Abstract
Purpose
Radiation therapy plays an essential role in the treatment of locally advanced lung cancer, but it inevitably leads to incidental and unnecessary dose to critical organs, including the lung, heart, and esophagus. Numerous radiation dose-volumetric parameters have been associated with increased risk of morbidity and mortality. The purpose of the present study is to quantify differences in normal tissue radiation exposure with intensity modulated radiation therapy (IMRT) compared with 3-dimensional conformal radiation therapy (3D-CRT).
Methods and materials
Twenty-four consecutive patients with locally advanced lung cancer undergoing definitive IMRT were enrolled on a phase 1 protocol. For each patient, an optimized 3D-CRT plan was also designed. Plans were normalized in terms of planning target coverage with a standard dose of 60 Gy in 2-Gy fractions with a subset of patients also receiving elective nodal irradiation to a dose of 44 Gy in 2-Gy fractions. Normal tissue dosimetric comparisons were made for the lung, heart, and esophagus.
Results
IMRT decreased incidental dose to the lungs, heart, and esophagus. For lung, both V20 Gy (21.5% vs 26.5%, P < .01) and mean lung dose (11.9 Gy vs 14.9 Gy, P < .01) were improved with IMRT without a corresponding increase in V5 Gy (P = .76). For heart, there was improvement in V5 (28.9% vs 33.7%, P < .01) but no difference in V30 Gy (9.8% vs 15.9%. P = .10). For esophagus, all dosimetric endpoints were improved (V20 Gy, V45 Gy, V60 Gy, mean dose). For example, V60 was 6.5% with IMRT compared with 21% with 3D-CRT (P < .01).
Conclusions
IMRT significantly decreased unnecessary dose to critical organs with equivalent coverage of planning target volumes. IMRT may therefore improve the tolerability of therapy.
Summary.
The purpose of this study was to compare normal tissue dosimetry between 3-dimensional conformal (3D-CRT) and intensity modulated radiation therapy (IMRT) plans in patients with locally advanced lung cancer. Twenty-four patients enrolled on a prospective phase 1 study using IMRT had 3D-CRT plans generated for comparison, with all plans normalized for target coverage. It was found that IMRT plans resulted in statistically and clinically significant improvements in doses to the lungs, heart, and esophagus.
Introduction
Concurrent chemotherapy and radiation therapy (RT) is an established treatment paradigm for locally advanced non–small cell lung cancer (NSCLC)1, 2 and limited stage small cell lung cancer.3 Thoracic RT is associated with side effects, risks, and even treatment-related mortality resulting from incidental dose to normal structures in the thorax. The esophagus can be a dose-limiting structure during thoracic RT. Radiation esophagitis may result in odynophagia, dysphagia, and, occasionally, treatment breaks and hospitalization. Several dose-volumetric metrics are predictive of esophagitis including V60, V20, and maximum dose.4, 5, 6, 7 Likewise, radiation pneumonitis can develop after completing treatment and is also associated with various dose-volumetric parameters such as V5, V20, V30, and mean lung dose.4, 8, 9, 10, 11, 12 Finally, emerging data from a large prospective study illustrate the implication of cardiac dose, in which heart V5 and V30 were found to be predictive of overall survival.13 These studies show that increasing dose to critical structures increases the risk of morbidity and even mortality.
The delivery of an adequate dose to intrathoracic tumors while minimizing dose to critical organs in the chest is a significant challenge for clinicians. Three-dimensional conformal radiation therapy (3D-CRT) is currently the most commonly used modality, but it has a limited ability to shield critical normal structures when they lie in close proximity to target volumes. On the other hand, intensity modulated RT (IMRT) is a more advanced method of radiation delivery and can provide concave dose distributions with the potential to decrease dose to surrounding organs without compromising target coverage.
