Abstract
Outcomes for acute myeloid leukemia (AML) patients who fail to achieve complete remission remain poor. Hematopoietic cell transplantation (HCT) has been shown to induce long-term survival in AML patients with active disease. HCT is largely performed with HLA- matched unrelated or HLA-matched related donors. Recently, HCT with HLA-haploidentical related donors has been identified as a feasible option when HLA-matched donors are not immediately available. However, there is little data comparing outcomes of AML patients with active disease who receive haploidentical versus traditionally matched HCT. We retrospectively analyzed data from 99 AML patients with active disease undergoing allogeneic HCT at a single institution. Forty-three patients received unrelated donor HCT, 32 patients received matched related donor HCT, and 24 patients received peripheral blood haploidentical HCT with post- transplant cyclophosphamide. We found no significant differences between treatment groups in terms of overall survival (OS), event-free survival (EFS), transplant-related mortality (TRM), cumulative incidence of relapse, and cumulative incidence of acute and chronic graft versus host disease (GvHD). We performed univariate regression analysis of variables that modified OS in all patients, and found only younger age at transplant and development of chronic GvHD significantly improved outcome. Although limited by our relatively small sample size, these results indicate that haploidentical HCT in active AML patients have comparable outcomes to HCT with traditionally matched donors. Haploidentical HCT can be considered in this population of high-risk patients when matched donors are unavailable, or when wait times for transplantation are unacceptably long.
Keywords: Acute Myeloid Leukemia, Active Disease, Haploidentical Transplant
INTRODUCTION
Acute myeloid leukemia (AML) is one of the most common hematologic malignancies in the non-pediatric patient population.1 Allogeneic hematopoietic cell transplantation (HCT) is the most curative therapeutic option in patients who have been able to achieve complete remission (CR) following induction chemotherapy.2, 3 However, only around 50% of young and 39% of elderly AML patients in poor prognostic groups are able to achieve CR with current intensive induction regimens.4 The prognosis of patients not achieving CR or who relapse and have minimal residual disease (MRD) or active disease at the time of allogeneic HCT remains dismal, and negligible for all patients who cannot proceed to HCT. In previous reports, overall survival (OS) in active AML patients undergoing HLA-matched related or HLA-matched unrelated HCT has ranged from 20–30%.5–8 Allogeneic HCT remains the best option for patients who otherwise fail to achieve remission due to refractory or relapsed disease.5
Recently, HCT with HLA-haploidentical related donors has emerged as a viable option for transplantation, with outcomes comparable to those of traditionally matched donors.9–11 The use of T-cell replete grafts with post-transplant cyclophosphamide has largely circumvented the unacceptably high rates of graft failure and infection seen after T-cell depleted haploidentical HCT,12, 13 and has been adopted at our institution for all haploidentical HCTs. Haploidentical HCT remains an important source of alternative donors, as a substantial proportion of patients in need of HCT will not have an optimally matched donor.14 This is especially true in minority populations in which HLA-matching of unrelated donors is particularly difficult.15
There has been evidence to suggest that increasingly mismatched HCTs may be associated with an increased graft versus leukemia (GvL) effect, resulting in improved outcomes in patients with high-risk disease.16 Previous studies have suggested lower rates of relapse in high-risk AML patients with the use of haploidentical HCT compared to matched unrelated or related HCT.17 Comparative outcomes in active disease however remain unknown. We retrospectively analyzed outcomes from active disease AML patients who underwent unrelated donor, related donor or haploidentical HCT. While haploidentical HCT has been shown to be feasible in active AML patients, there is little data on how outcomes of haploidentical HCT compare to those of traditionally matched donor HCT.18
MATERIALS AND METHODS
Study Population
All adult patients with active disease AML who underwent allogeneic HCT at Washington University Medical Center in St. Louis from 2012 to 2015 were included for analysis. Active disease was defined as ≥5% blasts in pre-transplantation bone marrow, presence of extramedullary disease at time of transplantation, or persistent abnormal cytogenetic findings on chromosome analysis or fluorescent in situ hybridization (FISH). Patients were excluded if they had undergone prior allogeneic HCT. This study was approved by the Institutional Review Board at Washington University School of Medicine, St. Louis.
