
- 16.Meade V.M., Burton M.A., Gray B.N., Self G.W. Distribution of different sized microspheres in experimental hepatic tumours. Eur J Cancer Clin Oncol. 1987;23:37–41. doi: 10.1016/0277-5379(87)90416-0. [DOI] [PubMed] [Google Scholar]
- 17.Mantravadi R.V., Spigos D.G., Tan W.S., Felix E.L. Intraarterial yttrium 90 in the treatment of hepatic malignancy. Radiology. 1982;142:783–786. doi: 10.1148/radiology.142.3.7063703. [DOI] [PubMed] [Google Scholar]
- 18.Kennedy A.S., Ball D., Cohen S.J. Multicenter evaluation of the safety and efficacy of radioembolization in patients with unresectable colorectal liver metastases selected as candidates for (90)Y resin microspheres. J Gastro Oncol. 2015;6:134–142. doi: 10.3978/j.issn.2078-6891.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewandowski R.J., Thurston K.G., Goin J.E. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135-150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J VascI Intervent Radiol. 2005;16:1641–1651. doi: 10.1097/01.RVI.0000179815.44868.66. [DOI] [PubMed] [Google Scholar]
- 20.Nace G.W., Steel J.L., Amesur N. Yttrium-90 radioembolization for colorectal cancer liver metastases: a single institution experience. Int J Surg Oncol. 2011;2011:571261. doi: 10.1155/2011/571261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abbott A.M., Kim R., Hoffe S.E. Outcomes of Therasphere radioembolization for colorectal metastases. Clin Colorectal Cancer. 2015;14:146–153. doi: 10.1016/j.clcc.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Ariel I.M., Padula G. Treatment of asymptomatic metastatic cancer to the liver from primary colon and rectal cancer by the intraarterial administration of chemotherapy and radioactive isotopes. J Surg Oncol. 1982;20:151–156. doi: 10.1002/jso.2930200304. [DOI] [PubMed] [Google Scholar]
- 23.Blanchard R.J. Treatment of liver tumours with yttrium-90 microspheres. Can J Surg. 1983;26:442–443. [PubMed] [Google Scholar]
- 24.Harbert J.C., Zeissman H.A. Therapy with Intraarterial microspheres. Nucl Med Annu. 1987:295–319. [Google Scholar]
- 25.Burton M.A., Gray B.N., Klemp P.F., Kelleher D.K., Hardy N. Selective internal radiation therapy: distribution of radiation in the liver. Eur J Cancer Clin Oncol. 1989;25:1487–1491. doi: 10.1016/0277-5379(89)90109-0. [DOI] [PubMed] [Google Scholar]
- 26.Wollner I., Knutsen C., Smith P. Effects of hepatic arterial yttrium 90 glass microspheres in dogs. Cancer. 1988;61:1336–1344. doi: 10.1002/1097-0142(19880401)61:7<1336::aid-cncr2820610711>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Herba M.J., Illescas F.F., Thirlwell M.P. Hepatic malignancies: Improved treatment with intraarterial Y-90. Radiology. 1988;169:311–314. doi: 10.1148/radiology.169.2.3174978. [DOI] [PubMed] [Google Scholar]
- 28.Anderson J.H., Angerson W.J., Willmott N., Kerr D.J., McArdle C.S., Cooke T.G. Regional delivery of microspheres to liver metastases: The effects of particle size and concentration on intrahepatic distribution. Br J Cancer. 1991;64:1031–1034. doi: 10.1038/bjc.1991.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray B.N., Burton M.A., Kelleher D.K., Anderson J., Klemp P. Selective internal radiation (SIR) therapy for treatment of liver metastases: Measurement of response rate. J Surg Oncol. 1989;42:192–196. doi: 10.1002/jso.2930420313. [DOI] [PubMed] [Google Scholar]
- 30.Gray B.N., Anderson J.E., Burton M.A. Regression of liver metastases following treatment with yttrium-90 microspheres. Aust N Z J Surg. 1992;62:105–110. doi: 10.1111/j.1445-2197.1992.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 31.Gray B.N., Van Hazel G., Buck M., Paton G., Burton M., Anderson J. Treatment of colorectal liver metastases with SIR-Spheres plus chemotherapy. GI Cancer. 2000;3:249–257. [Google Scholar]
- 32.Gray B., Van Hazel G., Hope M. Randomised trial of SIR-Spheres plus chemotherapy vs. chemotherapy alone for treating patients with liver metastases from primary large bowel cancer. Ann Oncol. 2001;12:1711–1720. doi: 10.1023/a:1013569329846. [DOI] [PubMed] [Google Scholar]
- 33.United States Food and Drug Administration. Medical devices, premarket approval Available at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/HowtoMarketYourDevice/PremarketSubmissions/PremarketApprovalPMA/ucm2007514.htm. Accessed August 22, 2016.