Although comparisons of 3D-CRT and IMRT for lung cancer have been reported in the literature, few have directly quantified the dosimetric benefit of IMRT.14, 15, 16, 17, 18, 19, 20, 21 The limitations and shortcomings of these studies likely influence current reimbursement policies and practice patterns in which 3D-CRT is the default approach; thus, quantifying the potential advantage with this technology is important. Using a unique, prospectively obtained dataset, we sought to assess this question.
Methods and materials
The institutional review board at Duke University Medical Center approved this study, which was conducted according to the principles set forth in the Declaration of Helsinki. As part of a prospective phase 1 study, patients with stage III NSCLC, stage II-III small cell lung cancer, or locally recurrent (N2 or N3) NSCLC after surgery were treated with concurrent cisplatin and etoposide with image guided IMRT.22 The primary endpoint of the phase 1 trial was determination of the maximum tolerated dose.
Radiation planning included custom immobilization, esophageal and intravenous contrast, and 4-dimensional computed tomography imaging to assess and account for respiratory motion. The primary tumor was expanded by 5 mm and the involved lymph nodes were expanded by 3 mm to generate a clinical target volume. The planning target volume (PTV) consisted of the clinical target volume with a 3-mm expansion. Elective nodal irradiation was optional (44 Gy, encompassing 1 nodal station beyond the involved sites[s]). Comprehensive elective nodal irradiation was not used in any case.
IMRT planning was used to avoid, as much as possible, incidental dose to the esophagus while using strict pulmonary constraints. Planning goals were to keep the spinal cord dose <45 Gy, lung V20 <35%, and esophageal V60 <25%, in this order of priority, with lung V5 Gy <50% and esophageal V20 Gy <50% whenever possible. Varian (Palo Alto, CA) Eclipse was used for treatment planning with treatment delivered on Varian linear accelerators using multiple fixed fields with a sliding window technique for beam modulation.
For the purposes of the present study, an optimized 3D-CRT plan was also designed for each patient. Various beam arrangements were used to maximize target coverage while minimizing the volume of normal tissue in the radiation fields and restricting the maximum spinal cord dose to <50 Gy. Field-in-field techniques were not used. Given that the phase 1 study was used for escalating doses of RT, for this analysis, all plans (IMRT and 3D-CRT) were standardized using a prescription dose of 60 Gy in 2-Gy fractions. In cases where elective nodal irradiation was used, a primary plan delivering 44 Gy to gross disease and elective nodal volumes was designed followed by boost plan delivering 16 Gy to gross disease, bringing the total dose to 60 Gy. To facilitate a fair comparison between IMRT and 3D-CRT approaches, all plans were normalized so that 100% of the designated dose covered 95% of the PTVs.
We compared dosimetric endpoints for the IMRT and 3D-CRT plans. For target coverage, gross tumor volume (GTV)60 V100% was assessed. PTV coverage was not analyzed because plans were normalized to equivalent coverage. Normal tissue dosimetric comparisons were made for lung (V20 Gy, V5 Gy, mean dose), esophagus (V20 Gy, V45 Gy, V60 Gy), and heart (V5 Gy, V30 Gy, V45 Gy, V60 Gy). Per International Commission on Radiation Units & Measurements 83,23 a global assessment of plan quality was made by comparing homogeneity indices (HIs) incorporating the near-maximum dose (D2), near-minimum dose (D98), and median dose (D50) to the target volume:
Conformity indices (CIs) were also compared incorporating the total volume of PTV covered by the 95% isodose (TV95), total volume of the PTV (TV), and total volume covered by the 95% isodose (V95):
Statistics
A statistical analysis was performed to compare the parameters of the target coverage metrics (GTV60) and normal tissue exposure including lungs, heart, and esophagus between IMRT and 3D-CRT methods. When the assumption of generalized linear model was satisfied, the generalized estimating equation approach was used to examine the effects of planning technique, elective nodal irradiation, and their interaction on outcome measures. In these models, an identical link and exchangeable working correlation structure were used with elective nodal irradiation (yes vs no) to assess between-subject effects and RT plan (IMRT vs 3D-CRT) as well as within-subject effects and RT plan. For those measures violating the assumption of normality, nonparametric tests, such as Wilcoxon rank sums exact test for between-subject effect and Wilcoxon signed-rank test for within-subject effect, were performed. Two-sided P values were reported. Differences were considered statistically significant if the 2-sided P value was ≤ .05. The analysis was performed using SAS for Windows, version 9.4 (SAS Institution Inc., Cary, NC).