Outcomes and Definitions
Study outcomes included overall survival (OS), event-free survival (EFS), cumulative incidence of relapse, and cumulative incidence of acute and chronic graft-versus-host disease (GvHD). OS was defined as time from transplantation to time of death from any cause or last follow-up. EFS was defined as survival without relapse or death. Treatment related mortality (TRM) was defined as any death prior to day +28 or any death while in continuous remission after day +28. Neutrophil engraftment was defined as the first day in which the absolute neutrophil count (ANC) was ≥500 for three consecutive days. Platelet engraftment was defined as the first day in which the platelet count was ≥20 for three consecutive days without need for platelet transfusion. Graft failure was defined as failure of neutrophil engraftment after HCT (primary), or loss of donor chimerism after initial engraftment with ≥95% recipient cells at any time, not due to relapsed disease (secondary). Relapse in patients achieving CR after HCT was defined as presence of ≥5% blasts in bone marrow. CMV reactivation was defined as presence of CMV DNA after at least 4 weeks of non-detectable levels during active surveillance.19 Functional status and comorbidities were evaluated using the Karnosfky Performance Score and Hematopoietic Cell Transplantation-Comorbidity Index (HCT-CI).20 Acute GvHD was graded according to International Bone Marrow Transplant Registry (IBMTR) staging guidelines.21 Chronic GvHD was graded according to National Institute of Health (NIH) consensus criteria.22
Statistical Analyses
Patient, disease, and transplant characteristics were collected from the electronic medical record in all qualifying patients as discussed above. Death in remission was considered a competing risk event for cumulative incidence of relapse. Graft failure, relapse, or death were considered as competing risk events for cumulative incidence of acute and chronic GvHD. Continuous variables between groups were compared with Mann-Whitney U-testing. Dichotomous variables between groups were compared with chi-squared testing or Fisher’s exact test, when appropriate. Cumulative incidence was measured with the cumulative incidence function. Time-to-event functions were measured using Kaplan-Meier curves and the log-rank test. Univariate Cox proportional hazards regression analysis was used to determine patient and disease variables that modified overall survival, with chronic GvHD treated as a time-dependent variable.
RESULTS
Patient Characteristics
A total of 99 patients with active AML were included in the analysis. Forty-three patients received an unrelated donor HCT, of which 6 had one HLA mismatch at the HLA-A, -B, -C, -DRB1, or -DQB1 locus (partially mismatched), and 2 had two HLA mismatches at the HLA-A, -B, -C, -DRB1, or -DQB1 locus (mismatched). Thirty-two patients received a matched related donor HCT. Twenty-four patients received haploidentical HCT. Seventy-five percent of the HLA-haploidentical related donors were mismatched at 5 HLA alleles (HLA-A, -B, -C, -DRB1, -DQB1) in both the graft versus host (GVH) and host versus graft (HVG) directions. Active disease as defined by ≥5% pre-transplantation bone marrow was present in 78% of patients, while active disease as defined by persistent cytogenetics or extramedullary disease was present in 20% and 2% of all patients, respectively. There were no significant differences between groups in distribution of active disease types (p=0.88). Median follow-up of survivors was 18 months.
Patient and disease characteristics are displayed in Table 1. There were no significant differences in patient and disease characteristics between groups. Median age of active AML patients receiving unrelated donor, related donor, and haploidentical HCT was 55, 60, and 54, respectively. Karnofsky performance scores were less than 90 in 72%, 66%, and 67% of unrelated donor, related donor, and haploidentical patients. High-risk cytogenetics as defined by European LeukemiaNet (ELN) scoring was present in 40%, 41%, and 50% of unrelated donor, related donor, and haploidentical patients.4 Fifty-one percent of unrelated donor patients had de novo AML, compared to 63% and 71% of related donor and haploidentical patients. At unrelated donor HCT, 51% of patients were primary induction failures and 49% were relapsed disease. Fifty-six percent of related donor HCT patients and 50% of haploidentical HCT patients were in primary induction failure at time of transplantation. For relapsed unrelated donor, related donor, and haploidentical patients, median duration of first remission (CR1) was 3.5, 2.9 and 5.3 months, respectively. Median blast percentage in pre-transplantation bone marrow biopsies were 18%, 9%, and 19.5% in unrelated donor, related donor, and haploidentical patients. Fifty-nine percent of patients had evidence of peripheral blasts at least one week prior to transplant (range:0–72%; median: 5%), with no significant differences in peripheral blast percentages between groups (p=0.87).
Table 1.
