Abstract
Purpose
The purpose of this study was to evaluate the impact of tumor motion on maximum standardized uptake value (SUVmax) and metabolic tumor volume (MTV) measurements in both 3-dimensional and respiratory-correlated, 4-dimensional positron emission tomography (PET) imaging. We also evaluated the effect of implementing different attenuation correction methods in 4-dimensional PET image reconstruction on SUVmax and MTV.
Methods and materials
An anthropomorphic thorax phantom with a spherical ball as a surrogate for a tumor was used. Different types of motion were imposed on the ball to mimic a patient's breathing motion. Three-dimensional PET imaging of the phantom without tumor motion was performed and used as the reference. The ball was then set in motion with different breathing motion traces and imaged with both 3- and 4-dimensional PET methods. The clinical 4-dimensional PET imaging protocol was modified so that 3 different types of attenuation correction images were used for reconstructions: the same free-breathing computed tomography (CT) for all PET phases, the same average intensity projection CT for all PET phases, and 4-dimensional CT for phase-matched attenuation correction. Tumor SUVmax and MTV values that were measured from the moving phantom were compared with the reference values.
Results
SUVmax that was measured in 3-dimensional PET imaging was different from the reference value by 20.4% on average for the motions that were investigated; this difference decreased to 2.6% with 4-dimensional PET imaging. The measurement of MTV in 4-dimensional PET also showed a similar magnitude of reduction of deviation compared with 3-dimensional PET. Four-dimensional PET with use of phase-matched 4-dimensional CT for attenuation correction showed less variation in SUVmax and MTV among phases compared with 4-dimensional PET with free-breathing CT or average intensity projection CT for attenuation correction.
Conclusions
Four-dimensional PET imaging reduces the impact of motion on measured SUVmax and MTV when compared with 3-dimensional PET imaging. Clinical 4-dimensional PET imaging protocols should consider phase-matched 4-dimensional CT imaging for attenuation correction to achieve more accurate measurements.
Summary.
This study involved phantom experiments to evaluate uncertainties in quantitative measurements of moving targets on positron emission tomography (PET) imaging. The study also investigated how much measurement accuracy can be improved with 4-dimensional PET imaging with different reconstruction approaches. The results of this study provide a quantitative basis for radiation oncology professionals to help decide when to image a moving tumor with PET.
Introduction
Positron emission tomography (PET) imaging has become an important tool in radiation therapy for target delineation,1, 2, 3 adaptive treatment,4 and outcome prediction.5, 6 Applications of PET imaging often rely on quantitative analysis of acquired images to extract functional metrics such as standardized uptake value (SUV) and metabolic tumor volume (MTV).7, 8 The measurement accuracy of such metrics directly affects treatment planning and outcome analysis. Various factors in PET imaging can affect image quality and subsequently quantitative analysis.9, 10, 11 Tumor motion is reported to be a major factor that can alter SUV and MTV measurements, especially in lung tumor imaging.12, 13, 14
Studies have reported the feasibility and clinical implementation of respiratory-correlated 4-dimensional PET imaging to reduce the impact of motion on acquired images for patients with lung cancer.15, 16 Comparison studies have shown that 4-dimensional PET imaging revealed differences of up to 50% compared with 3-dimensional PET imaging in measurements of maximum SUV (SUVmax) and MTV.16, 17 For current imaging equipment, different techniques have been implemented to reconstruct 4-dimensional PET images with one major development: different attenuation correction (AC) images are used for reconstruction of each phase of the 4-dimensional PET image.17, 18, 19, 20 Free-breathing helical computed tomography (FB CT) is a fast and simple method to acquire an AC image; however, it lacks breathing phase correspondence to the PET image. Average intensity projection CT (Ave-IP CT) takes longer to acquire and subjects patients to higher radiation exposure, but it may reduce the overall phase discrepancy between CT and PET. Finally, respiratory-correlated 4-dimensional CT imaging provides AC images that can be matched to each phase of the 4-dimensional PET image.