Results
Between September 2009 and September 2014, 24 patients were enrolled on a prospective phase 1 study evaluating IMRT for locally advanced lung cancer. Patient characteristics can be found in Table 1. Elective nodal irradiation (ENI; 44 Gy) to immediately adjacent nodal basins at risk was pursued in 54% (13/24) of these patients. The clinical results of this study have been previously published.22
Table 1.
Patient characteristics (n = 24)
| Characteristic | n (%) |
|---|---|
| Age, y | |
| Median | 64 |
| Range | 49-74 |
| Sex | |
| Female | 12 (50) |
| Male | 12 (50) |
| Histology | |
| Non–small cell | 21 (88) |
| Small cell | 3 (12) |
| Stage | |
| IIIA | 8 (33) |
| IIIB | 10 (42) |
| Xa | 6 (25) |
| Largest target lesion, cm | |
| Median | 4 |
| Range | 2-12 |
Locally recurrent after surgery with N2 or N3 disease.
For the IMRT plans, a median of 8 beams (range, 7-13) was used. For those treated with ENI, the same number of beams was used for the initial and boost plans. For the purposes of this analysis, an accompanying and optimized 3D-CRT plan was generated for each patient treating the same volumes as the IMRT plan. The median number of unique beams (with or without ENI) for the 3D-CRT fields was 4 (range, 3-5).
Dose normalization provided 95% PTV coverage in all patients. The V100% for GTV60 was 100% with all IMRT plans. The V100% for GTV60 was 100% in 21/24 3D-CRT plans and 98% in 3/24 plans. Because of tumor geometry in relation to the spinal cord, necessitating very tight margins on gross disease, 3 different 3D-CRT plans were unable to achieve full GTV coverage.
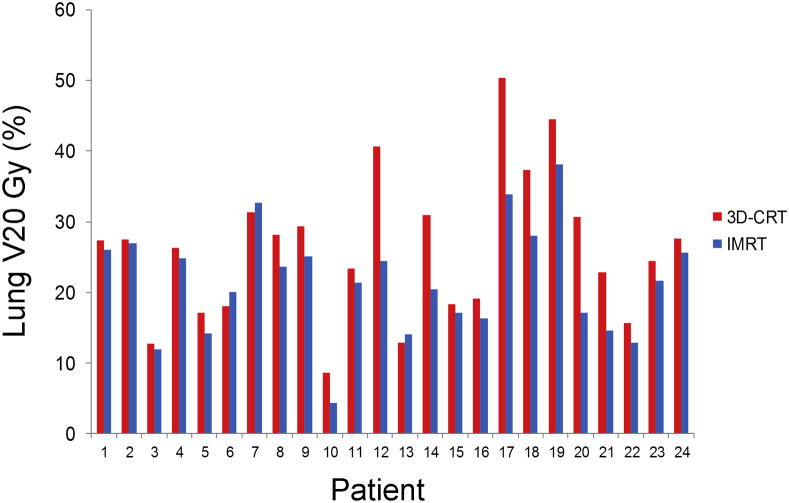
IMRT plans were associated with lower radiation exposure to the lungs. Statistically significant differences were noted in both lung V20 Gy and mean lung dose (Table 2). The mean lung V20 Gy was 21.5% with IMRT versus 26.1% with 3D-CRT (P < .01) (Fig 1). Corresponding values for mean lung dose were 11.9 Gy and 14.9 Gy, respectively (P < .01). There were no differences in V5 Gy (42% vs 45.3%, P = .76).