- 34.US Department of Health and Human Services. Public Health Service. Food and Drug Administration. SIR-Spheres: Summary of safety and effectiveness data 2002. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf/P990065b.pdf. Accessed August 22, 2016.
- 35.Houle S., Yip T.K., Shepherd F.A. Hepatocellular carcinoma: Pilot trial of treatment with Y-90 microspheres. Radiology. 1989;172:857–860. doi: 10.1148/radiology.172.3.2549567. [DOI] [PubMed] [Google Scholar]
- 36.Andrews J.C., Walker S.C., Ackermann R.J., Cotton L.A., Ensminger W.D., Shapiro B. Hepatic radioembolization with yttrium-90 containing glass microspheres: Preliminary results and clinical follow-up. J Nucl Med. 1994;35:1637–1644. [PubMed] [Google Scholar]
- 37.US Department of Health and Human Services. Public Health Service. Food and Drug Administration. TheraSphere: Summary of safety and probable benefit 1999. Available at: http://www.accessdata.fda.gov/cdrh_docs/pdf/H980006b.pdf. Accessed August 22, 2016.
- 38.Dancey J.E., Shepherd F.A., Paul K. Treatment of nonresectable hepatocellular carcinoma with intrahepatic 90Y-microspheres. J Nuclear Med. 2000;41:1673–1681. [PubMed] [Google Scholar]
- 39.US Food and Drug Administration. Humanitarian device exemption: Question and answers. Available at: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm389154.htm. Accessed August 22, 2016.
- 40.SIR-Spheres Package Insert. SIRTeX Medical Limited; North Sydney, Australia: 2014. [Google Scholar]
- 41.Gray B, inventor; Sirtex Medical Limited, assignee. Polymer based radionuclide containing particulate material. International application number PCT/AU2001/001370. February 5, 2002.
- 42.Sirtex Medical Inc. SIR-Spheres Microspheres Training Manual Physicians and Institutions. Woburn, MA: SIRTeX Medical Limited.
- 43.Archer S.G., Gray B.N. Vascularization of small liver metastases. Br J Surg. 1989;76:545–548. doi: 10.1002/bjs.1800760607. [DOI] [PubMed] [Google Scholar]
- 44.Burrill J., Hafeli U., Liu D.M. Advances in radioembolization - Embolics and isotopes. Nuclear Medi Radiat Ther. 2011;2:107. [Google Scholar]
- 45.Trudnowski R.J. Specific gravity of blood and plasma at 4 and 37 degrees C. Clin Chem. 1974;20:615–616. [PubMed] [Google Scholar]
- 46.Sirtex Medical Limited . SIRTeX Medical Limited; North Sydney, Australia: 2015. Sirtex Annual Report 2015. [Google Scholar]
- 47.Sirtex Medical Inc . SIRTeX Medical Limited; Woburn, MA: 2012. Sirtex Ordering and Dose Cancellation Policy for SIR-Spheres® microspheres. [Google Scholar]
- 48.Ehrhardt G.J., Day D.E. Therapeutic use of 90Y microspheres. Int J Rad Appl Instrum B. 1987;14:233–242. doi: 10.1016/0883-2897(87)90047-x. [DOI] [PubMed] [Google Scholar]
- 49.TheraSphere Yttrium-90 Microspheres Package Insert, v12 Surrey, UK: Biocompatibles UK Ltd. Available at: https://www.btg-im.com/getattachment/Therasphere/US/Products/Indications/TheraSphere-Package-Insert_USA_v13.pdf.aspx.