The purpose of this study is to evaluate the impact of tumor motion on SUVmax and MTV measurements in both 3- and 4-dimensional PET imaging. Sinusoidal motion patterns and actual patients' breathing motions with different motion amplitudes were investigated in this study. A clinical PET/CT simulator was used for 4-dimensional PET imaging, which had only one AC option (FB CT) in the clinical scanning protocol at the time of the experiment. Despite the theoretical advantage of phase-matched AC with 4-dimensional CT in 4-dimensional PET reconstruction, previous studies reported mixed results when comparing different AC methods.17, 18 In this study, we manually modified a 4-dimensional PET imaging protocol in our PET/CT scanner in order to evaluate images that were reconstructed with FB CT as the AC image compared to those reconstructed using Ave-IP CT or 4-dimensional CT.
Methods and materials
Data acquisition
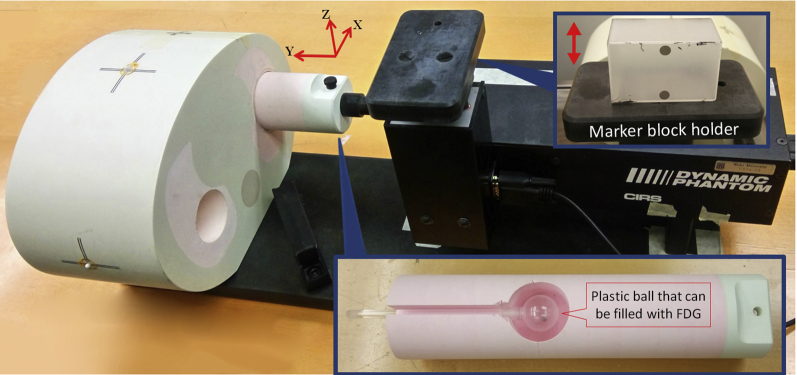
An anthropomorphic thorax phantom with a spherical plastic ball inside the lung portion (CIRS Dynamic Thorax Phantom, CIRS Inc., Norfolk, VA) was used for the study (Fig 1). The plastic ball, with an inner diameter of 2.5 cm, was filled with 18F-fluorodeoxyglucose (FDG) solution to mimic tumor uptake. The activity concentration of the FDG solution at the time of PET scanning was approximately 0.040 MBq/mL. Different motions were imposed on the ball along the superior-inferior direction through an external motor to mimic a patients' breathing motion.
Figure 1.
CIRS dynamic thorax phantom. The cylindrical insert in the lung portion can move in the Y direction. A spherical plastic ball filled with 18F-fluorodeoxyglucose solution can be placed in the hole in this cylindrical insert to mimic the tumor uptake. The marker block holder is used to place the Varian RPM marker block for motion tracking. The holder motion is in the Z direction and mechanically coupled with the cylindrical insert motion.
