Introduction
Superior vena cava syndrome (SVCS) may be caused by malignant diseases and has an incidence that ranges from 0% to 30%. SVCS can be a presenting finding or occur at disease recurrence.1, 2 Leading etiologies include lymphomas, germ cell tumors, rhabdomyosarcoma, Ewing sarcoma, and neuroblastoma. Depending on the symptom severity and underlying malignancy, treatment options include supportive care, chemotherapy, surgical resection, and radiation therapy.3 Wilms tumor is the most common pediatric renal malignancy and is known to metastasize to the lungs, lymph nodes, liver, and bone marrow. However, SVCS in patients with Wilms tumor is rare.4
We report a case of a pediatric patient with a relapsed Wilms tumor treated with radiation therapy using 10 mV photons in flattening filter-free (10XFFF) mode directed at a large anterior mediastinal mass causing SVCS and critical airway obstruction. During treatment, an optical surface monitoring system (OSMS) continuously monitored the patient's position. The combination of these 2 technologies minimized treatment delivery time and circumvented the need for anesthesia in a child who otherwise would not have been able to tolerate standard palliative radiation therapy.
Clinical case
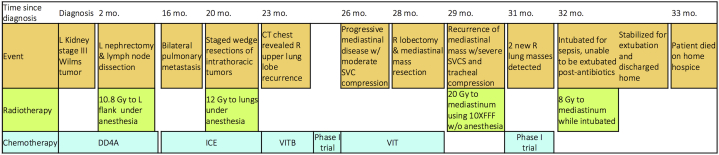
An 18-month old boy presented with a large, left, renal, stage III Wilms tumor without anaplasia and with favorable histology. He received neoadjuvant chemotherapy, left radical nephrectomy, and retroperitoneal lymph node dissection followed by adjuvant left flank radiation therapy (Fig 1).
Figure 1.
Timeline of patient history and medical interventions. DD4A, vincristine, doxorubicin, dactinomycin; ICE, ifosfamide, carboplatin, etoposide; VITB, vinblastine, irinotecan, temozolomide, bevacizumab; VIT, vinblastine, irinotecan, temozolomide.
Nine months after chemotherapy, he relapsed with bilateral pulmonary metastases. After salvage chemotherapy, staged bilateral thoracoscopic wedge resections of the remaining lung nodules revealed Wilms tumor with diffuse anaplasia. Subsequently, whole lung irradiation was performed.
The patient remained on chemotherapy for 3 months until he recurred with a 6 cm lesion of the right upper lobe with hilar extension. He showed significant improvement with a new chemotherapy regimen and a phase I immunotherapy drug, but later progressed with mediastinal disease. The patient then had a right upper lobectomy and debulking of the mediastinal mass.
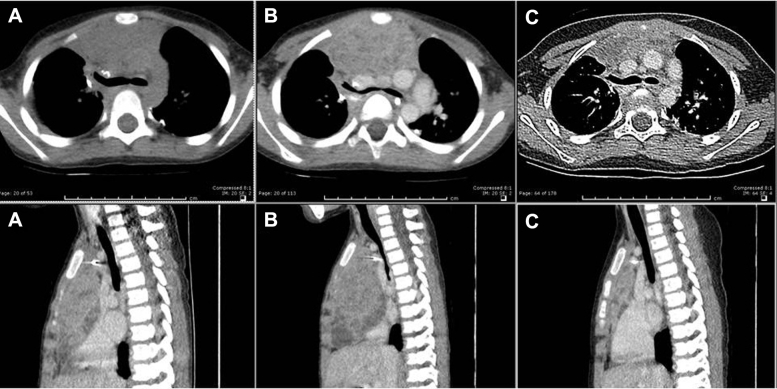
A month later, he presented with orthopnea, stridor, wheezing, facial edema, and a computed tomography (CT) scan of the chest revealed a large anterior mediastinal mass compressing the trachea, both mainstem bronchi, and the heart (Fig 2A). The patient was deemed unfit for additional anesthesia and surgery because of the SVCS and thus was evaluated for palliative radiation therapy.
Figure 2.
Paired axial and sagittal computed tomography chest scans showing the anterior mediastinal mass 1 week before radiation therapy (A), at the time of computed tomography simulation (B), and 4 weeks after radiation therapy (C).
Before the simulation, the patient was unable to remain supine for >30 seconds. An emergent CT simulation revealed further compression of the trachea and heart (Fig 2B). The gross tumor volume was delineated and expanded to generate the planning target volume (PTV).
To minimize the treatment delivery time, a single anterior-posterior (AP) field with 10XFFF mode at 2400 mU/min dose rate was planned rather than a conventional 6 mV beam at 600 mU/min. A dose of 20 Gy was prescribed to a depth of 5.5 cm in 5 fractions (Fig 3). Daily localization kV X-ray images and an OSMS (AlignRT, Vision RT Inc., Columbia, MD) were used to ensure accurate positioning. The beam hold feature was activated for intrafraction movement beyond a set tolerance of 3 mm. This was particularly important during the critical time period after image acquisition and before the start of treatment. Treatment times are listed in Table 1.