- 50.Lewandowski R.J., Minocha J., Memon K. Sustained safety and efficacy of extended-shelf-life (90)Y glass microspheres: long-term follow-up in a 134-patient cohort. Eur J Nucl Med Mol Imaging. 2014;41:486–493. doi: 10.1007/s00259-013-2575-8. [DOI] [PubMed] [Google Scholar]
- 51.Ibrahim S.M., Lewandowski R.J., Ryu R.K. Radiographic response to yttrium-90 radioembolization in anterior versus posterior liver segments. Cardiovasc Intervent Radiol. 2008;31:1124–1132. doi: 10.1007/s00270-008-9348-y. [DOI] [PubMed] [Google Scholar]
- 52.Jernigan S.R., Osborne J.A., Mirek C.J., Buckner G. Selective internal radiation therapy: Quantifying distal penetration and distribution of resin and glass microspheres in a surrogate arterial model. J Vasc Interv Radiol. 2015;26:897–904. doi: 10.1016/j.jvir.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 53.Basciano C.A., Kleinstreuer C., Kennedy A.S. Computational fluid dynamics modeling of 90Y microspheres in human hepatic tumors. J Nuclear Med Radiat Ther. 2011;01(01) [Google Scholar]
- 54.Piana P.M., Bar V., Doyle L. Early arterial stasis during resin-based yttrium-90 radioembolization: Incidence and preliminary outcomes. HPB (Oxford) 2014;16:336–341. doi: 10.1111/hpb.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kennedy A.S., McNeillie P., Dezarn W.A. Treatment parameters and outcome in 680 treatments of internal radiation with resin 90Y-microspheres for unresectable hepatic tumors. Int J Radiat Oncol Biol Phys. 2009;74:1494–1500. doi: 10.1016/j.ijrobp.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Bilbao J.I., de Martino A., de Luis E. Biocompatibility, inflammatory response, and recannalization characteristics of nonradioactive resin microspheres: Histological findings. Cardiovasc Intervent Radiol. 2009;32:727–736. doi: 10.1007/s00270-009-9592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krumbhaar E.B. Hemolysis due to intravenous injection of distilled water. JAMA. 1914;62:992–993. [Google Scholar]
- 58.Rother R.P., Bell L., Hillmen P., Gladwin M.T. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: A novel mechanism of human disease. JAMA. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 59.Schechter A.N. Hemoglobin research and the origins of molecular medicine. Blood. 2008;112:3927–3938. doi: 10.1182/blood-2008-04-078188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.US Department of Health and Human Services. Public Health Service. Food and Drug Administration. October 2014 PMA Approvals 2014. Available at: http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/ucm431264.htm. Accessed August 22, 2016.