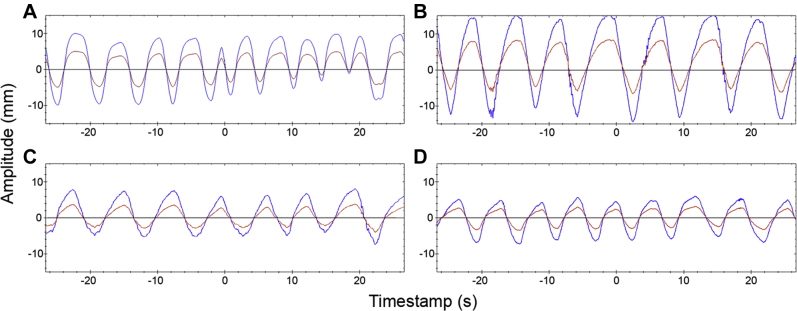
A Siemens Biograph mCT PET/CT simulator (Siemens Medical Solutions, Knoxville, TN) was used for PET-CT imaging. First, 3-dimensional PET-CT imaging of the phantom without tumor motion was performed to acquire reference images. Then, 6 different breathing motions were imposed on the ball, including 2 simulated sinusoidal patterns and 4 actual patients' breathing traces (Fig 2); Figure 2 shows the actual patients' breathing traces. The amplitude of the tumor motion in these traces was measured with 4-dimensional CT imaging and was 18.6 mm, 24.0 mm, 12.3 mm, and 12.0 mm for traces 2(A) to 2(D), respectively. The amplitudes of the 2 simulated sinusoidal motions were 15.4 mm and 30.6 mm, respectively, with period of 5 s/cycle for both motions. Finally, the phantom was imaged with 3- and 4-dimensional PET modes for each breathing motion. The 4-dimensional PET imaging protocol in the scanner uses the Varian RPM system (Varian Medical Systems, Palo Alto, CA) for respiratory gating and helical FB CT for AC. The acquisition times for 3- and 4-dimensional PET were 2 minutes and 8 minutes per bed position, respectively. An additional 4-dimensional CT scan for each breathing motion was acquired to make different types of CT images available for AC in the modified reconstruction protocols.
Figure 2.
Four actual patients' breathing traces used in the target motions. The amplitudes of motion (measured by 4-dimensional computed tomography imaging) in (A) to (D) were 18.6 mm, 24.0 mm, 12.3 mm, and 12.0 mm, respectively. The blue curve represents the actual motion of the target (spherical ball) in the superior-inferior direction, and the red curve represents the motion of the marker block holder (used for 4-dimensional imaging) in the anterior-posterior direction.
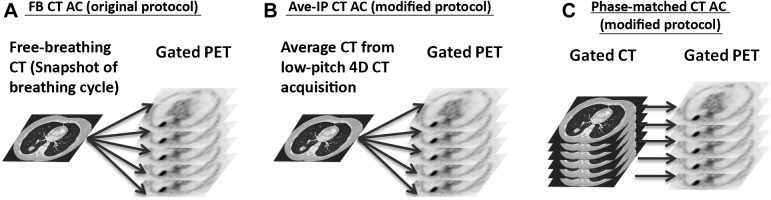
The 4-dimensional scan was performed with a pitch of 0.09, tube rotation time of 0.5 seconds at 120 kV, and 40 mAs/rotation. The 4-dimensional CT images were retrospectively reconstructed in 10 respiratory phases based on the breathing trace that was recorded by Varian RPM with phase-based gating. The Ave-IP CT in this study was created by averaging the untagged CT images from low-pitch 4-dimensional acquisition by Siemens software; thus, the breathing trace information was not needed for Ave-IP CT reconstruction. Ten phases of 4-dimensional PET images were reconstructed with phase-based gating and 3 different types of AC image: the same FB CT for all PET phases, the same Ave-IP CT for all PET phases, and 4-dimensional CT for phase-matched AC. Figure 3 shows the principal differences between the original protocol with only FB CT as the AC image and the 2 modified protocols with Ave-IP CT and phase-matching AC images. The other reconstruction parameters remained the same for all PET image reconstructions (including 3-dimensional PET imaging), which used the ordered subset expectation–maximization algorithm with spatial resolution modeling and time-of-flight (2 iterations, 21 subsets, 0.407 × 0.407 × 0.200 cm3/voxel, 0.5 cm full-width at half-maximum filtration).
Figure 3.
Difference in attenuation corrections between the current 4-dimensional positron emission tomography (PET) imaging protocol on the Siemens PET-CT scanner and the modified protocols for this study. (A) The same computed tomography (free breathing CT) is applied to every phase of the gated PET for attenuation correction in the original protocol. (B) Average intensity projection CT was acquired and applied to every phase of the gated PET for attenuation correction in a modified protocol. (C) Gated 4-dimensional CT was acquired and a phase-matched attenuation correction was applied for the gated 4-dimensional PET image in a modified protocol.