Table 2.
Dosimetric comparisons between IMRT and 3D-CRT
| Parameter | IMRT |
3D-CRT |
P value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Lung | |||||
| V5 Gy | 42.0 | 11.4 | 45.3 | 16.9 | .76 |
| V20 Gy | 21.5 | 7.8 | 26.1 | 10.2 | <.01 |
| Mean dose | 11.9 | 3.9 | 14.9 | 5.0 | <.01 |
| Esophagus | |||||
| V20 Gy | 35.2 | 12.7 | 50.4 | 19.3 | <.01 |
| V45 Gy | 16.0 | 11.2 | 37.5 | 16.5 | <.01 |
| V60 Gy | 6.5 | 8.1 | 21.0 | 15.3 | <.01 |
| Mean dose | 18.3 | 6.7 | 27.8 | 10.1 | <.01 |
| Heart | |||||
| V5 Gy | 28.9 | 28.6 | 33.7 | 26.4 | <.01 |
| V30 Gy | 9.8 | 12.4 | 15.9 | 20.0 | .10 |
| V45 Gy | 4.5 | 6.2 | 9.2 | 13.4 | .08 |
| V60 Gy | 1.0 | 1.43 | 3.2 | 6.3 | .17 |
| Heterogeneity Index | 0.14 | 0.07 | 0.11 | 0.04 | .26 |
| Conformity Index | 0.58 | 0.13 | 0.30 | 0.13 | <.01 |
3D-CRT, 3-dimensional conformal radiation therapy; IMRT, intensity modulated radiation therapy; SD, standard deviation.
Figure 1.
Distribution and comparison of lung V20 Gy for intensity modulated radiation therapy (IMRT) and 3-dimensional conformal radiation (3D-CRT) plans.
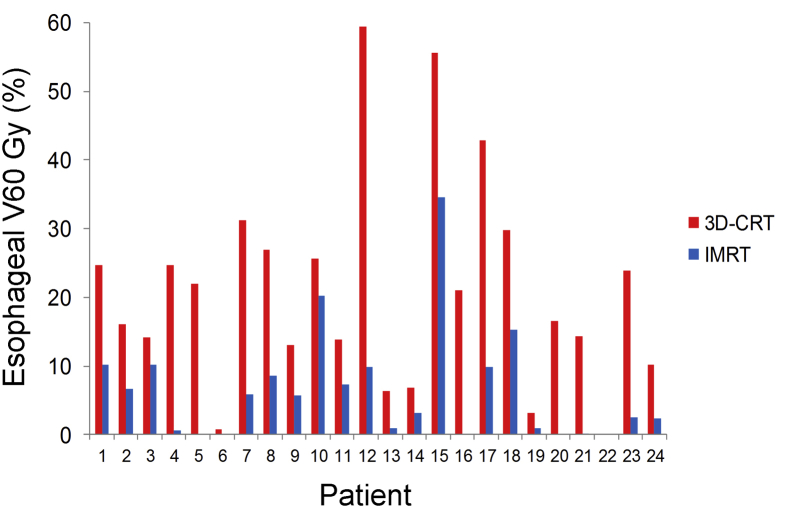
All esophageal metrics were improved with IMRT. For example, mean esophageal dose was 18.3 Gy with IMRT versus 27.8 Gy with 3D-CRT (P < .01). Similarly, mean V60 Gy was 6.5% with IMRT versus 21% with 3D-CRT (P < .01) (Fig 2).
Figure 2.
Distribution and comparison of esophagus V60 Gy for IMRT and 3D-CRT plans. See Fig 1 for abbreviations.