- 61.Ahmadzadehfar H., Meyer C., Pieper C.C. Evaluation of the delivered activity of yttrium-90 resin microspheres using sterile water and 5 % glucose during administration. EJNMMI Res. 2015;5:54. doi: 10.1186/s13550-015-0133-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato K., Lewandowski R.J., Bui J.T. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): Assessment of hepatic arterial embolization. Cardiovasc Intervent Radiol. 2006;29:522–529. doi: 10.1007/s00270-005-0171-4. [DOI] [PubMed] [Google Scholar]
- 63.Gulec S.A., Mesoloras G., Stabin M. Dosimetric techniques in 90Y-microsphere therapy of liver cancer: The MIRD equations for dose calculations. J Nuclear Med. 2006;47:1209–1211. [PubMed] [Google Scholar]
- 64.Kennedy A., Nag S., Salem R. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: A consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys. 2007;68:13–23. doi: 10.1016/j.ijrobp.2006.11.060. [DOI] [PubMed] [Google Scholar]
- 65.Vauthey J.N., Abdalla E.K., Doherty D.A. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233–240. doi: 10.1053/jlts.2002.31654. [DOI] [PubMed] [Google Scholar]
- 66.SIR-Spheres Microspheres Activity Calculator. North Sydney, Australia: SIRTeX Medical Ltd. Available at: http://apps01.sirtex.com/smac/. Accessed August 22, 2016.
- 67.Sangro B., Gil-Alzugaray B., Rodriguez J. Liver disease induced by radioembolization of liver tumors: Description and possible risk factors. Cancer. 2008;112:1538–1546. doi: 10.1002/cncr.23339. [DOI] [PubMed] [Google Scholar]
- 68.TheraSphere Interactive Dose Ordering Calculator (iDOC). London, United Kingdom: BTG International Group. Available at: https://www.btg-im.com/therasphere-idoc?utm_source=BTG+TheraSphere+iDOC+Newsletter&utm_campaign=fb0822c30f-therasphere_idoc_launch-march012015&utm_medium=email&utm_term=0_4a75385604-fb0822c30f-113854301. Accessed August 22, 2016.
- 69.Salem R., Thurston K.G. Radioembolization with 90Yttrium microspheres: A state-of-the-art brachytherapy treatment for primary and secondary liver malignancies. Part 1: Technical and methodologic considerations. J VascI Intervent Radiol. 2006;17:1251–1278. doi: 10.1097/01.RVI.0000233785.75257.9A. [DOI] [PubMed] [Google Scholar]
- 70.Memon K., Lewandowski R.J., Mulcahy M.F. Radioembolization for neuroendocrine liver metastases: Safety, imaging, and long-term outcomes. Int J Radiat Oncol Biol Phys. 2012;83:887–894. doi: 10.1016/j.ijrobp.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahnken A.H., Spreafico C., Maleux G., Helmberger T., Jakobs T.F. Standards of practice in transarterial radioembolization. Cardiovasc Intervent Radiol. 2013;36:613–622. doi: 10.1007/s00270-013-0600-8. [DOI] [PubMed] [Google Scholar]
- 72.ACR-SIR . 2014. ACR-SIR Practice Parameter for Radioembolization With Microsphere Brachytherapy Device (RBMD) for Treatment of Liver Malignancies.http://www.acr.org/∼/media/d8086059b5f541e9b64e55d68a71693b.pdf Available at: Accessed August 22, 2016. [Google Scholar]
- 73.Camacho J.C., Kokabi N., Xing M., Prajapati H.J., El-Rayes B., Kim H.S. Modified response evaluation criteria in solid tumors and European Association for The Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolization. J VascI Intervent Radiolog. 2014;25:256–665. doi: 10.1016/j.jvir.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 74.Saxena A., Bester L., Chua T.C., Chu F.C., Morris D.L. Yttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: A preliminary assessment of this novel treatment option. Ann Surg Oncol. 2010;17:484–491. doi: 10.1245/s10434-009-0777-x. [DOI] [PubMed] [Google Scholar]