Data analysis
SUVmax and MTV were measured from the PET images. Values of these metrics that were measured from the static phantom with no motion were used as references to evaluate the accuracy of different imaging methods when tumor motion was introduced to the phantom. The MTV was calculated with 2 different thresholds21: absolute SUV value of 2.5 (Vol-2.5) and 40% of SUVmax (Vol-40%). To compare the accuracy of metrics that were measured in 3- versus 4-dimensional PET, the measurements that were derived from 3-dimensional PET were evaluated against the measurements from the end of the exhalation phase of 4-dimensional PET, which was reconstructed with the end of the exhalation phase 4-dimensional CT for AC. The end of the exhalation phase was chosen on the basis of a previous study,17 which showed that the end of the exhalation phase of the 4-dimensional PET had minimum variability when considering only a subset of 4-dimensional PET with phase-matched AC. Comparison results were reported as percent accuracy relative to the reference metric values, with mean ± standard deviation (SD) taken over measurements from all 6 motion patterns. To compare the accuracy of metrics measured from 4-dimensional PET imaging with 3 different types of CT images for AC, results were reported as percent accuracy relative to the reference metric values with mean ± SD taken over measurements from all breathing phases and from all 6 motion patterns. The statistical analysis was performed with nonparametric paired Wilcoxon tests.
Results
3- versus 4-dimensional PET
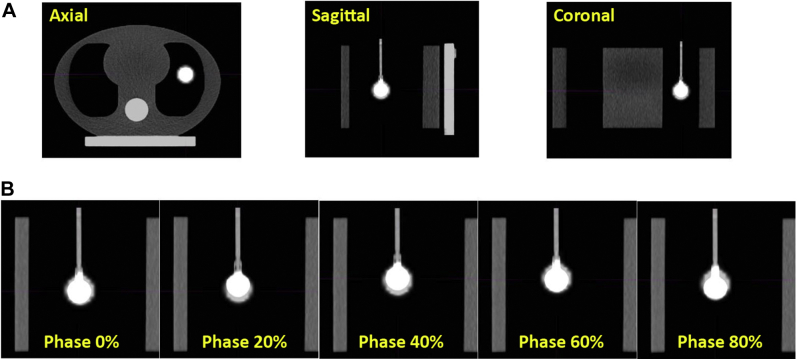
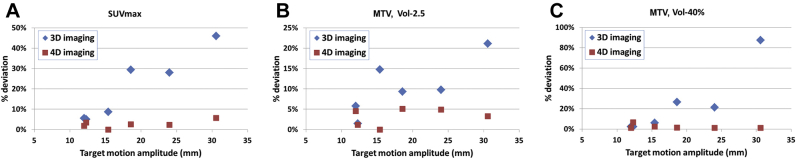
Figure 4 shows the 3-dimensional PET/CT image of the nonmoving static phantom (ie, reference image) and an example 4-dimensional PET/CT image of the moving phantom (sinusoidal motion with 15.4 mm amplitude). The percentage difference between the tumor SUVmax measured with 3-dimensional PET imaging of the moving phantom and the reference value was 20.4% ± 16.7% (mean ± SD; range, 5.0%-46.0%), whereas the difference between SUVmax measured at the end of the exhalation phase of 4-dimensional PET and the reference value was 2.6% ± 1.9% (range, 0.0%-5.7%), which was significantly different (P = .031, Wilcoxon test).
Figure 4.
Three- and 4-dimensional positron emission tomography and computed tomography (PET/CT) images of the phantom. (A) Three-dimensional PET/CT image of the nonmoving phantom where the CT and PET images are fused together. Three different views (axial, sagittal, and coronal) across the tumor are shown. (B) Four-dimensional PET/CT image of the moving phantom (sinusoidal motion with 15.4 mm amplitude). The 4-dimensional PET image was reconstructed with phase-matched attenuation correction. Sagittal views of PET/CT fusion from 5 of 10 breathing phases are shown.