Patient and Disease Characteristics
| UD (%) | RD (%) | Haplo (%) | p value | |
|---|---|---|---|---|
| N | 43 | 32 | 24 | |
| Age, years | 0.09 | |||
| Median | 55 | 60 | 54 | |
| Range | 23–73 | 32–72 | 21–73 | |
| Karnofsky Performance Scale | 0.85 | |||
| 100 | 0 (0) | 2 (6) | 2 (8) | |
| 90 | 12 (28) | 9 (28) | 6 (25) | |
| 80 | 18 (42) | 14 (44) | 9 (38) | |
| <70 | 13 (30) | 7 (22) | 7 (29) | |
| HCT-CI Risk | 0.70 | |||
| 0 | 5 (12) | 4 (13) | 1 (4) | |
| 1 –2 | 5 (12) | 5 (16) | 2 (8) | |
| ≥3 | 33 (77) | 23 (72) | 21 (88) | |
| ELN Risk | 0.83 | |||
| High | 17 (40) | 13 (41) | 12 (50) | |
| Intermediate | 24 (56) | 17 (53) | 10 (42) | |
| Low | 2 (4) | 2 (6) | 2 (8) | |
| Disease Etiology | 0.27 | |||
| De Novo | 22 (51) | 20 (63) | 17 (71) | |
| Secondary | 21 (49) | 12 (38) | 7 (29) | |
| Disease Status at Transplant | 0.99 | |||
| Primary Induction Failure | 22 (51) | 18 (56) | 12 (50) | |
| Relapse Refractory | 21 (49) | 14 (44) | 12 (50) | |
| Active Disease Subtype | 0.88 | |||
| Morphology | 37 (86) | 24 (75) | 20 (83) | |
| Cytogenetics | 6 (15) | 6 (18) | 4 (17) | |
| Extramedullary Disease | 0 (0) | 2 (6) | 0 (0) | |
| Median Relapse to Transplant, months | 4 | 2 | 6 | 0.24 |
| Median Duration of CR1, months | 4 | 3 | 5 | 0.34 |
| Median Pre-Transplant Blast% in BM | 18 (0–72) | 9 (0–87) | 19.5 (0–84) | 0.13 |
| Median Pre-Transplant Blast% in blood | 5 (0–32) | 4 (0–72) | 7 (0–60) | 0.87 |
Abbreviations: CR1: first complete remission; BM: bone marrow
Transplantation Characteristics
Transplant characteristics are displayed in Table 2. G-CSF mobilized peripheral blood grafts were used in all patients, except for one matched unrelated donor patient who received a non-mobilized bone marrow graft. There were no significant differences in donor-recipient sex mismatch or CMV status between groups. Seventy-nine percent of unrelated donor patients received myeloablative conditioning compared to 66% of related and 67% of haploidentical patients. The most common myeloablative (MA) regimen in unrelated and related donor transplants was fludarabine and busulfan. The most common reduced intensity conditioning (RIC) regimens were fludarabine plus cyclophosphamide and low-dose total body irradiation (TBI), or fludarabine and melphelan. All haploidentical HCT recipients received G-CSF mobilized T-cell replete peripheral blood grafts and post-transplant cyclophosphamide. GvHD prophylaxis consisted of methotrexate and a calcineurin inhibitor in 85% and 81% of unrelated and related donor patients, respectively, while all haploidentical patients received mycophenolate and a calcineurin inhibitor.
Table 2.