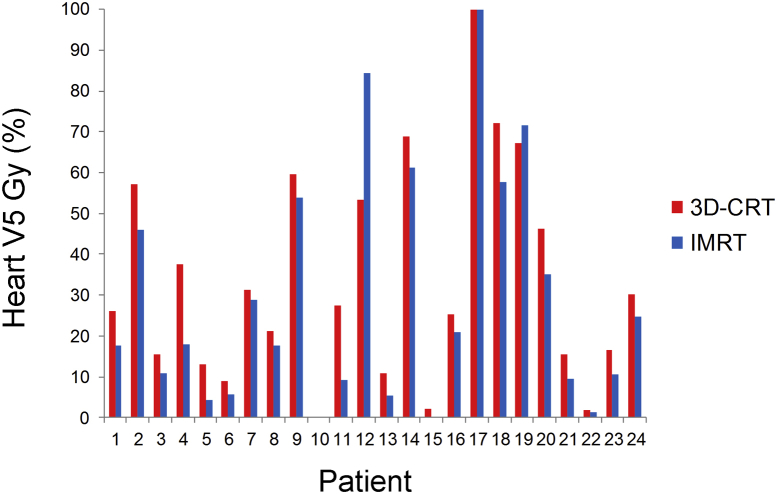
Overall, heart doses were low with both IMRT and 3D-CRT. Mean heart V30 Gy was 9.8% with IMRT and 15.9% with 3D-CRT (P = .1). Nonetheless, IMRT significantly decreased heart V5 Gy. Mean heart V5 Gy was 28.9 Gy with IMRT versus 33.7 Gy with 3D-CRT (P < .01) (Fig 3). There were no differences with other heart dose metrics (Table 2).
Figure 3.
Distribution and comparison of heart V5 Gy for IMRT and 3D-CRT plans. See Fig 1 for abbreviations.
The mean conformality index was improved with IMRT (0.58 vs 0.13, P < .01), but the heterogeneity index was not different (0.14 vs 0.11, P = .26). There were no observed interactions in normal tissue dosimetric values between patients receiving or not receiving elective nodal irradiation in relation to plan (IMRT vs 3D-CRT).
Discussion
Although using IMRT for the treatment of lung cancer has been increasing for more than a decade,15 3D-CRT remains the standard of care, as reflected in current treatment guidelines.24 This may reflect historical practice patterns or lack of robust data demonstrating the potential advantages of more sophisticated treatment techniques such as IMRT. Incidental radiation exposure to intrathoracic organs leads to significant morbidity and even mortality. Further, clinically meaningful dose-volumetric parameters have been clearly defined for normal intrathoracic organs. A direct comparison of IMRT and 3D-CRT in a uniform patient population is needed to address this critical question.
Results from this unique dataset demonstrate the superiority of IMRT globally in regard to normal tissue exposure. Regarding lung dose-volumetric comparisons, IMRT resulted in statistically and clinically meaningful reductions in V20 Gy and mean lung dose, both important metrics of pneumonitis risk. Crucially, this was achieved without compromising lung V5 Gy. This may reflect our general IMRT approach of primarily using beams in an approximate anteroposterior/posteroanterior axis in lieu of circumferentially oriented beams.
In terms of esophageal doses, IMRT was again superior for all endpoints, including V20 Gy, V45 Gy, V60 Gy, and mean dose. This may reflect our planning priorities. In a study of 1082 patients, high-grade esophageal toxicity was associated with a V60 ≥17%.25 IMRT was able to meet this criterion, whereas 3D-CRT was not. Finally, for heart dose, IMRT proved superior in regard to V5 Gy with trends toward improved V30 Gy and V45 Gy. The significance of cardiac dose was highlighted in the recently published randomized trial conducted by the Radiation Therapy Oncology Group.13 In a post hoc analysis, it was found that increasing V5 Gy and V30 Gy both predicted increased mortality.