- 75.Kennedy A., Bester L., Salem R. Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): Guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford) 2015;17:29–37. doi: 10.1111/hpb.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fendler W.P., Lechner H., Todica A. Safety, efficacy, and prognostic factors after radioembolization of hepatic metastases from breast cancer: A large single-center experience in 81 patients. J Nuclear Med. 2016;57:517–523. doi: 10.2967/jnumed.115.165050. [DOI] [PubMed] [Google Scholar]
- 77.Bangash A.K., Atassi B., Kaklamani V. 90Y radioembolization of metastatic breast cancer to the liver: Toxicity, imaging response, survival. J VascI Intervent Radiol. 2007;18:621–628. doi: 10.1016/j.jvir.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 78.Xing M., Prajapati H.J., Dhanasekaran R. Selective internal yttrium-90 radioembolization therapy (90Y-SIRT) versus best supportive care in patients with unresectable metastatic melanoma to the liver refractory to systemic therapy: Safety and efficacy cohort study. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000109. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 79.Memon K., Kuzel T.M., Vouche M., Atassi R., Lewandowski R.J., Salem R. Hepatic yttrium-90 radioembolization for metastatic melanoma: A single-center experience. Melanoma Res. 2014;24:244–251. doi: 10.1097/CMR.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 80.Abdelmaksoud M.H., Louie J.D., Hwang G.L., Kothary N., Minor D.R., Sze D.Y. Yttrium-90 radioembolization of renal cell carcinoma metastatic to the liver. J Vasc Interv Radiol. 2012;23:323–330. doi: 10.1016/j.jvir.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Murthy R., Mutha P., Lee J.H., Oh Y. Yttrium-90-labeled microsphere radioembolotherapy of liver-dominant metastases from thoracic malignancies. J Vasc Interv Radiol. 2008;19:299–300. doi: 10.1016/j.jvir.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 82.Gibbs P., Do C., Lipton L. Phase II trial of selective internal radiation therapy and systemic chemotherapy for liver-predominant metastases from pancreatic adenocarcinoma. BMC Cancer. 2015;15:802. doi: 10.1186/s12885-015-1822-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Hazel G., Blackwell A., Anderson J. Randomised phase 2 trial of SIR-Spheres plus fluorouracil/leucovorin chemotherapy versus fluorouracil/leucovorin chemotherapy alone in advanced colorectal cancer. J Surg Oncol. 2004;88:78–85. doi: 10.1002/jso.20141. [DOI] [PubMed] [Google Scholar]
- 84.Sharma R.A., Van Hazel G.A., Morgan B. Radioembolization of liver metastases from colorectal cancer using yttrium-90 microspheres with concomitant systemic oxaliplatin, fluorouracil, and leucovorin chemotherapy. J Clin Oncol. 2007;25:1099–1106. doi: 10.1200/JCO.2006.08.7916. [DOI] [PubMed] [Google Scholar]
- 85.van Hazel G.A., Heinemann V., Sharma N.K. SIRFLOX: Randomized Phase III Trial Comparing First-Line mFOLFOX6 (Plus or Minus Bevacizumab) Versus mFOLFOX6 (Plus or Minus Bevacizumab) Plus Selective Internal Radiation Therapy in Patients With Metastatic Colorectal Cancer. J Clin Oncol. 2016;34:1723–1731. doi: 10.1200/JCO.2015.66.1181. [DOI] [PubMed] [Google Scholar]
- 86.Dutton S.J., Kenealy N., Love S.B. FOXFIRE protocol: an open-label, randomised, phase III trial of 5-fluorouracil, oxaliplatin and folinic acid (OxMdG) with or without interventional Selective Internal Radiation Therapy (SIRT) as first-line treatment for patients with unresectable liver-only or liver-dominant metastatic colorectal cancer. BMC Cancer. 2014;14:497. doi: 10.1186/1471-2407-14-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.BTG International Inc; Biocompatibles UK Ltd. Efficacy Evaluation of TheraSphere Following Failed First Line Chemotherapy in Metastatic Colorectal Cancer (EPOCH). Available at: https://clinicaltrials.gov/ct2/show/NCT01483027. Accessed August 22, 2016.