The deviations of Vol-2.5 from the reference value were 10.4% ± 6.9% in 3-dimensional imaging and significantly different at 3.2% ± 2.1% in 4-dimensional imaging (P = .031, Wilcoxon test). The deviations of Vol-40% from the reference value were 24.4% ± 32.5% in 3-dimensional imaging and not significantly different at 2.3% ± 2.2% in 4-dimensional imaging (P = .156, Wilcoxon test). The SUVmax was underestimated in the 3-dimensional PET compared with the reference value, although it was slightly overestimated at the end of the exhalation phase of 4-dimensional PET in all motion patterns. Vol-2.5 was overestimated in both 3- and 4-dimensional PET imaging, and Vol-40% was overestimated in 3-dimensional PET but showed mixed patterns in 4-dimensional PET among different motion patterns. Figure 5 shows the relationship between the measurement deviation of PET metric values from the reference and the amplitude of the target motion. The deviation in 3-dimensional PET imaging generally increased with increased motion amplitude. This kind of relationship was not seen in 4-dimensional PET imaging, which suggests that 4-dimensional imaging successfully mitigated the impact of motion on PET metrics measurements.
Figure 5.
Deviation of maximum standardized uptake value and metabolic tumor volume from the reference values as a function of motion amplitude, for 3- and 4-dimensional positron emission tomography (PET) images. X-axis: amplitude of target motion. Y-axis: percentage deviation of measured metrics from the reference values. Each symbol represents a result from one breathing motion. Four-dimensional values are for the end of the exhalation phase.
Impact of CT image used for AC in 4D PET
Table 1 summarizes the variations of measured PET metrics throughout different phases of the 4-dimensional PET image. The variation was determined by comparing the numbers from each phase with the reference values. Three different AC methods are compared in Table 1, showing that 4-dimensional PET with AC that uses phase-matched 4-dimensional CT had minimal variation in measured SUVmax and MTV numbers throughout the entire breathing cycle. However, AC with FB CT and Ave-IP CT both produced larger variations (Wilcoxon test, P < .001 for all metric comparisons between phase-matched 4-dimensional CT and FB CT and between phase-matched 4-dimensional CT and Ave-IP CT). The variation of these metrics between phases increased with increased motion amplitude when each set of motion data was analyzed separately. For the smallest target motion (amplitude of 12.0 mm), mean variations of SUVmax were 4.5%, 4.0%, and 1.5% for AC with FB CT, Ave-IP CT, and phase-matched 4-dimensional CT, respectively. These numbers increased to 13.0%, 7.3%, and 6.5%, respectively, in the images with the largest target motion (amplitude of 30.6 mm). Vol-2.5 and Vol-40% also showed a similar trend between phase-specific variation and target motion amplitude.
Table 1.
Variation in SUVmax, Vol-2.5, and Vol-40% as a function of AC method implemented in 4-dimensional PET reconstruction (mean ± SD calculated over all phases and over all six motion patterns)
| CT used for AC | SUVmax | Vol-2.5 | Vol-40% |
|---|---|---|---|
| FB CT | 8.7% ± 5.6% | 4.1% ± 3.2% | 5.8% ± 4.5% |
| Ave-IP CT | 6.1% ± 3.0% | 5.8% ± 5.3% | 6.5% ± 6.7% |
| Phase-matching 4-dimensional CT | 4.1% ± 3.3% | 2.9% ± 2.9% | 2.6% ± 0.7% |
AC, attenuation correction; Ave-IP CT, average intensity projection computed tomography; CT, computed tomography; FB CT, free-breathing computed tomography; PET, positron emission tomography; SD, standard deviation; SUVmax, maximum standardized uptake value.