There is a relative paucity of similar analyses for patients undergoing definitive RT for lung cancer. In a study by Grills et al, 18 consecutive patients were planned with 1 of 4 techniques: (1) IMRT, (2) 3D-CRT, (3) 3D-CRT limited to 2 or 3 beams, and (4) 3D-CRT incorporating ENI.21 Through comparative analyses, it was found that with any of the 3D techniques, either tumor control probability or normal tissue complication probability was compromised when compared with IMRT. The benefit was greatest in patients who were node-positive. Interpretation of this study is confounded by several variables. First, the study was small and included a heterogeneous mix of patients (stages I-IIIB). Second, the plans were normalized for tumor control probability based on a theoretical equation that incorporated isocenter dose as the only dosimetric parameter.26 This is problematic when comparing IMRT with 3D-CRT plans that are inherently heterogeneous and will inevitably have higher point doses. Furthermore, the median number of beams for the IMRT plans was only 4.6 (range, 3-6). This may explain the relatively small improvement with IMRT when compared with 3D-CRT, particularly when the analysis was limited to plans achieving acceptable target coverage.
A recent analysis by Woodford et al compared normal tissue doses among 3 plans for 10 patients with N2 NSCLC: (1) 3D-CRT, (2) IMRT, and (3) IMRT with cardiac constraints.20 With a prescription dose of 60 Gy and normalization for target coverage, IMRT significantly reduced the dose to the esophagus and heart compared with 3D-CRT. In the cardiac-sparing plan, where the heart was a specific avoidance structure with a goal to minimize V5 Gy and V30 Gy, heart V30 Gy could be further improved. There was a trend toward improved V5 Gy. There was no improvement in mean lung dose or V20 Gy with IMRT. This study highlights that IMRT is operator-dependent and the use of dose constraints (among other factors) will influence plan quality.
Several retrospective analyses compare dosimetric and clinical endpoints between cohorts of patients treated with either 3D-CRT or IMRT. A series from Liao et al compared outcomes for patients treated with IMRT (n = 91) or 3D-CRT (n = 318).14 V20 Gy was significantly reduced with IMRT, whereas V5 Gy was increased. IMRT reduced the risk of grade ≥3 radiation pneumonitis nearly 3-fold. Further work from the same group compared acute toxicity rates for 3D-CRT, IMRT, and proton beam therapy, showing incremental improvements in rates of both esophagitis and pneumonitis for protons.19 A study by Noh et al examined 77 consecutive patients with N3 disease, 45 (58%) of whom were treated with IMRT.18 Although the cohort treated with IMRT was more likely to have supraclavicular lymph node involvement and larger target volumes, there was no difference in toxicity. Dosimetric comparisons in this unmatched group showed higher mean lung dose and V5 for IMRT, but no difference in V20. Esophageal and spinal cord dose parameters were similar, whereas mean heart dose was higher with IMRT. Although these studies suggest dosimetric and/or clinical benefits for IMRT, the conclusions regarding IMRT are limited by confounding variables and dissimilarities between comparator groups.
There have also been population-based analyses. A Surveillance, Epidemiology, and End Results Medicare study found that the use of IMRT has been increasing since at least 2002.15 Multivariable analysis revealed that both 3D-CRT and IMRT offered survival benefits compared with 2-dimensional RT; however, a comparison of 3D-CRT and IMRT showed no differences in toxicity or survival. In a similar Surveillance, Epidemiology, and End Results Medicare study, it was again shown that the IMRT use has been increasing, particularly in patients with higher comorbidity scores and in those receiving chemotherapy.17 Despite these differences, there was no difference is survival.
A recently reported meta-analysis of retrospective data assessed the impact of IMRT compared with 3D-CRT on endpoints including overall survival, radiation pneumonitis, and radiation esophagitis.27 Although IMRT reduced the risk of radiation pneumonitis with a hazard ratio of 0.74 (0.59-0.93), there was an increased risk of radiation esophagitis with a hazard ratio of 2.47 (1.95-3.14). There was no difference in overall survival.
Care should be taken when considering the results of these studies. Population studies are limited in the granularity of data and the ability to account for confounding variables. Data from the meta-analysis were largely extracted from retrospective and population-based studies. Furthermore, the benefits of IMRT are limited by the quality and clinical validity of the dose-volumetric constraints used, which were not available.