Transplant Characteristics
| UD (%) | RD (%) | Haplo (%) | p value | |
|---|---|---|---|---|
| Donor-recipient Sex | 0.26 | |||
| MM | 19 (44) | 7 (22) | 9 (37) | |
| MF | 5 (11) | 11 (34) | 6 (25) | |
| FM | 13 (30) | 8 (25) | 6 (25) | |
| FF | 6 (14) | 6 (19) | 3 (13) | |
| Donor-recipient CMV status (%) | 0.47 | |||
| +/+ | 9 (21) | 11 (34) | 7 (29) | |
| +/− | 8 (19) | 2 (6) | 7 (29) | |
| −/+ | 13 (30) | 8 (25) | 5 (21) | |
| −/− | 13 (30) | 11 (34) | 5 (21) | |
| Degree of HLA Match* (%) | <0.001 | |||
| Well-Matched | 35 (81) | 32 (100) | N/A | |
| Partially-Matched | 6 (14) | 0 (0) | 24 (100) | |
| Mismatched | 2 (5) | 0 (0) | N/A | |
| Graft Type (%) | 1.00 | |||
| Peripheral blood | 42 (98) | 32 (100) | 24 (100) | |
| Bone Marrow | 1 (2) | 0 (0) | 0 (0) | |
| Type of Conditioning (%) | 0.36 | |||
| Myeloablative | 34 (79) | 21 (66) | 16 (67) | |
| Reduced Intensity Conditioning | 9 (21) | 11 (34) | 8 (23) | |
| Conditioning Regimen (%) | <0.001 | |||
| Clo/Bu | 15 (35) | 6 (19) | 0 (0) | |
| Bu/Cy | 14 (33) | 8 (25) | 0 (0) | |
| Flu/Bu ± Other | 12 (28) | 12 (38) | 3 (13) | |
| Cy/TBI ± Other | 1 (2) | 5 (16) | 0 (0) | |
| Flu/TBI | 1 (2) | 0 (0) | 13 (54) | |
| Flu/Melph | 0 (0) | 1 (3) | 2 (8) | |
| Flu/Cy/TBI | 0 (0) | 0 (0) | 6 (25) | |
| GvHD Prophylaxis (%) | <0.001 | |||
| (Tacro or Siro) + MTX | 31 (72) | 26 (81) | 0 (0) | |
| (Tacro or Siro) + MTX ± Other | 6 (13) | 0 (0) | 0 (0) | |
| (Tacro or Siro) + MMF ± Other | 5 (11) | 4 (13) | 24 (100) | |
| Other | 1 (2) | 2 (6) | 0 (0) |
Well-matched: no identifiable HLA mismatch at HLA-A, -B, -C, -DRB1, or -DQB1 locus; Partially-matched: one identifiable HLA mismatch at HLA-A, -B, -C, -DRB1, or -DQB1 locus; Mismatched: greater than one identifiable HLA mismatch at HLA-A, -B, -C, -DRB1, or -DQB1 locus
Engraftment Outcomes
No significant differences in engraftment outcomes were found (Table 3). Neutrophil recovery by day 30 occurred in 91%, 91%, and 83% of unrelated donor, related donor, and haploidentical patients. Platelet recovery by day 30 occurred in 84%, 84%, and 75% of unrelated donor, related donor, and haploidentical patients. Full donor chimerism, as defined by short-tandem repeat (STR) testing on Day 30 bone marrow, was present in 65%, 76%, and 71% of evaluable unrelated donor, related donor, and haploidentical patients. There were no significant differences in CMV reactivation or graft failure between groups.
Table 3.
Transplant Outcomes
| UD (CI 95%) | RD (CI 95%) | Haplo (CI 95%) | p | |
|---|---|---|---|---|
| Neutrophil Engraftment at Day 30 | 91 (81 – 100) | 91 (80 – 100) | 83 (67 –99) | 0.07 |
| Platelet Engraftment at Day 100 | 84 (72 – 96) | 84 (71 – 97) | 75 (57 – 93) | 0.20 |
| Full Donor Chimerism at Day 30 | 65 | 76 | 71 | 0.49 |
| Graft Failure at 1 year | 2 | 0 | 4 | 1.00 |
| CMV Reactivation at 1 year | 60 (42 – 78) | 43 (21 – 65) | 63 (40 – 86) | 0.40 |
| Acute GvHD (Grades 2–4) | 57 (41 – 73) | 36 (18 – 54) | 58 (36 – 80) | 0.11 |
| Acute GvHD (Grades 3–4) | 30 (17 – 43) | 23 (8 – 38) | 28 (8 – 48) | 0.74 |
| Chronic GvHD at 1 year | 20 (8 – 32) | 17 (3 – 31) | 13 (0 – 26) | 0.73 |
| Severe Chronic GvHD at 1 year | 15 (4 – 26) | 10 (0 – 20) | 10 (0 – 24) | 0.61 |
Abbreviations: UD = Unrelated Donor; RD = Related Donor; Haplo = haploidentical; CI = Cumulative Incidence
GvHD Outcomes
Acute and chronic GvHD outcomes are displayed in Table 3. The cumulative incidence of grades 3–4 acute GvHD at day 100 were 30% (CI 17–43%) in unrelated donor patients, 23% (CI 8–38%) in related donor patients, and 28% (CI 8–48%) in haploidentical patients. There were no significant differences in the cumulative incidences of grades 3–4 acute GvHD between groups (p=0.74). Similarly, there were no significant differences in the cumulative incidences of severe chronic GvHD at 1 year between groups. Overall cumulative incidences of severe chronic GvHD at 1 year were 15% (CI 4–26%) in unrelated donor patients, 10% (CI 0–20%) in related donor patients, and 10% (CI 0–24%) in haploidentical patients.