- 88.BTG International Inc; Biocompatibles UK Ltd. Efficacy Evaluation of TheraSphere in Patients With Inoperable Liver Cancer (STOP-HCC). Available at: https://clinicaltrials.gov/ct2/show/NCT01556490. Accessed August 22, 2016.
- 89.BTG International Inc; Biocompatibles UK Ltd. Efficacy Evaluation of TheraSphere to Treat Inoperable Liver Cancer With Blockage of the Portal Vein (YES-P) [Available from: https://clinicaltrials.gov/ct2/show/NCT01887717. Accessed August 22, 2016.
- 90.Singapore General Hospital; National Cancer Centre (Singapore); National Medical Research Council (Singapore); Singapore Clinical Research Institute; Sirtex Medical. Study to Compare Selective Internal Radiation Therapy (SIRT) Versus Sorafenib in Locally Advanced Hepatocellular Carcinoma (HCC) (SIRveNIB). Available at: https://clinicaltrials.gov/ct2/show/NCT01135056. Accessed August 22, 2016.
- 91.University of Magdeburg; Bayer; Sirtex Medical. Sorafenib and Micro-therapy Guided by Primovist Enhanced MRI in Patients With Inoperable Liver Cancer (SORAMIC). Available at: https://clinicaltrials.gov/ct2/show/NCT01126645. Accessed August 22, 2016.
- 92.Assistance Publique - Hôpitaux de Paris; Ministry of Health (France). SorAfenib Versus RADIOEMBOLIZATION in Advanced Hepatocellular Carcinoma (SARAH). Available at: https://clinicaltrials.gov/ct2/show/NCT01482442. Accessed August 22, 2016.
- 93.Sirtex Medical Inc. SIR-Spheres Ongoing Clinical Studies. Available at: http://www.sirtex.com/ap/clinicians/ongoing-clinical-studies/. Accessed August 22, 2016.
- 94.Center Eugene Marquis. Efficacy Study of Intra-hepatic Administration of Therasphere® in Association With Intravenous Chemotherapy to Treat Cholangiocarcinoma (MispheC). Available at: https://clinicaltrials.gov/ct2/show/NCT01912053. Accessed August 22, 2016.
- 95.Sirtex Medical. FOLFOX6m Plus SIR-Spheres Microspheres vs FOLFOX6m Alone in Patients With Liver Mets From Primary Colorectal Cancer (FOXFIREGlobal). Available at: https://clinicaltrials.gov/ct2/show/NCT01721954. Accessed August 22, 2016.
- 96.Universiteit Antwerpen. Comparing HAI-90Y (SIR-spheres)+Chemotx LV5FU2 Versus Chemotx LV5FU2 Alone to Treat Colorectal Cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT01895257?term=sir-step&rank=1. Accessed August 22, 2016.
- 97.Center Eugene Marquis. Internal Radiation Therapy for Hepatocellular Carcinomas With Therasphere: Optimized Dosimetry Versus Standard Dosimetry. Available at: https://clinicaltrials.gov/ct2/show/NCT02582034. Accessed August 22, 2016.
- 98.Northwestern University; National Cancer Institute. Chemoembolization Versus Radioembolization in Treating Patients With Liver Cancer That Cannot Be Treated With Radiofrequency Ablation Or Surgery. Available at: https://clinicaltrials.gov/ct2/show/NCT00956930. Accessed August 22, 2016.
- 99.University Hospital G. Transarterial RAdioembolization Versus ChemoEmbolization for the Treatment of Hepatocellular Carcinoma (HCC) (TRACE). Available at: https://clinicaltrials.gov/ct2/show/NCT01381211. Accessed August 22, 2016.
- 100.Johannes Gutenberg University Mainz. Selective Internal Radiotherapy (SIRT) Versus Transarterial Chemoembolisation (TACE) for the Treatment of Cholangiocellular Carcinoma (CCC). Available at: https://clinicaltrials.gov/ct2/show/NCT01798147. Accessed August 22, 2016.