There are potential limitations to the presented study. First, the disparity in the number of beams used between IMRT (median, 8) and 3D-CRT (median, 4) could potentially bias results toward the former. Although this may be true, 4-field plans are used most commonly for 3D-CRT at our institution as well as in others and therefore represent a “real-life” comparator to IMRT. Another limitation is the use of ENI, which results in larger treatment volumes and which, again, may bias results toward IMRT. Although ENI is not consistently used for NSCLC, it still used by some practitioners, particularly in the setting of conventional doses (60 Gy). We did not observe any interactions in normal tissue dosimetric values between patients receiving or not receiving ENI in relation to plan (IMRT vs 3D-CRT).
A unique strength of this study is that all patients had locally advanced lung cancer treated on a prospective study; therefore, all IMRT plans were generated with a priori established and strict dosimetric constraints, allowing for an optimal dosimetric comparison with 3D-CRT plans. Further, a direct comparison was possible because the only variable was treatment modality (IMRT or 3D-CRT). The primary limitation of the study was its small size. Clinical endpoints could not be assessed because patients were all treated with IMRT. Although elective nodal irradiation was used in about half of the patients, this was not shown to have an interactive effect on the observed outcomes.
Conclusions
Using prospective data, this study demonstrated clinically meaningful dosimetric advantages of IMRT in reducing dose to normal tissues in the definitive treatment of patients with lung cancer. Reducing dose to surrounding normal organs will decrease treatment-related toxicity and increase compliance with a course of therapy, both of which will serve to improve the therapeutic index.
Footnotes
Conflicts of interest: None.
References
- 1.Curran W.J., Jr., Paulus R., Langer C.J. Sequential vs. concurrent chemoradiation for stage III non–small cell lung cancer: randomized phase III trial RTOG 9410. J Natl Cancer Inst. 2011;103:1452–1460. doi: 10.1093/jnci/djr325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auperin A., Le Pechoux C., Rolland E. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non–small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 3.Turrisi A.T., 3rd, Kim K., Blum R. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 4.Kim T.H., Cho K.H., Pyo H.R. Dose-volumetric parameters of acute esophageal toxicity in patients with lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:995–1002. doi: 10.1016/j.ijrobp.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Rose J., Rodrigues G., Yaremko B., Lock M., D'Souza D. Systematic review of dose-volume parameters in the prediction of esophagitis in thoracic radiotherapy. Radiother Oncol. 2009;91:282–287. doi: 10.1016/j.radonc.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Wei X., Liu H.H., Tucker S.L. Risk factors for acute esophagitis in non–small-cell lung cancer patients treated with concurrent chemotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:100–107. doi: 10.1016/j.ijrobp.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Singh A.K., Lockett M.A., Bradley J.D. Predictors of radiation-induced esophageal toxicity in patients with non–small-cell lung cancer treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2003;55(2):337–341. doi: 10.1016/s0360-3016(02)03937-8. [DOI] [PubMed] [Google Scholar]