Discussion
In this study, the accuracy of PET metric measurements in a moving target was evaluated with different imaging techniques. Four-dimensional imaging in comparison with 3-dimensional imaging showed improved accuracy in measuring tumor SUVmax and volume with more pronounced improvements in the targets moving with larger amplitudes. Nonparametric paired Wilcoxon tests showed significant differences in measurement accuracy between 3- and 4-dimensional PET for SUVmax and Vol-2.5 (P < .05) but failed to show significance for Vol-40% (P > .05). The power of the statistical analysis was low in this comparison due to the small sample size of the study, but it is clear in Figure 5 that 4-dimensional PET reduced the impact of motion on measured SUVmax and MTV when compared with 3-dimensional PET.
These results are in accordance with the findings of Nagel et al,19 who also showed improvement in the imaging of moving objects with respiration-correlated PET/CT scanning. In contrast to their study, our experiments used measured metrics from 3-dimensional PET imaging of the nonmoving static phantom as the reference values so that variations introduced by PET imaging characteristics (eg, resolution, dose cross calibration, reconstruction, etc.) will have the same effect on reference and moving phantom imaging. The deviation of measured metric values in the moving phantom then is mainly attributed to the motion of the phantom.
In this study, we also compared different AC methods in the 4-dimensional PET reconstruction. The results showed that AC with the same FB CT for all different phases of 4-dimensional PET is not as accurate as phase-matched reconstruction with 4-dimensional CT. Pönisch et al18 performed experiments on a Siemens Biograph PET/CT scanner, which was modified to extend its normal capability to include phase-matched AC, and reached a similar conclusion that use of a 4-dimensional CT is superior to that of a 3-dimensional CT in 4-dimensional PET reconstruction. Although Pönisch et al18 only investigated sinusoidal motion patterns in their work, this study extended the research to more motions, including 4 actual patients' breathing traces. Nyflot et al17 conducted a study on the impact of AC method on 4-dimensional PET/CT imaging with a GE Discovery STE PET/CT scanner (GE Healthcare, Waukesha, WI). In contrast to the results from our study, they concluded that the phase-matched AC with 4-dimensional CT is not necessarily the optimal correction method due to image artifacts and differences in CT and the PET phase sorting. In our study, the performance of different AC methods was tested with a Siemens PET/CT scanner, which led to differences in image acquisition and reconstruction between this work and the study by Nyflot et al,17 such as the low-pitch helical acquisition of 4-dimensional CT with the Siemens system versus the cine axial acquisition of the GE system and the different filter and/or kernel models that were used in the PET reconstruction. In addition to the intrinsic differences between the 2 scanners, there were differences in experimental settings, such as the number of phase bins for 4-dimensional images, range of motion amplitude, phantoms used, and method of generating Ave-IP CT images. This warrants further investigation of 4-dimensional PET AC methods across different scanners, breathing patterns, phantoms, and PET acquisition and image reconstruction techniques.
This study only included phantom experiments and lacks evaluation with actual patient data. However, the ground truth reference values of SUVmax and MTV are only available with phantom data by stopping the tumor motion in the phantom. With actual patient imaging, this is not achievable. Moreover, as a step toward patient imaging, 4 of the breathing traces that were used in the experiments in this study were derived from the monitoring of actual patient breathing.
Phase-matched AC provides better SUVmax and MTV accuracy in 4-dimensional PET imaging but requires a higher imaging dose from 4-dimensional CT. The reference CT dose index for a thorax scan in our Siemens scanner is 3.26 mGy for 3-dimensional imaging and 26.22 mGy for 4-dimensional imaging. Benefits and risks should be carefully evaluated when adding increasing radiation dose to the patients. AC with free breathing CT did not produce results as accurate as those of the phase-matched AC but still reduced the deviation in measured metrics dramatically compared with 3-dimensional PET imaging. If additional 4-dimensional CT imaging is of concern in terms of patient dose and scanning time, an FB CT is still an acceptable means of AC for 4-dimensional PET imaging with up to 6.5% loss in accuracy relative to phase-matched AC for motion amplitudes and patterns that are similar to those in this study. On the basis of the results of our experiments, Ave-IP CT did not show improvements in the accuracy of PET metrics compared with FB CT.