Figure 3.
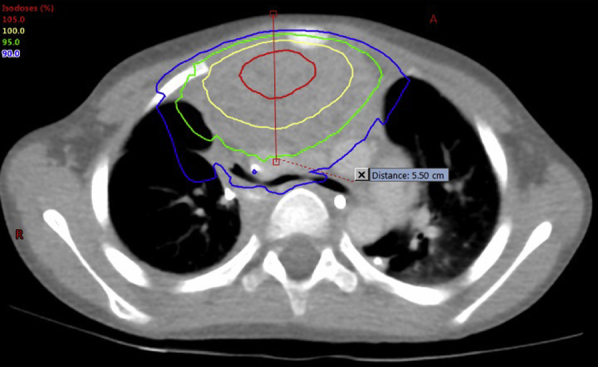
Isodose lines displayed on axial computed tomography simulation image.
Table 1.
Treatment times (in seconds)
| Fraction | Initial set-up, image guidance, and repositioning time | Beam time | Total time | |
|---|---|---|---|---|
| Course #3 10XFFF non-anesthesia | 1 | 557.11 | 11.89 | 569 |
| 2 | 780.92 | 12.08 | 793 | |
| 3 | 242.28 | 11.72 | 254 | |
| 4 | 454.29 | 11.71 | 466 | |
| 5 | 615.19 | 11.81 | 627 | |
| Course #4 6X with anesthesia | 1 | 1022 | 102 | 1124 |
| 2 | 935 | 55 | 990 |
10XFFF, 10 mV photons in flattening filter-free.
After 4 days, the patient experienced significant improvement in breathing and reduced facial swelling. He remained asymptomatic for a month and a follow-up CT scan of the chest showed a significant reduction in the mass (Fig 2C). However, 2 new lung metastases adjacent to the PTV were noted.
A week later, the patient was admitted to an outside hospital for Bacillus pumilis sepsis and required intubation. After he was transferred back to our pediatric intensive care unit, his infection and sepsis resolved, but he could not be weaned off the ventilator because of the residual mediastinal mass. The patient was offered a course of palliative re-irradiation under sedation, with 8 Gy in 2 fractions with a single 6 mV AP field. Within a few days, he experienced symptomatic improvement and was extubated. He returned home on hospice care, where he died a month later.
Discussion
Our institution has long had a guideline for the assessment and management of children with anterior mediastinal masses and critical airways. Like other investigators,5, 6 we assess severity using risk factors such as severe narrowing or complete occlusion of one or both mainstem bronchi, >50% reduction of the tracheal cross-sectional area, pericardial or pleural effusion, and signs or symptoms of superior vena cava obstruction. Patients who are at a higher risk should be initiated on steroids, tumor lysis prevention, and diagnosis-relevant chemotherapy and receive an urgent radiation oncology consultation.
Palliative radiation therapy is used frequently in adults, but few published reports have addressed it in children.7, 8, 9, 10 Rahn et al7 reported 45 children who had a symptomatic improvement at a median of 1 week after radiation therapy initiation, of whom 13% presented with dyspnea or chest pain. In 19 children with respiratory compromise from thoracic or abdominal disease, Bertsch et al9 observed a favorable clinical response rate of 72%.
The majority of children <7 years of age require anesthesia for radiation therapy to facilitate setup and minimize inadvertent movements.7, 11, 12 However, children with mediastinal masses are challenging because their airways are smaller and more compliant and compressible. The effects of airway and vascular compression in patients with SVCS can be exacerbated by anesthesia.13, 14 Deaths with anesthesia in SVCS have been reported, so alternative options are often necessary.
The 10XFFF mode was originally developed to shorten treatment times for stereotactic radiation surgery or radiation therapy.15 In this case, we demonstrate that 10XFFF in combination with OSMS enabled palliative treatment in a child who could not tolerate radiation therapy without anesthesia. Because the patient had difficulty remaining still, it was important to minimize time for setup, image guidance, and beam delivery. Via continuous surveillance, OSMS ensured accurate and precise positioning and repositioning during several necessary breaks before treatment. Without this technology, any perceived patient movements would require setup verification with skin marks or imaging, thus resulting in a lengthier process. The potential time savings of 10XFFF can be negated by plans that require gantry rotation.
An alternative treatment plan could have been a single, flattened beam with large margins without image guidance. However, we chose a more conformal hypofractionated approach to minimize the lung dose and reduce acute toxicity. With similar goals, Rahn et al7 treated 34% of their patients using advanced radiation techniques such as IMRT.