- 8.Palma D.A., Senan S., Tsujino K. Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: An international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;85:444–450. doi: 10.1016/j.ijrobp.2012.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradley J.D., Hope A., El Naqa I. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:985–992. doi: 10.1016/j.ijrobp.2007.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tucker S.L., Liu H.H., Liao Z. Analysis of radiation pneumonitis risk using a generalized Lyman model. Int J Radiat Oncol Biol Phys. 2008;72:568–574. doi: 10.1016/j.ijrobp.2008.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hernando M.L., Marks L.B., Bentel G.C. Radiation-induced pulmonary toxicity: A dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 12.Graham M.V., Purdy J.A., Emami B. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 13.Bradley J.D., Paulus R., Komaki R. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non–small-cell lung cancer (RTOG 0617): A randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Z.X., Komaki R.R., Thames H.D., Jr. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non–small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76:775–781. doi: 10.1016/j.ijrobp.2009.02.032. [DOI] [PubMed] [Google Scholar]
- 15.Harris J.P., Murphy J.D., Hanlon A.L., Le Q.T., Loo B.W., Jr., Diehn M. A population-based comparative effectiveness study of radiation therapy techniques in stage III non–small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88:872–884. doi: 10.1016/j.ijrobp.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Hsia T.C., Tu C.Y., Chen H.J. A population-based study of primary chemoradiotherapy in clinical stage III non–small cell lung cancer: Intensity-modulated radiotherapy versus 3D conformal radiotherapy. Anticancer Res. 2014;34:5175–5180. [PubMed] [Google Scholar]
- 17.Chen A.B., Li L., Cronin A., Schrag D. Comparative effectiveness of intensity-modulated versus 3D conformal radiation therapy among medicare patients with stage III lung cancer. J Thorac Oncol. 2014;9:1788–1795. doi: 10.1097/JTO.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 18.Noh J.M., Kim J.M., Ahn Y.C. Effect of radiation therapy techniques on outcome in N3-positive IIIB non–small cell lung cancer treated with concurrent chemoradiotherapy. Cancer Res Treat. 2016;48:106–114. doi: 10.4143/crt.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sejpal S., Komaki R., Tsao A. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer. 2011;117:3004–3013. doi: 10.1002/cncr.25848. [DOI] [PubMed] [Google Scholar]
- 20.Woodford K., Panettieri V., Ruben J.D., Senthi S. Limiting the risk of cardiac toxicity with esophageal-sparing intensity modulated radiotherapy for locally advanced lung cancers. J Thorac Dis. 2016;8:942–949. doi: 10.21037/jtd.2016.03.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grills I.S., Yan D., Martinez A.A., Vicini F.A., Wong J.W., Kestin L.L. Potential for reduced toxicity and dose escalation in the treatment of inoperable non–small-cell lung cancer: A comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57:875–890. doi: 10.1016/s0360-3016(03)00743-0. [DOI] [PubMed] [Google Scholar]
- 22.Kelsey C.R., Das S., Gu L., Dunphy F.R., 3rd, Ready N.E., Marks L.B. Phase 1 dose escalation study of accelerated radiation therapy with concurrent chemotherapy for locally advanced lung cancer. Int J Radiat Oncol Biol Phys. 2015;93:997–1004. doi: 10.1016/j.ijrobp.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 23.International Commission on Radiation Units & Measurements . Oxford University Press; Oxford: 2010. Prescribing, recording, and reporting photon-beam intensity modulated radiotherapy (IMRT). ICRU Report 83. [Google Scholar]
- 24.Ettinger D.S., Wood D.E., Akerley W. Non–small cell lung cancer, version 6.2015. J Natl Compr Canc Netw. 2015;13:515–524. doi: 10.6004/jnccn.2015.0071. [DOI] [PubMed] [Google Scholar]
- 25.Palma D.A., Senan S., Oberije C. Predicting esophagitis after chemoradiation therapy for non–small cell lung cancer: an individual patient data meta-analysis. Int J Radiat Oncol Biol Phys. 2013;87:690–696. doi: 10.1016/j.ijrobp.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 26.Martel M.K., Ten Haken R.K., Hazuka M.B. Estimation of tumor control probability model parameters from 3-D dose distributions of non–small cell lung cancer patients. Lung Canc. 1999;24:31–37. doi: 10.1016/s0169-5002(99)00019-7. [DOI] [PubMed] [Google Scholar]
- 27.Hu X., He W., Wen S. Is IMRT superior or inferior to 3DCRT in radiotherapy for NSCLC? A meta-analysis. PLoS One. 2016;11:e0151988. doi: 10.1371/journal.pone.0151988. [DOI] [PMC free article] [PubMed] [Google Scholar]