Survival Outcomes and Relapse Rates
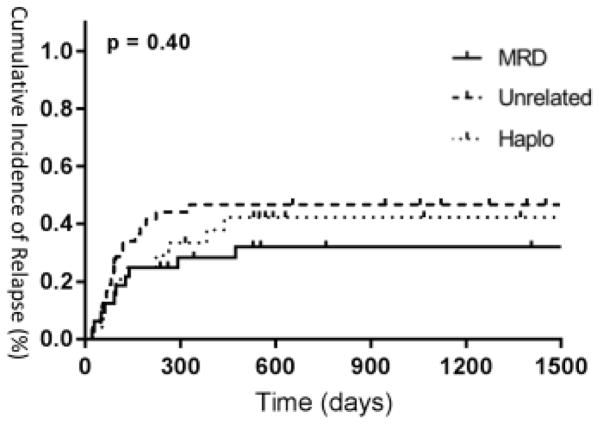
There were no significant differences between unrelated donor, related donor, and haploidentical transplants in terms of relapse rates, EFS, TRM, and OS. In patients who subsequently achieved CR after HCT, one-year relapse rates were 48% (CI 0.3232–0.6368) in unrelated donor patients, 28% (CI 0.1232–0.4368) in related donor patients, and 33% (CI 0.134–0.526) in haploidentical patients. In all relapsed patients (N=30), median survival after relapse was 67 days in unrelated donor patients (N=15), 22 days in related donor patients (N=7), and 43 days in haploidentical patients (N=8), with no significant differences between groups (p=0.55). Twenty-eight percent of patients in the entire cohort received donor lymphocyte infusion (DLI) after relapse, with no patient receiving prophylactic DLI. There were no significant differences in DLI treatment between groups (p=0.23).
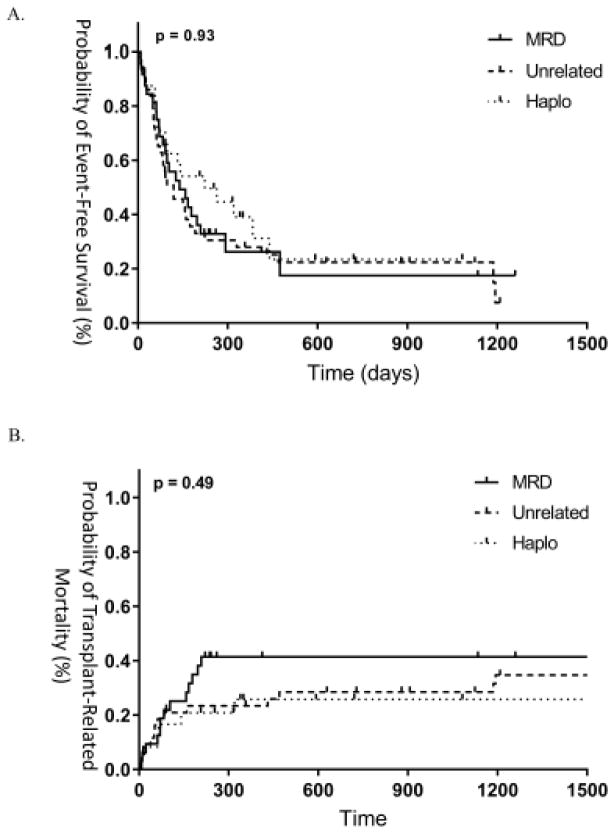
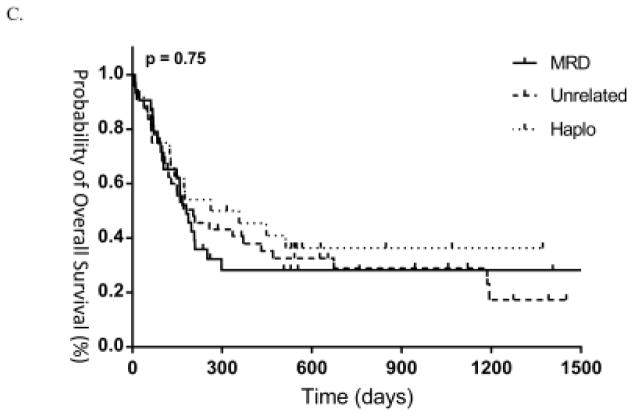
One and two-year EFS were 28% and 22% in unrelated donor patients, 27% and 18% in related donor patients, and 39% and 23% in haploidentical patients (Figure 2a). Similarly, TRM at 1 and 2 years was 23% and 29% in unrelated donor patients, 42% and 42% in related donor patients, and 26% and 26% in haploidentical patients (Figure 2b). OS at 1 and 2 years was 41% and 29% in unrelated donor patients, 28% and 28% in related donor patients, and 45% and 36% in haploidentical patients (Figure 2c). OS between active disease patients as defined by morphology or persistent cytogenetics were not significantly different, consistent with previous findings in the literature (p=0.67).23 As such, we found similar results in outcomes when performing subgroup analysis of patients with active disease as defined by morphology only, with no differences in OS between groups (p=0.21).