Phase mismatch exists between 4-dimensional CT and 4-dimensional PET.17 The 4-dimensional PET/CT data that were acquired in this study showed a relatively small mismatch at the end of exhalation (50%) and inhalation (0%) phases but had a larger mismatch between these 2 phases (Fig 4B). Future studies are needed to investigate how to quantify the mismatch and how it correlates with the accuracy of PET metric measurements. Studies to minimize the difference in phase sorting between CT and PET should provide more accurate phase-matched CT for PET reconstruction, which in theory should benefit the quality of 4-dimensional PET imaging.
Conclusions
Four-dimensional PET imaging reduces the impact of motion on measured SUVmax and MTV compared with 3-dimensional PET imaging. A 4-dimensional scanning protocol should be considered to improve the accuracy of SUVmax and MTV measurements of moving tumors when these metrics are critical for treatment and outcome assessment. AC that uses non-gated CT in 4-dimensional PET imaging is not optimal for quantitative analysis but can still provide improvement over 3-dimensional PET imaging. Clinical 4-dimensional PET imaging protocols should consider phase-matched 4-dimensional CT imaging, if available, to achieve better accuracy in PET metric measurements.
Footnotes
Sources of support: None.
Conflicts of interest: Yunfeng Cui and James Bowsher have nothing to disclose. Jing Cai reports grants from NIH-NCI outside of the submitted work. Fang-Fang Yin reports grants from NIH-NCI and Varian Medical Systems outside of the submitted work.
References
- 1.Konert T., Vogel W., MacManus M.P. PET/CT imaging for target volume delineation in curative intent radiotherapy of non-small cell lung cancer: IAEA consensus report 2014. Radiother Oncol. 2015;116:27–34. doi: 10.1016/j.radonc.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Grills I.S., Yan D., Black Q.C., Wong C.Y., Martinez A.A., Kestin L.L. Clinical implications of defining the gross tumor volume with combination of CT and 18FDG-positron emission tomography in non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;67:709–719. doi: 10.1016/j.ijrobp.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 3.Bradley J., Bae K., Choi N. A phase II comparative study of gross tumor volume definition with or without PET/CT fusion in dosimetric planning for non-small-cell lung cancer (NSCLC): primary analysis of Radiation Therapy Oncology Group (RTOG) 0515. Int J Radiat Oncol Biol Phys. 2012;82:435–441. doi: 10.1016/j.ijrobp.2010.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillham C., Zips D., Pönisch F. Additional PET/CT in week 5-6 of radiotherapy for patients with stage III non-small cell lung cancer as a means of dose escalation planning? Radiother Oncol. 2008;88:335–341. doi: 10.1016/j.radonc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 5.van Elmpt W., Ollers M., Dingemans A.M., Lambin P., De Ruysscher D. Response assessment using 18F-FDG PET early in the course of radiotherapy correlates with survival in advanced-stage non-small cell lung cancer. J Nucl Med. 2012;53:1514–1520. doi: 10.2967/jnumed.111.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Usmanij E.A., de Geus-Oei L.F., Troost E.G. 18F-FDG PET early response evaluation of locally advanced non-small cell lung cancer treated with concomitant chemoradiotherapy. J Nucl Med. 2013;54:1528–1534. doi: 10.2967/jnumed.112.116921. [DOI] [PubMed] [Google Scholar]