Although 10XFFF is ideal for the treatment of smaller volumes and may not uniformly cover a larger PTV, the dosimetric compromise may not be clinically meaningful in palliative cases. Pronounced peaked profiles occur when using 10XFFF with field sizes larger than 7 × 7 cm.16 In our case, we used a 13 × 14 cm field with the 95% isodose line covering the majority of the PTV (Fig 3).
In summary, we demonstrated the potential utility of 10XFFF with OSMS for palliative radiation therapy in pediatric patients and omitting anesthesia when not feasible. This treatment approach allowed for rapid improvement in the patient's quality of life and allowed our patient to return home on hospice care.
References
- 1.Ingram L., Rivera G.K., Shapiro D.N. Superior vena cava syndrome associated with childhood malignancy: Analysis of 24 cases. Med Pediatr Oncol. 1990;18:476–481. doi: 10.1002/mpo.2950180608. [DOI] [PubMed] [Google Scholar]
- 2.Acker S.N., Linton J., Tan G.M. A multidisciplinary approach to the management of anterior mediastinal masses in children. J Pediatr Surg. 2015;50:875–878. doi: 10.1016/j.jpedsurg.2014.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Yu J.B., Wilson L.D., Detterbeck F.C. Superior vena cava syndrome—A proposed classification system and algorithm for management. J Thorac Oncol. 2008;3:811–814. doi: 10.1097/JTO.0b013e3181804791. [DOI] [PubMed] [Google Scholar]
- 4.Fong K.W., Lee A.C.W., Wong Y.C., Lee W.K., Tsui K.Y. Wilms tumor presenting as superior vena cava syndrome. Med Pediatr Oncol. 2002;38:135–136. doi: 10.1002/mpo.1291. [DOI] [PubMed] [Google Scholar]
- 5.Perger L., Lee E.Y., Shamberger R.C. Management of children and adolescents with a critical airway due to compression by an anterior mediastinal mass. J Pediatr Surg. 2008;43:1990–1997. doi: 10.1016/j.jpedsurg.2008.02.083. [DOI] [PubMed] [Google Scholar]
- 6.Lam J.C., Chui C.H., Jacobsen A.S., Tan A.M., Joseph V.T. When is a mediastinal mass critical in a child? An analysis of 29 patients. Pediatr Surg Int. 2004;20:180–184. doi: 10.1007/s00383-004-1142-6. [DOI] [PubMed] [Google Scholar]
- 7.Rahn D.A., Mundt A.J., Murphy J.D., Schiff D., Adams J., Murphy K.T. Clinical outcomes of palliative radiation therapy for children. Pract Radiat Oncol. 2015;5:183–187. doi: 10.1016/j.prro.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Panoff J., Simoneaux R.V., Shah N. Radiation therapy at end of life in children. J Palliat Med. 2015;18:167–169. doi: 10.1089/jpm.2014.0219. [DOI] [PubMed] [Google Scholar]
- 9.Bertsch H., Rudoler S., Needle M.N. Emergent/urgent therapeutic irradiation in pediatric oncology: patterns of presentation, treatment, and outcome. Med Pediatr Oncol. 1998;30:101–105. doi: 10.1002/(sici)1096-911x(199802)30:2<101::aid-mpo6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Bhasker S., Bajpai V., Turaka A. Palliative radiotherapy in paediatric malignancies. Singapore Med J. 2008;49:998–1001. [PubMed] [Google Scholar]
- 11.McMullen K.P., Hanson T., Bratton J., Johnstone P.A.S. Parameters of anesthesia/sedation in children receiving radiotherapy. Radiat Oncol Lond Engl. 2015;10:65. doi: 10.1186/s13014-015-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caussa L., Hijal T., Michon J., Helfre S. Role of palliative radiotherapy in the management of metastatic pediatric neuroblastoma: a retrospective single-institution study. Int J Radiat Oncol Biol Phys. 2011;79:214–219. doi: 10.1016/j.ijrobp.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Narang S., Harte B.H., Body S.C. Anesthesia for patients with a mediastinal mass. Anesthesiol Clin N Am. 2001;19:559–579. doi: 10.1016/s0889-8537(05)70247-9. [DOI] [PubMed] [Google Scholar]
- 14.Harless J., Ramaiah R., Bhananker S.M. Pediatric airway management. Int J Crit Illn Inj Sci. 2014;4:65–70. doi: 10.4103/2229-5151.128015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scorsetti M., Alongi F., Castiglioni S. Feasibility and early clinical assessment of flattening filter free (FFF) based stereotactic body radiotherapy (SBRT) treatments. Radiat Oncol Lond Engl. 2011;6:113. doi: 10.1186/1748-717X-6-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Georg D., Knöös T., McClean B. Current status and future perspective of flattening filter free photon beams. Med Phys. 2011;38:1280–1293. doi: 10.1118/1.3554643. [DOI] [PubMed] [Google Scholar]