Figure 2.
(A) EFS, (B) TRM and (C) OS between groups.
In this cohort, many of the clinical and disease characteristics previously associated with poor outcomes were not significantly associated with OS, including de novo versus secondary AML (p = 0.68), primary induction failure versus relapsed disease (p = 0.54), relapse duration > 6 months (p = 0.24), cytogenetic risk (p = 0.89), Karnofsky performance status < 90 (p = 0.53), HCT-CI (p = 0.58), use of RIC versus MA conditioning (p = 0.22), blast percentage in pre-transplantation bone marrow (p = 0.16), and the number of mismatched alleles (p = 0.57). Only age at transplant significantly impacted OS (p = 0.03). Older age at transplant was associated with poor OS (HR: 1.03, 95% CI 1.00–1.05). Additionally, in patients surviving to day +80, the development of chronic GvHD was associated with significantly better OS (HR: 0.22, 95% CI 0.10–0.50).
DISCUSSION
To our knowledge, this is the first study to compare outcomes of AML patients in active disease undergoing transplantation from HLA- haploidentical, unrelated, and related donors. We demonstrate that haploidentical HCT is similar to HCT from traditionally matched donors, with comparable TRM, EFS, and OS. There were no significant differences between groups in terms of relapse rates and incidence of acute and chronic GvHD.
Some studies have suggested an increased GvL effect in HLA-mismatched patients undergoing transplantation, although conflicting data exists.11, 16, 24 Relapse rates in one-locus-mismatched HCT are lower compared to matched HCT, which offsets the adverse effects of HLA mismatch on transplant-related mortality in patients with high-risk disease.25 Compared to traditionally matched donors, Wang et al. found lower cumulative incidences of relapse and improved OS in high-risk AML patients undergoing haploidentical HCT with the GIAC (G: G-CSF mobilization; I: intensified immunosuppression; A: ATG administration; C: combination peripheral blood stem cell and bone marrow graft use) approach.17 There is a theoretical increased GvL effect in patients with active AML transplanted with a haploidentical donor. While we found a weak trend in 2-year OS rates in haploidentical patients (36% compared to 29% and 28% in unrelated and related donor patients, respectively), these differences were not statistically significant. We also did not find an effect of HLA-mismatch degree on OS and EFS, although analysis is limited by the small variety of HLA-mismatch seen in our dataset. Interestingly, we found that patients transplanted with a related donor had the lowest relapse rates despite also having the lowest OS rates, although our results were not statistically significantly. This is likely accounted by the increased TRM seen in patients who receive HCT with a related donor, and further studies with a larger cohort of patients are necessary to investigate these trends. We additionally found no significant differences in post-relapse survival between groups, in contrast to prior studies demonstrating inferior post-relapse survival in patients receiving haploidentical transplant.26, 27 This may be due to the similar rates of DLI use between groups, as decreased use of DLI was thought to contribute to the poorer post-relapse survival outcomes seen in haploidentical patients.27 However, our sample size is also small, and post-relapse survival in active disease patients specifically has not been investigated.