- 7.Lee P., Kupelian P., Czernin J., Ghosh P. Current concepts in F18 FDG PET/CT-based radiation therapy planning for lung cancer. Front Oncol. 2012;2:71. doi: 10.3389/fonc.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang W., Zhou T., Ma L. Standard uptake value and metabolic tumor volume of 18F-FDG PET/CT predict short-term outcome early in the course of chemoradiotherapy in advanced non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2011;38:1628–1635. doi: 10.1007/s00259-011-1838-5. [DOI] [PubMed] [Google Scholar]
- 9.Boellaard R., Krak N.C., Hoekstra O.S., Lammertsma A.A. Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: A simulation study. J Nucl Med. 2004;45:1519–1527. [PubMed] [Google Scholar]
- 10.Lockhart C.M., MacDonald L.R., Alessio A.M., McDougald W.A., Doot R.K., Kinahan P.E. Quantifying and reducing the effect of calibration error on variability of PET/CT standardized uptake value measurements. J Nucl Med. 2011;52:218–224. doi: 10.2967/jnumed.110.083865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westerterp M., Pruim J., Oyen W. Quantification of FDG PET studies using standardised uptake values in multi-centre trials: effects of image reconstruction, resolution and ROI definition parameters. Eur J Nucl Med Mol Imaging. 2006;34:392–404. doi: 10.1007/s00259-006-0224-1. [DOI] [PubMed] [Google Scholar]
- 12.Greco C., Rosenzweig K., Cascini G.L., Tamburrini O. Current status of PET/CT for tumour volume definition in radiotherapy treatment planning for non-small cell lung cancer (NSCLC) Lung Cancer. 2007;57:125–134. doi: 10.1016/j.lungcan.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 13.Park S.J., Ionascu D., Killoran J. Evaluation of the combined effects of target size, respiratory motion and background activity on 3D and 4D PET/CT images. Phys Med Biol. 2008;53:3661–3679. doi: 10.1088/0031-9155/53/13/018. [DOI] [PubMed] [Google Scholar]
- 14.Liu C., Pierce L.A., Alessio A.M., Kinahan P.E. The impact of respiratory motion on tumor quantification and delineation in static PET/CT imaging. Phys Med Biol. 2009;54:7345–7362. doi: 10.1088/0031-9155/54/24/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aristophanous M., Berbeco R.I., Killoran J.H. Clinical utility of 4D FDG-PET/CT scans in radiation treatment planning. Int J Radiat Oncol Biol Phys. 2012;82:e99–e105. doi: 10.1016/j.ijrobp.2010.12.060. [DOI] [PubMed] [Google Scholar]
- 16.Nehmeh S.A., Erdi Y.E., Pan T. Four-dimensional (4D) PET/CT imaging of the thorax. Med Phys. 2004;31:3179–3186. doi: 10.1118/1.1809778. [DOI] [PubMed] [Google Scholar]
- 17.Nyflot M.J., Lee T.-C., Alessio A.M. Impact of CT attenuation correction method on quantitative respiratory-correlated (4D) PET/CT imaging. Med Phys. 2015;42:110–120. doi: 10.1118/1.4903282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pönisch F., Richter C., Just U., Enghardt W. Attenuation correction of four dimensional (4D) PET using phase-correlated 4D-computed tomography. Phys Med Biol. 2008;53:N259–N268. doi: 10.1088/0031-9155/53/13/N03. [DOI] [PubMed] [Google Scholar]
- 19.Nagel C.C., Bosmans G., Dekker A.L. Phased attenuation correction in respiration correlated computed tomography/positron emitted tomography. Med Phys. 2006;33:1840–1847. doi: 10.1118/1.2198170. [DOI] [PubMed] [Google Scholar]
- 20.Killoran J.H., Gerbaudo V.H., Mamede M., Ionascu D., Park S.J., Berbeco R. Motion artifacts occurring at the lung/diaphragm interface using 4D CT attenuation correction of 4D PET scans. J Appl Clin Med Phys. 2011;12:3502. doi: 10.1120/jacmp.v12i4.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nestle U., Kremp S., Schaefer-Schuler A. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-small cell lung cancer. J Nucl Med. 2005;46:1342–1348. [PubMed] [Google Scholar]