DLI for relapse after haploidentical HCT results in durable response rates and acceptable levels of GVHD toxicity when appropriately dosed, although overall remission rates after relapse in AML remain low.28, 29 Recently, the use of prophylactic DLI has been found to favorably influence disease progression and progression-free survival in active AML patients after haploidentical transplant.18 In a study by Jaiswal et al., 18-month OS rates were reported as 70% in active AML patients undergoing DLI after MA conditioning, compared to 35% in MA patients without DLI.18 Although the incidence of chronic GvHD was higher in the MA-DLI group, the authors found no differences in incidence rates of acute GvHD. In our cohort, 28% of patients received a DLI after relapse, although our sample size was too limited to definitively evaluate whether DLI had a beneficial impact in each transplant group. Further investigation is needed to determine whether additional forms of immunotherapy in combination with haploidentical HCT can result in superior outcomes in patients with active AML.
There were no significant differences in acute and chronic GvHD between groups. In prior retrospective studies, incidence of chronic GvHD has been found to be lower in haploidentical HCT, an effect mediated by either the use of post-transplant cyclophosphamide or the more frequent use of bone marrow grafts.9, 10 Interestingly, in haploidentical HCT using peripheral blood stem cells, this difference in chronic GvHD rates between transplant groups has not been seen, similar to the results of our analysis.11 The effect of graft source on GVHD risk and engraftment outcomes has become an area of interest after haploidentical transplant. In particular, there is growing experience in the use of G-CSF mobilized bone marrow grafts, which may offer lower GVHD rates and more rapid engraftment compared to G-CSF mobilized peripheral blood or non-mobilized bone marrow.30
We found a beneficial impact of chronic GvHD on OS, consistent with prior studies demonstrating lower relapse rates in patients who develop chronic GvHD.31, 32 Few other variables on univariate analysis were associated with outcomes, with only increased age at transplant showing worse OS. This may be partially explained by the increased likelihood of RIC conditioning in patients older than 60 (p<0.001), although interestingly, we found no significant differences in OS between active AML patients receiving RIC and MA conditioning. The lack of association between survival and traditional predictors of risk, such as HCT-CI and cytogenetic risk, may reflect the generally poor outcomes of active AML patients.
Our study has several limitations. We had a relatively small sample size, which limits the ability to detect small but significant differences between groups. There was also significant heterogeneity between cohorts in regards to conditioning regimens and GvHD prophylaxis. However, the patients were otherwise treated with identical supportive care measures at a single institution. Patients with persistent cytogenetic disease were included in our active disease cohort given data in the literature suggesting similar outcomes.23 We confirmed in our cohort that there were no significant differences in OS between morphologic and cytogenetic definitions of active disease, and our results were unchanged on subgroup analysis with active disease patients by morphology only. We also had a relatively short follow-up, with median follow-up of survivors at 18 months. However, outcomes in AML after transplant are historically poor, and in our cohort specifically, we found that 96% of deaths occurred before 18 months in our non-surviving patients.9 It is possible that further differences between groups would be detected with longer follow-up, although the number of future events that would occur are likely be diminished.
Our results indicate that haploidentical HCT performed in patients with active AML have similar outcomes to HCT performed using traditionally matched unrelated and related donors. Haploidentical HCT remains a viable alternative in active disease patients, and has the further advantage of decreased waiting times for transplantation. Larger, multi-institution studies are warranted to both validate and extend our findings in this group of high-risk patients.
Figure 1.
Cumulative incidence of relapse between groups.
Highlights.
AML patients not able to achieve remission or relapsed after being previously in remission continue to have extremely poor prognosis.
Due to the relative urgency of proceeding to transplant in active AML patients, haploidentical transplantation is particularly an attractive option as most patients have readily available HLA haploidentical donors.
Use of haploidentical hematopoietic cell transplantation with post-transplant cyclophosphamide is associated with post -transplant survival comparable to matched unrelated or matched sibling donor transplantation.
Footnotes
Financial disclosures: none
Conflicts of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Zittoun RA, Mandelli F, Willemze R, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. The New England journal of medicine. 1995;332:217–223. doi: 10.1056/NEJM199501263320403. [DOI] [PubMed] [Google Scholar]
- 3.Cassileth PA, Harrington DP, Appelbaum FR, et al. Chemotherapy compared with autologous or allogeneic bone marrow transplantation in the management of acute myeloid leukemia in first remission. The New England journal of medicine. 1998;339:1649–1656. doi: 10.1056/NEJM199812033392301. [DOI] [PubMed] [Google Scholar]
- 4.Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craddock C, Labopin M, Pillai S, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia. 2011;25:808–813. doi: 10.1038/leu.2011.13. [DOI] [PubMed] [Google Scholar]
- 7.Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM) Bone marrow transplantation. 2000;26:1157–1163. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
- 8.Biggs JC, Horowitz MM, Gale RP, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80:1090–1093. [PubMed] [Google Scholar]
- 9.Bashey A, Zhang X, Sizemore CA, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31:1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 10.Ciurea SO, Zhang MJ, Bacigalupo AA, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashidi A, DiPersio JF, Westervelt P, et al. Comparison of Outcomes after Peripheral Blood Haploidentical versus Matched Unrelated Donor Allogeneic Hematopoietic Cell Transplantation in Patients with Acute Myeloid Leukemia: A Retrospective Single-Center Review. Biol Blood Marrow Transplant. 2016;22:1696–1701. doi: 10.1016/j.bbmt.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8:377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 13.Luznik L, O’Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gragert L, Eapen M, Williams E, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. The New England journal of medicine. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Besse K, Maiers M, Confer D, Albrecht M. On Modeling Human Leukocyte Antigen-Identical Sibling Match Probability for Allogeneic Hematopoietic Cell Transplantation: Estimating the Need for an Unrelated Donor Source. Biol Blood Marrow Transplant. 2016;22:410–417. doi: 10.1016/j.bbmt.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Kasamon YL, Luznik L, Leffell MS, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010;16:482–489. doi: 10.1016/j.bbmt.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Liu DH, Xu LP, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant. 2011;17:821–830. doi: 10.1016/j.bbmt.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Jaiswal SR, Zaman S, Chakrabarti A, et al. Improved Outcome of Refractory/Relapsed Acute Myeloid Leukemia after Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation with Myeloablative Conditioning and Early Prophylactic Granulocyte Colony-Stimulating Factor-Mobilized Donor Lymphocyte Infusions. Biol Blood Marrow Transplant. 2016;22:1867–1873. doi: 10.1016/j.bbmt.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 20.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone marrow transplantation. 1995;15:825–828. [PubMed] [Google Scholar]
- 22.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Araki D, Wood BL, Othus M, et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34:329–336. doi: 10.1200/JCO.2015.63.3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drobyski WR, Klein J, Flomenberg N, et al. Superior survival associated with transplantation of matched unrelated versus one-antigen-mismatched unrelated or highly human leukocyte antigen-disparate haploidentical family donor marrow grafts for the treatment of hematologic malignancies: establishing a treatment algorithm for recipients of alternative donor grafts. Blood. 2002;99:806–814. doi: 10.1182/blood.v99.3.806. [DOI] [PubMed] [Google Scholar]
- 25.Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000) Blood. 2003;102:1541–1547. doi: 10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- 26.McCurdy SR, Kasamon YL, Kanakry CG, et al. Comparable composite endpoints after HLA-matched and HLA-haploidentical transplantation with posttransplantation cyclophosphamide. Haematologica. 2016 doi: 10.3324/haematol.2016.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solh M, Zhang X, Connor K, et al. Post-relapse survival after haploidentical transplantation vs matched-related or matched-unrelated hematopoietic cell transplantation. Bone marrow transplantation. 2016;51:949–954. doi: 10.1038/bmt.2016.62. [DOI] [PubMed] [Google Scholar]
- 28.Zeidan AM, Forde PM, Symons H, et al. HLA-haploidentical donor lymphocyte infusions for patients with relapsed hematologic malignancies after related HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2014;20:314–318. doi: 10.1016/j.bbmt.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghiso A, Raiola AM, Gualandi F, et al. DLI after haploidentical BMT with post-transplant CY. Bone marrow transplantation. 2015;50:56–61. doi: 10.1038/bmt.2014.217. [DOI] [PubMed] [Google Scholar]
- 30.Ji SQ, Chen HR, Wang HX, et al. G-CSF-primed haploidentical marrow transplantation without ex vivo T cell depletion: an excellent alternative for high-risk leukemia. Bone marrow transplantation. 2002;30:861–866. doi: 10.1038/sj.bmt.1703769. [DOI] [PubMed] [Google Scholar]
- 31.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. The New England journal of medicine. 1981;304:1529–1533. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 32.Ringden O, Pavletic SZ, Anasetti C, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]